Abstract
Injury to retinal ganglion cell (RGC) axons triggers rapid activation of Jun N-terminal kinase (JNK) signaling, a major prodeath pathway in injured RGCs. Of the multiple kinases that can activate JNK, dual leucine kinase (Dlk) is known to regulate both apoptosis and Wallerian degeneration triggered by axonal insult. Here we tested the importance of Dlk in regulating somal and axonal degeneration of RGCs following axonal injury. Removal of DLK from the developing optic cup did not grossly affect developmental RGC death or inner plexiform layer organization. In the adult, Dlk deficiency significantly delayed axonal-injury induced RGC death. The activation of JUN was also attenuated in Dlk deficient retinas. Dlk deficiency attenuated the activation of the somal pool of JNK but did not prevent activation of the axonal pool of JNK after axonal injury, indicating that JNK activation in different cellular compartments of an RGC following axonal injury is regulated by distinct upstream kinases. In contrast to its robust influence on somal degeneration, Dlk deficiency did not alter RGC axonal degeneration after axonal injury as assessed using physiological readouts of optic nerve function.
Introduction
Axonal injury is a hallmark of several neurodegenerative disorders and can lead to permanent functional impairment as a result of neuronal cell death and axonal degeneration. In glaucoma, an early critical site of insult to retinal ganglion cell (RGC) axons occurs in the region of the lamina as they exit the eye (Anderson and Hendrickson, 1974; Howell et al., 2007b; Quigley et al., 1983; Schlamp et al., 2006). Axonal injury initiates a cascade of signaling events both proximally and distally to the site of insult to trigger somal and axonal degeneration respectively (Abe and Cavalli, 2008; Coleman, 2005; Howell et al., 2012). The somal and axonal degeneration pathways in glaucoma appear to be molecularly distinct (Howell et al., 2012; Whitmore et al., 2005). Bax deficiency prevents RGC death following axonal injury, but BAX is not required for axonal degeneration (Howell et al., 2007a; Howell et al., 2011; Li et al., 2000; Libby et al., 2005). The WldS mutation delays axonal degeneration but does not prevent somal degeneration of RGCs following axonal injury (Beirowski et al., 2008; Lorber et al., 2012). It remains unknown how axonal injury triggers different effectors to regulate degeneration of these distinct cellular compartments. A key to understanding glaucomatous neurodegeneration will be to identify and critically test the importance of molecules activated by axonal injury in regulating both somal and axonal degeneration.
Previously we have shown that Jun N-terminal kinase (JNK) is activated in RGC axons following axonal injury (Fernandes et al., 2012). Of the multiple kinases that can activate JNK, dual leucine kinase (DLK; also known as MAP3K12) is known to be activated by axonal injury and regulate cell death triggered by pathological JNK activation in neurons. DLK is expressed in axons (Eto et al., 2010; Hirai et al., 2005; Xiong et al., 2010) and has been shown to function in retrograde injury signaling to the soma following axonal injury (Xiong et al., 2010). DLK is required for activation of the stress-induced pool of JNK but does not alter physiological JNK activity in neurons (Ghosh et al., 2011). Genetic deletion of Dlk abolishes the axonal injury induced accumulation of JNK and activation of JUN in the cell body as well as transcriptional responses to axonal injury (Watkins et al., 2013; Xiong et al., 2010). DLK has also been implicated in axonal degeneration (Ghosh et al., 2011; Miller et al., 2009). DLK promotes degeneration of both embryonic DRG axons in vitro following axotomy or neurotrophic deprivation as well as adult sciatic nerves in vivo following transection (Ghosh et al., 2011; Miller et al., 2009). In contrast, DLK functions as an inhibitor of Wallerian degeneration of Drosophila motorneuron axons following injury (Xiong and Collins, 2012). These contrasting functions of DLK likely reflect inherent differences in the requirement of specific molecules in regulating axonal degeneration in different neuronal subtypes. This complexity highlights the importance of testing a molecule’s role in RGC axonal degeneration in the context of a glaucoma-relevant insult.
Here we characterize both somal and axonal degeneration of RGCs following controlled optic nerve crush (CONC) in Dlk deficient animals. Consistent with published reports (Watkins et al., 2013; Welsbie et al., 2013), Dlk deficiency significantly attenuated the number of dying RGCs after CONC. The activation of JNK and its canonical substrate, JUN, was significantly attenuated in Dlk deficient retinas. However, while Dlk deficiency abolished the activation of JNK in RGC somas, JNK was still activated in RGC axons both proximal and distal to the site of injury in Dlk deficient mice. Surprisingly, Dlk deficiency had no effect on the degeneration of the axon distal to the site of injury. Collectively, these data support a role for DLK in somal but not axonal degeneration of RGCs following optic nerve crush. Furthermore, these data suggest that distinct upstream kinases regulate activation of JNK in distinct cellular compartments.
Materials and Methods
Mice
Dlk deficiency results in perinatal lethality in mice (Hirai et al., 2006). Therefore, to study Dlk’s function in the developing and adult retina a floxed allele of Dlk (Dlkfl; Miller et al., 2009) was recombined using a retinal expressed cre, Tg(Six3-cre)69Frty mice (referred to as Six3-cre; Furuta et al., 2000) or conditionally in the adult using a tamoxifen inducible cre, Tg(CAG-cre/Esr1)5Amc (referred to as Cre-ER™; Hayashi and McMahon, 2002) respectively. Both of these cre-recombinases deleted Dlk with a high efficiency in the retina as judged by western blot analysis (Suppl. Fig. 1; protein level was reduced by >95% in both the Six3-cre and Cre-ER™ lines). Conditional deletion of Dlk in the adult was performed as previously described for other similarly constructed floxed alleles (Harder et al., 2012). Adult mice older than 45 days received an intraperitoneal injection of tamoxifen (5mg/40g mouse, dissolved in corn oil) once a day for 5 consecutive days. After the fifth injection the mice were left alone for 10 days to allow time for the deletion and degradation of DLK. For all experiments, mice wildtype for the floxed allele with or without cre were used as controls. In experiments involving the conditional deletion of Dlk using tamoxifen, the control mice were also injected with tamoxifen. Mice with recombined floxed alleles are referred to as Dlk−/− and mice containing wildtype alleles or non-recombined floxed alleles are refereed to as Dlk+/+ or wildtype. Six3-cre was used to delete the floxed allele of Dlk during retinal development and was used for both the optic nerve explant culture and electrophysiology experiments, since in both these cases we sought to test the role of DLK intrinsically in RGC axons. In all other experiments, Cre-ER™ was used to delete floxed Dlk alleles. For experiments assessing JUN, a floxed allele of Jun (Junfl; Behrens et al., 2002) was deleted with the Six3-cre. Six3-cre causes complete Jun recombination in the vast majority of RGCs (89.7% of RGCs; Fernandes et al., 2013). Mice deficient in both Jnk2 (Mapk9tm1Flv, Jackson Laboratory stock number 004321) and Jnk3 (Mapk10tm1Flv, Jackson Laboratory stock number 004322) are referred to as Jnk2/3−/− or Jnk2/3 deficient mice in the text. The original WldS allele (Lunn et al., 1989; Mack et al., 2001) was backcrossed into C57BL/6J > 20 generations. Mice were housed in a 12-hour light dark cycle and were fed chow and water ad libitum. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology’s statement on the use of animals in ophthalmic research and were approved by the University of Rochester’s University Committee on Animal Resources.
Optic nerve injury
Controlled optic nerve crush (CONC) was performed as previously described (Harder and Libby, 2011; Libby et al., 2005). In brief, optic nerves were crushed for approximately 5 seconds just behind the eye using self-closing forceps (Roboz RS-5027). For control eyes, unmanipulated or contralateral eyes that had a sham surgery performed (where the optic nerve was exposed but not crushed) were used as controls. Mice were harvested at the indicated time-points (2 hrs, 5 days, 7 days, or 35 days) following CONC.
Immunohistochemistry and Cell Counts
Experiments were performed as previously described (Fernandes et al., 2012). Whole eyes were fixed in 4% paraformaldehyde (PFA) for 2 hours and then the anterior segment was removed and the posterior eye cup was processed for cryosectioning or whole mount immunostaining. Immunohistochemistry was performed on 14μm retinal sections. Sections were incubated with primary antibodies overnight at 4°C. The following primary antibodies were used on sections: rabbit anti-pJNK (Cell Signaling, 4668S, 1:250; note, this antibody recognizes all three JNK isoforms phosphorylated at Thr183 and Tyr185 sites), goat anti-CHAT (Chemicon, AB144P, 1:500), rabbit anti-calretinin (Oncogene, PC254L, 1:1000), and mouse anti-Neurofilament (Abcam, ab24575, 1:1000). The following day the sections were washed and incubated with Alexafluor-conjugated secondary antibodies (Invitrogen). Sections were then counterstained with DAPI and then coverslipped in Fluorogel in TRIS buffer (Electron Microscopy Sciences). For whole mount immunostaining, retinas were incubated for with primary antibodies for 72 hours at 4°C. The following primary antibodies were used: rabbit anti-cCASP3 (RD, AF835, 1:1000), mouse anti-TUJ1 (Covance, MMS-435P, 1:1000), goat anti-POU4F2 (Santacruz, sc-6026, 1:250). Following the incubation with primary antibodies, the retinas were washed and then were incubated with Alexafluor-conjugated secondary antibodies (Invitrogen) for 24 hours at 4°C. Slides were mounted RGC side up. Because RGC density varies with respect to retinal location, for RGC counting images were obtained from eight 20x fields around the peripheral retina (two from each quadrant). Each field was approximately 220 μm from the peripheral edge of the retina (one half of a 20X field in from the peripheral margin). The number of immunolabeled cells in each image was quantified using the cell-counter tool in ImageJ. For quantification of nerve fiber layer axon bundles, the thickness of 8 random TUJ1+ axon bundles were measured approximately 300-400μm from the optic disc. Note for all histological measurements and cell counts, the experimenter was masked to genotype and experimental group.
Western Blotting
Experiments were performed as previously described (Fernandes et al., 2012). Approximately 30μg of protein was loaded in each lane (Nanodrop was used to quantify protein concentrations from retinal protein lysates). Proteins of interest were detected with the following primary antibodies: rabbit anti-DLK (1:300, (Hirai et al., 2002)), rabbit anti-JUN (Cell Signaling, 9165S; 1:500), rabbit anti-pJUN (Cell Signaling, 9261S, 1:500), mouse anti-GAPDH, Calbiochem CB1001, 1:3000), and mouse anti-α-tubulin (Sigma, T5168, 1:1000). After overnight incubation with primary antibody at 4C, membranes were washed and then incubated with secondary antibodies: HRP-conjugated anti-rabbit IgG (Biorad Laboratories, 170-6515, 1:10,000) HRP-conjugated anti-mouse IgG (Biorad Laboratories, 170-6516, 1:10,000) for 1 hour at room temperature. Immunoreactive bands were detected using an enhanced chemiluminescence reagent kit (Supersignal West Dura Extended Substrate, Pierce, 34075 or Immun-star, BioRad 170-5070). Densitometric analysis was used to determine the relative abundance of proteins using Quantity One software (BioRad). Amounts of protein were normalized to the loading control and then expressed relative to appropriate control group for the experiment.
Optic nerve explant culture
Optic nerves were dissected free by transecting them close to the eye and at the chiasm. Optic nerves were then incubated in artificial cerebrospinal fluid (ACSF) aerated with 95% oxygen/5% CO2 at room temperature for 4 hours. ACSF was prepared with the following constituents (in mM): 125 NaCl, 1.25 NaH2PO4, 25 glucose, 25 NaHCO3, 2.5 CaCl2, 1.3 MgCl2, and 2.5 KCl. Nerves were then fixed in 4% PFA for 2 hours following which they were permeabilized with 100% methanol for 5 minutes. Nerves were rinsed in PBS and then cryoprotected by immersion in 30% sucrose overnight. 10μm cryosections were obtained and then processed for immunohistochemistry as described above for retinal sections.
Electrophysiology
Animals were sacrificed at either 5 or 7 days following CONC and the optic nerves were dissected free by transecting the nerve close to the eye and at the chiasm. Following dissection both optic nerves were incubated for a minimum of 60 minutes in ACSF aerated with 95% oxygen/5% CO2 at room temperature. Optic nerves were then transferred to a temperature controlled recording chamber containing a continuous flow of oxygenated ACSF. Suction pipette electrodes with flared tips were filled with ACSF and were used to draw the nerve in at both the simulating end (retinal end) and the recording end (chiasm end). A stimulus isolation icrosec pulse. The method of Stys et. al. (Stys et al., 1991) was used to measure the resistance of the recording pipette, both before (R1) and after (R2) the nerve was inserted. R2 could be varied by altering the level of suction, and thereby changing the electrical seal of the nerve in the pipette. Stys et al. (Stys et al., 1991) reported that the compound action potential (CAP) area was a linear function of R2, and we found that this held for the CAP amplitude as well. We further found that if the CAP was measured with the ratio R2/R1 constant, the amplitude of the signal was independent of the particular glass pipette used, to within 5%. Thus, as a means of normalizing the data, all measurements of amplitude were made with R2/R1 = 1.7, a value that was easily reached with gentle suction for all nerves examined. All recordings were performed at 25°C and 37°C. Note that relative changes between these genotypes were similar at both temperatures. Only the data from 25°C is presented. Records were digitized and analyzed off-line.
Statistical Analysis
At least 3 retinas were analyzed for each genotype for all experimental conditions. During the analysis of cell counts the experimenter was masked to genotype and experimental group. Statistical significance of cell counts was determined using the unpaired Student’s t-test with significance reported at P values < 0.05. Experiments comparing differences across more than two genotypes at a single timepoint were analyzed using a one-way ANOVA followed by the Bonferroni post hoc test for group comparisons with P < 0.05 reported as significant. Experiments with more than 2 groups and comparing differences across more than one time point were analyzed using a two-way ANOVA followed by the Bonferroni post hoc test for group comparisons with significance determined at P values < 0.05.
Results
Dlk deficiency does not appear to alter RGC development
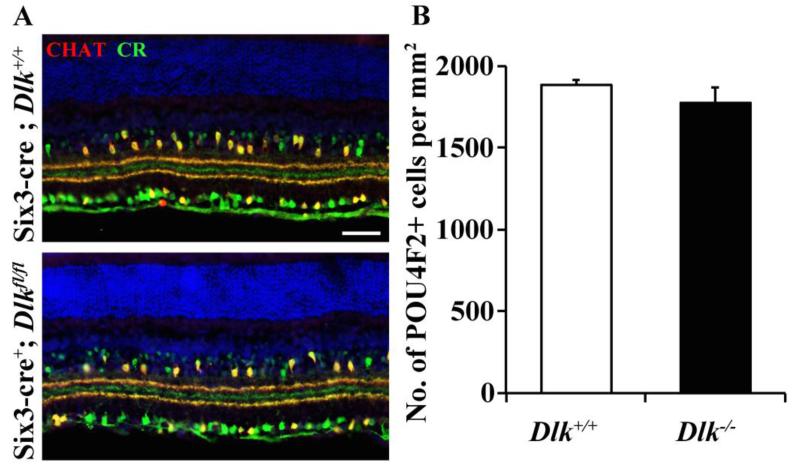
DLK is expressed during development and has been shown to regulate diverse aspects of neuronal development including neuron migration (Hirai et al., 2002), developmental cell death (Ghosh et al., 2011), dendritic morphology (Wang et al., 2013b) and synapse development (Klinedinst et al., 2013). To determine if Dlk was required for development of the retina, a floxed allele of Dlk was conditionally deleted in the retina using an early retinal cre, Six3-cre (Six3-cre is expressed in the developing optic cup starting from E9.0-9.5; Furuta et al., 2000). Developmental deletion of Dlk also did not alter the stratification of RGC and amacrine cell arbors in the inner plexiform layer (Fig. 1A). Deletion of Dlk throughout retinal development did not alter developmental RGC death as assessed by the number of POU4F2 labeled surviving RGCs in the postnatal retina (Fig. 1B). Thus, deficiency in Dlk does not appear to alter RGC development.
Figure 1. Dlk deficiency does not appear to significantly affect RGC development.
(A) Representative images from wildtype and Dlk deficient (Six3-cre+; Dlkfl/fl) retinas. Deficiency in Dlk throughout retinal development (Six3-cre) did not alter the gross lamination in the inner plexiform layer, as judged by normal organization of calretinin (CR, green) and choline acetyl transferase (CHAT, red) processes (3 retinas of each genotype were assessed). (B) Cell counts of POU4F2 labeled RGCs in the adult retina show that embryonic deletion of Dlk in the retina does not alter developmental cell death of RGCs (P = 0.316; N=6 for each genotype). Scale bar: A, 50 μm.
Dlk deficiency attenuates RGC death following axonal injury
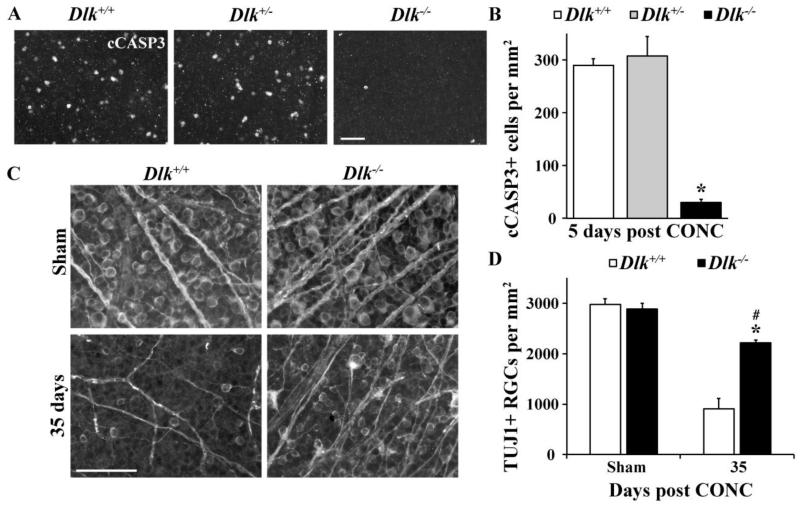
Recently, it has been shown that Dlk deficiency significantly delays RGC death after axonal injury (Watkins et al., 2013; Welsbie et al., 2013). Using a Dlkfl allele, recombined using a tamoxifen inducible cre (Cre-ER™; note non recombined floxed alleles and wildtype alleles are referred to as +, and recombined and inactivated floxed alleles as −) we also found significant protection for RGCs after axonal injury (controlled optic nerve crush; CONC). The number of dying RGCs labeled with the apoptotic marker, cleaved caspase 3 (cCASP3) 5 days after CONC was significantly attenuated in Dlk−/− mice compared to controls (Fig. 2A,B; P < 0.001; n ≥ 8 for each genotype). There was no difference in the number of cCASP3+ cells in Dlk+/- mice compared to Dlk+/+ mice at this time point (Fig. 2A,B; P=0.61). RGC survival, as assessed using the RGC specific marker, TUJ1 (Cui et al., 2003), was significantly increased in Dlk−/− mice at 35 days, a time point when the majority of RGCs have died in wildtype retinas (Fig. 2C,D; P < 0.001; n ≥ 4 for each genotype). However, it is important to note that at 35 days after CONC there was a significant reduction in RGC survival in Dlk−/− mice compared to sham injured Dlk−/− mice (Fig. 2C,D; P =0.02; n ≥ 4 for each genotype). These results confirm the previous reports showing that DLK is an important prodeath molecule in RGCs after axonal injury.
Figure 2. Dlk deficiency delays RGC death after axonal injury.
(A) Representative images from Dlk+/+, Dlk+/- and Dlk−/− flat mounted retinas stained with anti-cleaved caspase 3 (cCASP3) 5 days after CONC, a time point where RGC death peaks (Harder et al., 2012). (B) Cell counts of cCASP3 positive cells at 5 days after CONC (N ≥ 6 for each genotype). Complete deficiency in Dlk significantly lessened the number of dying RGCs after CONC compared to wildtype retinas (P < 0.001). The number of dying cells was similar in Dlk+/- mice compared to wildtype retinas (P = 0.61). To determine if deficiency in Dlk affected the long term survival of RGCs after axonal injury, the number of surviving RGCs (TUJ1+ cells) were counted at 35 days after CONC, a time point when the majority of RGCs have died in wildtype retinas (C,D). (C) Representative images from Dlk+/+ and Dlk−/− flat mounted retinas stained for the RGC marker TUJ1 35 days after CONC. (D) TUJ1 cell counts show that significantly more RGCs survive 35 days after CONC in Dlk deficient retinas compared to wildtype retinas (* P < 0.001). However, at 35 days after CONC, there is significant decrease in RGC numbers in Dlk deficient retinas compared to sham-injured retinas (# P < 0.05). N ≥ 4 for each genotype and condition (sham, CONC); scale bar: A, C, 50 μm.
Dlk deficiency prevents activation of the somal but not axonal pool of JNK following axonal injury
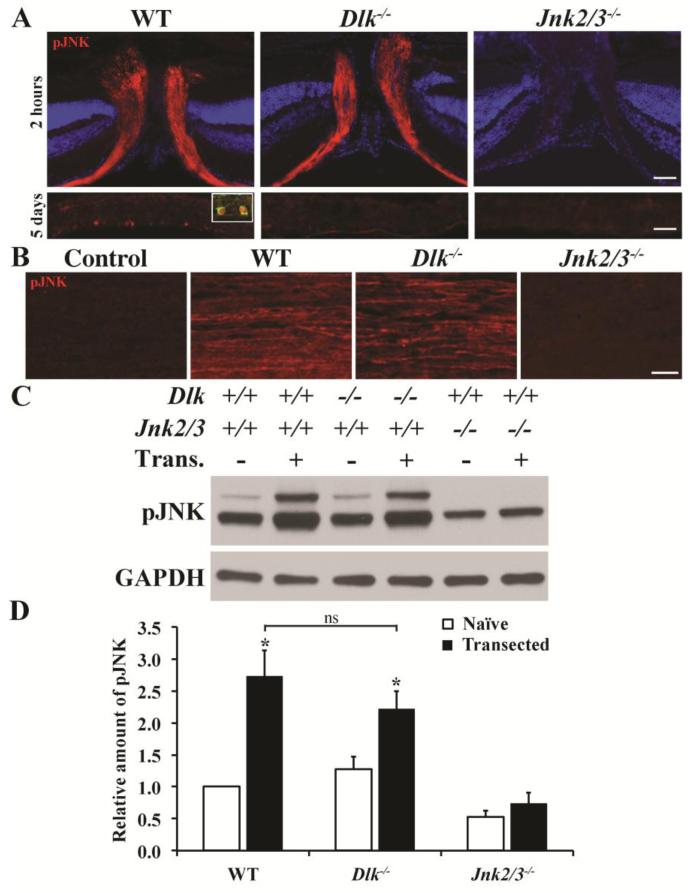
DLK is a MAP3K known to be upstream of JNK phosphorylation. We previously showed that JNK is activated in the proximal segment of RGC axons soon after CONC (proximal is defined as between the site of injury and the cell body; Fernandes et al., 2012). To determine whether DLK regulates injury-induced JNK activation in RGCs after insult, retinal cross sections that included the optic nerve head (the area where RGC axons exit the eye) were stained for the presence of pJNK. As previously shown (Fernandes et al., 2012), pJNK was present in the proximal segment of RGC axons 2 hours after CONC within the optic nerve head in wildtype retinas and Jnk2/3 deficiency dramatically reduces the presence of pJNK (Fig. 3A). Dlk deficiency did not prevent the activation of JNK in RGC axons proximal to the site of injury. In contrast, DLK appears to be required for JNK activation in RGC somas. Five days after axonal injury pJNK fills RGC somas in wildtype retinas, but was not present in RGCs of Dlk−/− mice (Fig. 3A). These data were surprising since DLK is known to be expressed in axons and regulate axonal injury signaling (Eto et al., 2010; Hirai et al., 2005; Xiong et al., 2010). However, it is possible that there is some level of pJNK activation that is controlled by DLK in the proximal axon that was not observed by immunohistochemistry. To confirm the finding that Dlk does not regulate JNK activation in RGC axons, another model of axonal injury was used where optic nerves were transected at both the retinal and the chiasm ends. In optic nerve explants cultured for 4 hours, robust activation of JNK was observed in wildtype axons (Fig. 3B). Similarly, in optic nerve explants from Dlk deficient mice JNK continued to be activated in RGC axons (Fig. 3B). To test if JNK activation induced by optic nerve transection in RGC axons was significantly increased in both wildtype and Dlk deficient optic nerves, pJNK levels in optic nerve explants cultured for 4 hours following transection (referred to as Trans. +) were compared to pJNK levels in optic nerves dissected from animals and immediately transferred to protein lysis buffer. A significant increase in JNK activation (pJNK) was observed in both cultured wildtype and Dlk deficient optic nerves (Fig. 3C,D). pJNK levels did not increase after 4 hours in culture in Jnk2/3 deficient optic nerves (Fig. 3C,D). Note, there was no significant difference in pJNK levels in optic nerves between wildtype and Dlk deficient optic nerves either before or after 4 hours in culture (Fig. 3D; P > 0.05). Thus, DLK does not appear to be required for JNK activation in RGC axons after axonal injury but does have a role in phosphorylating or sustaining JNK activation in RGC somas.
Figure 3. Dlk deficiency does not prevent JNK activation in RGC axons after CONC.
(A) Immunohistochemistry of retinal sections containing the optic nerve head (ONH) 2 hours after CONC shows robust pJNK staining in RGC axons of wildtype mice. Dlk deficiency did not prevent JNK activation in RGC axons in the ONH. As previously shown, combined deficiency of Jnk2 and Jnk3 prevents pJNK detection in RGC axons (Fernandes et al., 2012). Five days after CONC robust pJNK staining is detected in RGC somas in wildtype retinas (inset shows pJNK expressed in a TUJ1+ RGC). Interestingly, despite the presence of pJNK in RGC axons in Dlk deficient mice, pJNK does not appear to be present in somas 5 days after CONC. pJNK was also not present in RGC somas in mice deficient in both Jnk2 and Jnk3. (B) To determine if Dlk is required to phosphorylate JNK in axons, optic nerves were transected both at the retinal and chiasm ends and the optic nerve segments were cultured. Optic nerve transection induces robust activation of JNK in wildtype nerves. In Dlk deficient nerves, pJNK was still detected indicating that Dlk is not required for activation of the axonal pool of JNK in RGCs following axonal injury. (C) Representative western blots showing levels of pJNK increase in optic nerve segments that are transected and cultured for 4 hours. (D) There was a significant increase in pJNK levels in both wildtype and Dlk deficient optic nerves but not Jnk2/3 deficient optic nerves after transection. Additionally, pJNK levels after transection were not significantly different between wildtype and Dlk deficient optic nerves. At least 3 retinas or optic nerves were assessed for each genotype and condition. *, P < 0.05; ns, not significant; Scale bar: A, 50 μm; B, 20μm.
Dlk deficiency attenuates the phosphorylation of JUN following axonal injury
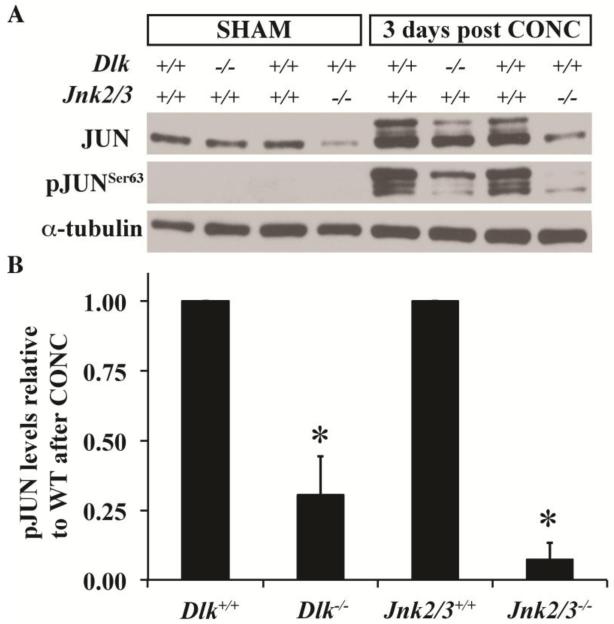
Previously, it has been shown that both Dlk deficiency (Huntwork-Rodriguez et al., 2013; Watkins et al., 2013) and Jnk2/3 deficiency (Fernandes et al., 2012) significantly lessened the amount of pJUN in retinas after optic nerve crush. Given the results showing differences in JNK activation between these two mutants, the effect on JUN induction was compared across these two genotypes 3 days after CONC. Jnk2/3 deficiency lessened the physiological levels (levels in uninjured retinas) of JUN. Consistent with DLK’s known role in pathological but not physiological regulation of JNK signaling (Ghosh et al., 2011), Dlk deficiency did not alter JUN levels in uninjured retinas (Fig. 4A). pJUN was not detected in uninjured retinas of any genotype (Fig. 4A). After axonal injury JUN accumulated in both Dlk and Jnk2/3 deficient retinas, though to a lesser extent than in wildtype retinas (Fig. 4A,B). The amount of pJUN 3 days after CONC was significantly less in Dlk and Jnk2/3 deficient retinas compared to wildtype retinas (Fig 4A,B). After CONC, 3 pJUN bands were detected with differences in electrophoretic mobility. These three different species reflect differences in how many phosphorylation residues within JUN are phosphorylated (Nateri et al., 2004). Interestingly, in the Jnk2/3 deficient mice, the lower molecular weight pJUN species was the most abundant form while in the Dlk deficient mice the higher molecular weight species was the most abundant (Fig. 4A). These data suggest that there are differences in JUN activation between the Dlk and Jnk2/3 deficiency. It is also important to note that in Jnk2/3 deficient mice, some pJUN was still observed, suggesting a role for JNK1 in the axonal injury response in RGCs (Fig. 4A,B).
Figure 4. Activation of JUN is attenuated in the Dlk deficient mice.
(A) Representative western blot showing the expression of JUN and pJUNSer63 in uninjured (Sham) retinas and in retinas 3 days post controlled optic nerve crush (CONC). Note that the Ser63 residue of JUN is phosphorylated by JNK. Levels of JUN in uninjured retinas appear to be similar in wildtype and Dlk deficient retinas. In Jnk2/3 deficient mice, JUN levels are less than in wildtype retinas, suggesting that JNK2 and/or 3 participate in regulating the physiological levels of JUN in uninjured Sham retinas. There is no detectable level of pJUNSer63 in uninjured retinas of any genotype (data not shown). Three days after CONC, JNK phosphorylation of JUN occurs in wildtype retinas. Levels of pJUNSer63 are reduced in Dlk deficient and Jnk2/3 deficient retinas. Interestingly, most of the JUN that is phosphorylated in Dlk deficient retinas appears to be phosphorylated at multiple sites as suggested by predominance of the higher molecular weight band in contrast to predominant lower molecular weight band observed in Jnk2/3 deficient retinas. (B) Quantitation of the western data shows that deficiency in Dlk or Jnk2/3 significantly reduces JUN phosphorylation 3 days after CONC. Note, the amount of pJUN 3 days after CONC is normalized to the loading control and is expressed relative to the injured wildtype. N = 3 for all conditions and genotypes. *, P < 0.05.
Preservation of the soma is linked to proximal axon segment sparing after CONC
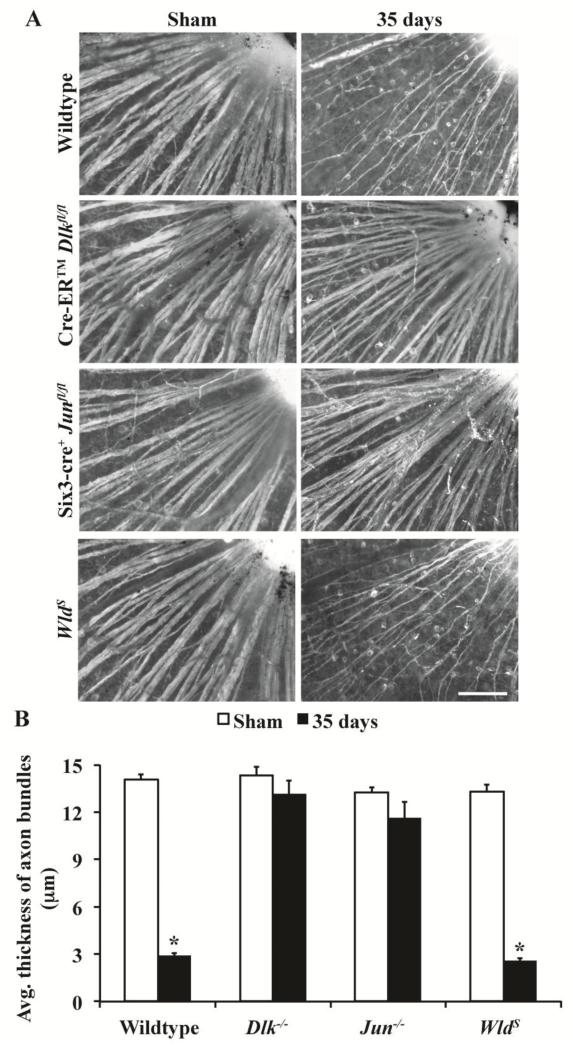
Dlk is known to be involved in axonal degeneration (Miller et al., 2009) and has been implicated in degeneration of the RGC axon segment proximal to the crush site after CONC (the segment of RGC axons between the RGC soma and the site injury; Watkins et al., 2013). Also, DLK-dependent axonal degeneration has been shown to be JUN-independent following NGF deprivation (Ghosh et al., 2011), suggesting DLK has a direct role in axonal degeneration. To determine whether the effect on proximal axonal segment survival was a direct role of DLK in regulating axonal degeneration or a consequence of increased somal survival, degeneration of the proximal segment of RGC axons was assessed in Jun and Dlk deficient retinas 35 days after CONC. Additionally, similar numbers of RGCs survive long-term in Jun and Dlk deficient animals, making it possible to test the influence of somal survival on proximal axon segment survival across these mutants. Jun deficient mice had 69.3±5.0% of TUJ1+ RGCs surviving at 35 days after CONC (n=5). This number was similar to the level of RGC survival observed for Dlk deficient mice at this time point (76.8±1.9%; see Fig. 1). Surprisingly, robust proximal axon survival was seen in both Jun and Dlk deficient retinas, suggesting that proximal axonal survival is linked to somal survival (Fig. 5A). The average thickness of intraretinal axon bundles (proximal to the site of insult) was significantly reduced in wildtype retinas 35 days after CONC in comparison to sham injured retinas (Fig. 5B; P < 0.001). In both Dlk and Jun deficient retinas, the thickness of proximal axon bundles did not significantly decrease (Fig. 5B; P > 0.05). In fact, these data are consistent with a previous report showing that axons survive up to the site of CONC in Bax deficient animals in which RGC death following axonal injury is prevented (Howell et al., 2007a). Additionally, in mice carrying the WldS mutation that is known to delay axonal but not somal degeneration (Wang et al., 2013a), proximal RGC axon segments degenerated 35 days after crush (Fig. 5 A,B). Thus, proximal axon segment survival appears to be linked to somal survival and cannot be used to assess a molecule’s role in degeneration of the distal axon segment separated from the cell body.
Figure 5. Axons do not degenerate proximal to injury site in Dlk deficient mice.
(A) Representative images adjacent to the optic nerve head of retinal flatmounts (RGC layer up) stained with the RGC marker, TUJ1. Dlk deficiency and Jun deficiency have similar numbers of surviving RGCs 35 days after injury (see above, Dlk−/−, 76.9%; Jun−/−, 69.3%; Dlk and Jun floxed alleles were recombined with Cre-ER™ and Six3-cre respectively). In Dlk deficient retinas, numerous RGC axons were present on the surface of the retina, suggesting that Dlk has a role in axonal degeneration. However, the axon bundle thickness appeared similar in Jun deficient retinas, suggesting that proximal RGC axonal survival is linked to somal survival. In contrast, there appeared to be extensive loss of proximal axon segments in WldS retinas, which do not have significant somal survival compared to wildtype retinas at this time point (Wang et al., 2013a). (B) Quantitative analysis of proximal axon bundle thickness shows that the proximal axon degenerates in both WT and WldS retinas 35 days after CONC. However, there is no significant reduction in the thickness of the axon bundle in either Dlk−/− or Jun−/− retinas at 35 days post CONC. At least 3 retinas were examined for each genotype and condition. *, P < 0.05; Scale bar, 100 μm.
DLK does not regulate axonal degeneration distal to the site of injury
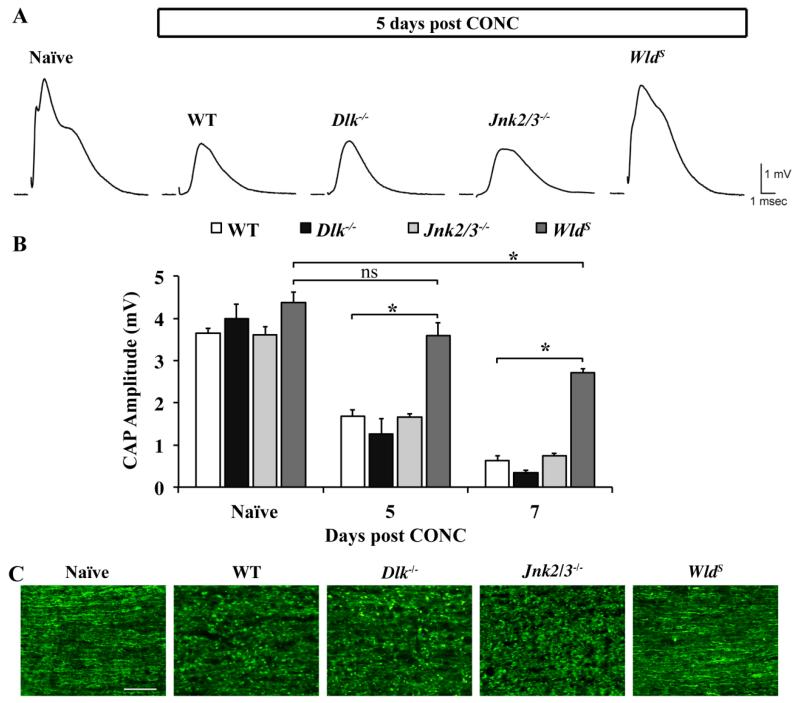
DLK has also been shown to be important for Wallerian degeneration of the axon segment distal to the site of insult in several neuronal cell types, including dorsal root ganglion neurons, olfactory neurons and sciatic nerves (Miller et al., 2009). Likewise, Dlk deficiency delays axonal degeneration of dorsal root ganglion axons following NGF deprivation (Ghosh et al., 2011). Thus, given DLK’s known role in axonal degeneration, it is still possible that DLK functions in degeneration of the optic nerve distal to the site of the crush. To test this possibility the function of Dlk deficient axons was tested following CONC (Fig. 6). Compound action potentials (CAP; a measure of axonal function (Baltan et al., 2010) were recorded from optic nerves harvested 5 and 7 days after CONC, time points where in wildtype mice there were significant reductions in CAP amplitudes. Dlk deficiency did not significantly alter the decrease in CAP amplitude observed at either 5 or 7 days compared to wildtype nerves (P > 0.05 for each time point; Fig 6). Thus, DLK does not have a critical role in regulating axonal degeneration after axonal injury in RGCs. JNKs have been shown to have a direct role in axonal degeneration in other systems (Miller et al., 2009). Also, there was DLK-independent activation of JNK2 and/or JNK3 in distal optic nerve segments after transection (see Fig. 3B). Therefore, to determine if JNK2/3 have direct role in regulating axonal degeneration, CAP were also recorded from Jnk2/3 deficient mice at 5 and 7 days after CONC. Jnk2/3 deficiency did not prevent the reduction in CAP amplitude observed at either 5 or 7 days after injury (Fig. 6). To demonstrate that this assay is measuring physiologically relevant aspects of axonal degeneration, WldS mice, which are known to be protected from axonal degeneration (Howell et al., 2007a; Lunn et al., 1989; Mack et al., 2001) were used as positive controls. The CAP amplitude was significantly higher in WldS optic nerves at both 5 and 7 days after CONC in comparison to wildtype nerves (Fig. 6A,B). To histologically verify that axons had degenerated in both Dlk and Jnk2/3 deficient optic nerves 5 days after CONC, longitudinal optic nerve sections were stained with neurofilament. There were substantial signs of axonal degeneration (fragmentation and beading) in wildtype, Dlk−/− and Jnk2/3−/− nerves (Fig 6C). Axon integrity appeared to be maintained at this time point in WldS mice (Fig. 6C). Together these data show that DLK is not required for distal axonal degeneration of adult RGCs after CONC.
Figure 6. Dlk deficiency does not appear to affect distal axonal degeneration.
To determine if Dlk was important in axonal degeneration distal to the site of injury, compound action potentials (CAP) were recorded from wildtype, Dlk−/−,, Jnk2/3−/−, and WldS optic nerves at 5 or 7 days after CONC. Dlk or Jnk2/3 deficiency did not rescue the reduction in amplitude of the CAP observed after CONC and both were significantly reduced compared to naïve control mice (P < 0.001 for each time point and genotype). As expected, optic nerves from WldS mice were protected from axon degeneration following CONC. The CAP amplitude of WldS optic nerves 5 days after CONC was not significantly different from the naïve control. Seven days after CONC there was a significant reduction in the CAP amplitude in WldS nerves compared to uninjured naïve control (P = 0.006). However, at both 5 and 7 days after CONC, the CAP of WldS nerves was significantly higher than wild-type nerves (P<0.001). (C) Representative images of longitudinal optic nerve sections show that there was extensive axonal fragmentation and beading in wildtype, Dlk−/− and Jnk2/3−/− optic nerves after CONC. However, optic nerves from WldS mice 5 days after CONC appeared intact, supporting the functional rescue observed in WldS mice at this time point. N ≥ 3 for each genotype and condition (sham, CONC). *, P < 0.05; ns, not significant; Scale bar: 50 μm.
Discussion
The degeneration of the distinct cellular compartments of a neuron is thought to be controlled by molecularly distinct processes (Beirowski et al., 2008; Howell et al., 2007a; Libby et al., 2005; Lorber et al., 2012). Nevertheless, it is possible that signaling events activated by axonal injury converge on distinct effectors to regulate somal and axonal degeneration. A few molecules have been identified that have been shown to regulate both somal and axonal degeneration (Kim et al., 2007; Mukherjee et al., 2013; Nikolaev et al., 2009; Osterloh et al., 2012). The MAP3K, DLK has also been implicated in both somal and axonal degeneration of dorsal root ganglion neurons following NGF deprivation (Ghosh et al., 2011; Miller et al., 2009). DLK has been shown to regulate both retrograde axonal injury signaling (Shin et al., 2012; Xiong et al., 2010) and somal degeneration following axonal injury (Watkins et al., 2013; Welsbie et al., 2013). Dlk deficiency delays Wallerian degeneration of sciatic nerves in vivo following transection (Miller et al., 2009). Therefore, DLK appears to be an important target upstream of both somal and axonal degeneration.
Previous studies have demonstrated a critical role for DLK in regulating RGC somal degeneration following axonal injury (Watkins et al., 2013; Welsbie et al., 2013). Our data also supports a role for DLK in somal degeneration. In some neurons Dlk has also been shown to be critical for axonal degeneration (Ghosh et al., 2011; Miller et al., 2009), a role that does not require JUN (thus is thought to be a role for the kinase directly in the axon degeneration pathway; Ghosh et al., 2011). Further, a previous report showed that in Dlk deficient mice the proximal axon segment (between the site of injury and the cell body) survives after axonal injury (Watkins et al., 2013). This has lead to the suggestion that DLK has a direct role in RGC axonal degeneration, similar to other systems. Using Jun and Dlk deficient retinas and WldS mice, we show that proximal axonal survival is a consequence of somal survival in vivo and that degeneration of the proximal axon segment is not compartmentalized from somal degeneration. Importantly, these data show that proximal axon survival cannot be used to assess a molecule’s role in distal axonal degeneration.
Different kinds of axonal insults trigger axonal degeneration via distinct mechanisms even in the same neuronal type (Chen et al., 2012; Miller et al., 2009). Additionally, developmental axonal degeneration that occurs during axonal pruning differs from injury-induced axonal degeneration (Hoopfer et al., 2006). Further, there could be inherent differences in the requirement of specific molecules in regulating axonal degeneration across different neuronal subtypes. It is important to note that based on studies using developing neurons and/or cultured neurons that DLK-JNK signaling has been hypothesized to have a major role in axonal degeneration in CNS disease. Deficiency in Dlk does not alter optic nerve degeneration (RGC axons) following axonal injury. Our data is the first to directly assess DLK’s role in axons of adult, injured CNS neurons in vivo and we show that DLK does not appear to have a critical function in axonal degeneration. Rather, its function appears to be restricted to soma. These observations highlight the necessity of testing Dlk’s role in neuronal disease in vivo.
Dlk deficiency phenocopies all aspects of Jun deficiency including proximal axon survival, delayed somal degeneration and the transcriptional regenerative response to axonal injury (Fernandes et al., 2013; Watkins et al., 2013). In fact, there is significant preservation of RGC somas and proximal axons (intra-retinal axons) in both Dlk and Jun deficient retinas 35 days after CONC. Together these data suggest that increased somal survival is tightly linked to preservation of the proximal axon segment after axonal injury. Interestingly, both Dlk and Jun deficiency have been shown to suppress the proregenerative response of RGCs to axonal injury (Watkins et al., 2013). Additionally, the pro-regenerative genes suppressed in Dlk deficient retinas are also attenuated in Jun deficient retinas (Fernandes et al., 2013; Watkins et al., 2013). Since canonically DLK is upstream of JUN and controls JUN activation in axonally injured RGCs (see above and Watkins et al., 2013; Welsbie et al., 2013), collectively these observations indicate that Dlk acts primarily through a JUN-dependent pathway to regulate somal degeneration and the transcriptional regenerative response to axonal injury in RGCs. Thus, it will be important to determine Jun regulated transcriptional networks in injured RGCs to understand the molecular pathways that control RGC degeneration and regeneration after axonal injury.
Following axonal injury, JNK is activated in three cellular compartments of an RGC, the soma, the axons proximal to the crush site and in axons distal to site of transection (Fig. 2). Our data indicate that Dlk is upstream of JNK activation only in the somal cellular compartment. This is consistent with another report that has shown that DLK regulates activation of JNK in the cell body of motorneurons but not in the neuropil or segmental nerves of Drosophila larvae (Xiong et al., 2010). The temporal separation between JNK activation in proximal axonal and somal compartments suggests that DLK plays a role in sustaining but not initiating JNK activation in RGCs following axonal injury. These effects could be a consequence of DLKs role in retrograde axonal injury signaling (Shin et al., 2012). In fact, a recent study showed that JNK-mediated phosphorylation of DLK plays a critical role in stabilizing DLK levels (Huntwork-Rodriguez et al., 2013). Further, DLK is known to be transported in axons following axonal injury (Xiong et al., 2010) and loss of function mutations in Dlk have been shown to result in defective axonal transport (Horiuchi et al., 2007). Dlk deficiency has also been shown to reduce retrograde axonal transport of activated transcription factors following axonal injury, supporting a role for DLK in retrograde axonal transport (Shin et al., 2012). Collectively, these observations support a mechanism wherein the pool of JNK activated in RGC axons by axonal injury stabilizes DLK levels in the axon, which upon retrograde transport from the axon to the soma activates the somal pool of JNK.
Our data suggest that distinct MAP3Ks regulate pathological activation of JNK in distinct cellular compartments of an RGC following axonal injury. Since axon injury is a major component of numerous CNS disease and traumatic brain injury, understanding the complex regulation of pathological JNK signaling will be an important step in understanding these diseases. To understand axonal injury-induced RGC death and to understand how different pathological pools of JNK in RGCs are regulated, it will be important to identify the distinct MAP3Ks upstream of JNK activation in RGC axons.
Supplementary Material
Highlights.
DLK is major regulator of JNK-JUN dependent RGC death after axonal injury.
DLK is not required for axonal activation of JNK in axonally injured RGCs.
DLK is not required for distal axonal degeneration of RGCs.
Dlk deficiency does not affect developmental RGC death or IPL organization
Acknowledgements
The authors would like to thank Syu-ichi Hirai for his generously providing DLK antibody, Drs DiAntonio (Dlkfl), Furuta (Six3-cre) and Wagner (Junfl) for generously providing mice, and Donna Shannon for technical help. This work was supported by The Glaucoma Foundation (RTL), EY018606 (RTL), T32 EY007125 (JMH), the Schmitt Program on Integrative Brain Research (PS and RTL), and a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology at the University of Rochester Medical Center. S.W.M. John is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–83. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–83. [PubMed] [Google Scholar]
- Baltan S, et al. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010;30:5644–52. doi: 10.1523/JNEUROSCI.5956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, et al. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. Embo J. 2002;21:1782–90. doi: 10.1093/emboj/21.7.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, et al. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28:1166–79. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Chen M, et al. Spatially coordinated kinase signaling regulates local axon degeneration. J Neurosci. 2012;32:13439–53. doi: 10.1523/JNEUROSCI.2039-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nature reviews. Neuroscience. 2005;6:889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Cui Q, et al. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Eto K, et al. Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci Res. 2010;66:37–45. doi: 10.1016/j.neures.2009.09.1708. [DOI] [PubMed] [Google Scholar]
- Fernandes KA, et al. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis. 2012;46:393–401. doi: 10.1016/j.nbd.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, et al. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp Eye Res. 2013;112:106–17. doi: 10.1016/j.exer.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, et al. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–2. [PubMed] [Google Scholar]
- Ghosh AS, et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol. 2011;194:751–64. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, et al. BCL2L1 (BCL-x) promotes survival of adult and developing retinal ganglion cells. Mol Cell Neurosci. 2012 doi: 10.1016/j.mcn.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Libby RT. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. Molecular neurodegeneration. 2011;6:50. doi: 10.1186/1750-1326-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hirai S, et al. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci. 2006;26:11992–2002. doi: 10.1523/JNEUROSCI.2272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, et al. MAPK-upstream protein kinase (MUK) regulates the radial migration of immature neurons in telencephalon of mouse embryo. Development. 2002;129:4483–95. doi: 10.1242/dev.129.19.4483. [DOI] [PubMed] [Google Scholar]
- Hirai S, et al. Expression of MUK/DLK/ZPK, an activator of the JNK pathway, in the nervous systems of the developing mouse embryo. Gene Expr Patterns. 2005;5:517–23. doi: 10.1016/j.modgep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–95. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Horiuchi D, et al. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–7. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007a;179:1523–37. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, et al. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007b;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429–44. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, et al. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork-Rodriguez S, et al. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol. 2013 doi: 10.1083/jcb.201303066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, et al. MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med. 2007;204:2063–74. doi: 10.1084/jem.20070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinedinst S, et al. Independent pathways downstream of the Wnd/DLK MAPKKK regulate synaptic structure, axonal transport, and injury signaling. J Neurosci. 2013;33:12764–78. doi: 10.1523/JNEUROSCI.5160-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp Eye Res. 2000;71:209–13. doi: 10.1006/exer.2000.0873. [DOI] [PubMed] [Google Scholar]
- Libby RT, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B, et al. Retinal ganglion cell survival and axon regeneration in WldS transgenic rats after optic nerve crush and lens injury. BMC Neurosci. 2012;13:56. doi: 10.1186/1471-2202-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, et al. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Mack TG, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nature neuroscience. 2009;12:387–9. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, et al. Activation of the innate signaling molecule MAVS by bunyavirus infection upregulates the adaptor protein SARM1, leading to neuronal death. Immunity. 2013;38:705–16. doi: 10.1016/j.immuni.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri AS, et al. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303:1374–8. doi: 10.1126/science.1092880. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, et al. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–9. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Osterloh JM, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–4. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, et al. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95:673–91. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, et al. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC neuroscience. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–22. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK, et al. Compound action potential of nerve recorded by suction electrode: a theoretical and experimental analysis. Brain Res. 1991;546:18–32. doi: 10.1016/0006-8993(91)91154-s. [DOI] [PubMed] [Google Scholar]
- Wang CH, et al. Protective role of Wallerian degeneration slow (Wld(s)) gene against retinal ganglion cell body damage in a Wallerian degeneration model. Exp Ther Med. 2013a;5:621–625. doi: 10.3892/etm.2012.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Bimodal control of dendritic and axonal growth by the dual leucine zipper kinase pathway. PLoS Biol. 2013b;11:e1001572. doi: 10.1371/journal.pbio.1001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA, et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A. 2013;110:4039–44. doi: 10.1073/pnas.1211074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS, et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A. 2013;110:4045–50. doi: 10.1073/pnas.1211284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore AV, et al. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–62. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Xiong X, Collins CA. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci. 2012;32:610–5. doi: 10.1523/JNEUROSCI.3586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191:211–23. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.