Abstract
Humans spontaneously imbue the world with social meaning: we see not only emotions and intentional behaviors in humans and other animals, but also anger in the movements of thunderstorms and willful sabotage in crashing computers. Converging evidence supports a role for the amygdala, a collection of nuclei in the temporal lobe, in processing emotionally and socially relevant information. Here, we report that a patient with bilateral amygdala damage described a film of animated shapes (normally seen as full of social content) in entirely asocial, geometric terms, despite otherwise normal visual perception. Control tasks showed that the impairment did not result from a global inability to describe social stimuli or a bias in language use, nor was a similar impairment observed in eight comparison subjects with damage to orbitofrontal cortex. This finding extends the role of the amygdala to the social attributions we make even to stimuli that are not explicitly social and, in so doing, suggests that the human capacity for anthropomorphizing draws on some of the same neural systems as do basic emotional responses.
The word “anthropomorphizing” suggests inaccurate judgments of what we perceive; however, this negative connotation must be reconciled with the ubiquity of the phenomenon in humans. Social psychology and, more recently, evolutionary psychology and cognitive neuroscience have stressed that social judgments can be automatic and serve an adaptive role in guiding our behavior in a complex social environment. Certain configural cues [e.g., the presence of eyes, (1)] and motion cues [e.g., contingent movement, (2)] seem to trigger an automatic attribution of animacy and/or agency. The anthropologist Stewart Guthrie suggests that humans evolved a propensity to anthropomorphize because failures to do so would have been more costly than overattribution (3). Cajoling one's computer, feeling sympathy for a dying houseplant, or being wary of forest trolls, while not entirely free of cost, are nonetheless better than failing to appease an angry dominant conspecific, alienating potential mates, or misperceiving a stalking predator as a rustling tree.
A classic stimulus for eliciting anthropomorphic attributions is a short film, constructed by the psychologists Heider and Simmel in the 1940s, which depicts the movements of three shapes for ≈2 min and is normally described as the social interactions of three human-like characters (4). Viewers commonly ascribe not only intentions to the moving shapes, but also emotions, gender, relationships, and personality traits. Subsequent studies using the original stimulus or similar films have shown that the tendency to describe it in anthropomorphic terms occurs across cultures (5, 6), is evident by age 5 (7), and is attenuated in autism (8-10).
The neural circuitry subserving the perception and processing of socially relevant stimuli has been a subject of considerable study in recent years (for reviews see refs. 11-14). Both lesion and functional neuroimaging studies have underscored the importance of the amygdala for processing emotional information. People with bilateral amygdala damage show impairments recognizing facial expressions of emotions (15, 16), and viewing emotional faces elicits amygdala activation in healthy subjects (17, 18), even when they are not aware of the stimuli (19, 20). Further support for the amygdala's automatic processing of emotional information comes from findings that it can be engaged independently of attention (ref. 21, but see ref. 22), and that electrophysiological responses within it are triggered at very short latencies (23).
The amygdala is also involved in processing more complex social information from visual stimuli. For instance, amygdala damage results in impairments detecting faux pas (24) and in judging trustworthiness (25), and amygdala activation is observed in healthy subjects when they view faces that look untrustworthy, even when no explicit judgment about trustworthiness is required in the task (26). These findings have led to two conclusions about the amygdala's role in social cognition: first, it participates both in basic emotional processing as well as in more complex social judgment; and second, this processing is often automatic and occurs below the level of conscious awareness. One interpretation of these dual conclusions is that they are causally related: one mechanism by which we interpret the social world may be through automatic, obligatory activation of emotional reactions.
If such processing depends on the amygdala for stimuli that are overtly emotional or social, a natural question is whether this dependency might extend to anthropomorphic social judgments about stimuli that are not explicitly human. In the current study, we analyzed the emotional and social attributions made by a rare subject with complete bilateral amygdala damage when shown the Heider and Simmel film mentioned above, and compared her responses to those of a group of demographically matched control subjects.
Methods
Subjects. Subject SM has complete, bilateral damage to the amygdala, as well as minor damage to adjacent anterior entorhinal cortex, due to Urbach-Wiethe disease (Fig. 1; see ref. 27 for detailed demographic and neuropsychological information). She has normal basic visual perception and attention, performs normally on tests of language ability, and has an IQ in the normal range, but also features a previously documented impairment in making certain emotional and social judgments about human faces (27). SM was compared with nine age-, gender-, and education-matched, neurologically healthy control subjects on the target task, and five age-, gender-, and education-matched healthy control subjects on the control task. In addition, she was compared with eight neurological comparison subjects with unilateral (one left, two right) or bilateral damage to orbitofrontal cortex (OFC). We chose these neurological comparison subjects with damage to OFC to provide a strong test for the specificity of the impairment to amygdala damage because damage to the OFC is also known to result in impaired emotional and social processing (28, 29). All subjects with OFC lesions had normal visual perception, memory, language, and IQ. Neurological subjects were selected from the Patient Registry of the Division of Cognitive Neuroscience, Department of Neurology, University of Iowa. All subjects gave informed consent, as approved by the University of Iowa Institutional Review Board.
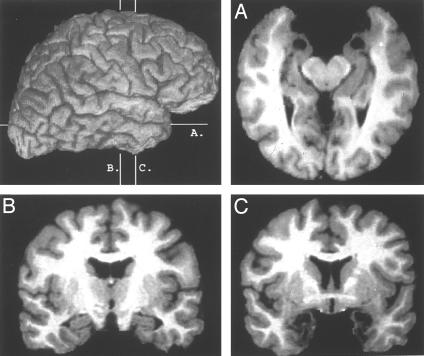
Fig. 1.
Structural MRI of SM's brain. Axial and coronal slices (A and C) show the lack of signal at the amygdala, but a coronal slice at the level of the hippocampus (B) shows that this structure is intact. (Photographs courtesy of the Human Neuroimaging and Neuroanatomy Laboratory, Department of Neurology, University of Iowa.)
Stimulus and Target Task. We used a video of the original Heider and Simmel film (4), which is silent, ≈90 s long, and depicts three shapes moving around the outline of a larger rectangle (Fig. 2 and Movie 1, which is published as supporting information on the PNAS web site). Subjects were seated 1.5 m from the TV screen and were told before the start of the film that they would see a silent movie “about two minutes long,” that they should pay attention and not talk while watching it, and that, when it was over, they would be asked to tell the experimenter what they saw. No further information about the content of the movie was given. Immediately after the presentation, the experimenter started a tape recorder and asked the subject, “Tell me what you saw,” again without any further prompts. After this initial description was recorded, subjects were shown the movie a second time and were administered a structured questionnaire that included explicit questions about the intentions, emotions, and personalities of the geometric figures in the film. After answering all of these questions, which took ≈20 min, subjects were asked to tell the story of the movie a second time. Thus, the experiment yielded two descriptions of the movie, one before and one after the questionnaire. The entire series of responses was tape-recorded and transcribed for analysis.
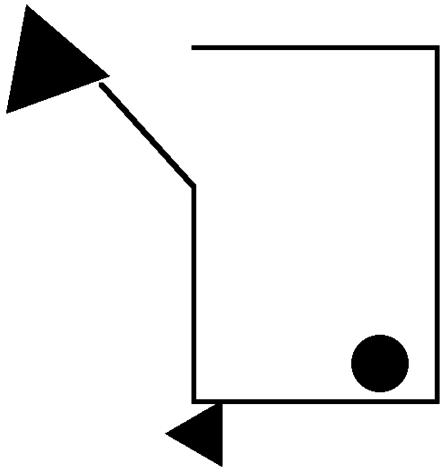
Fig. 2.
A scene from the classic Heider and Simmel (4) movie.
It is important to note that the initial description occurred with no cues from the experimenter that the movie should be described in anthropomorphic or affective terms: we did not ask subjects to tell a story and did not provide any indication that the shapes could be considered as animate characters. The second description was recorded after an extended series of questions that may have led subjects to consider the video in social terms if they had not already. Therefore, in the opening approach to the data analysis, we analyzed the initial and final descriptions separately.
SM completed the entire experiment on two occasions separated by ≈18 months; all other subjects were tested once.
Transcripts of subjects' narratives were analyzed by using the computer program linguistic inquiry and word count 2001[liwc2001; (30)], which counts the total number of words in each sample as well as percentages of words per sample in each of 74 categories. We focused on three a priori categories, two relevant to emotion attribution and/or anthropomorphizing (Affective or Emotional Processes, hereafter “Affect,” and Social Processes) and a control category (Motion, hereafter “Movement”).
The Affect category contains 615 words drawn from two subcategories called Positive Emotions (e.g., “happy,” “joy,” and “win”) and Negative Emotions (e.g., “hate,” “enemy,” and “nervous”). The second target category, Social Processes, is made up of 314 words, including social pronouns (e.g., “he”), communication verbs (“share”), and references to family, friends, and other humans. The Movement category includes words such as “move” and “go.” We compared SM's word use in these three categories with those of control subjects using Z-scores with 95% confidence intervals. In addition, as an overall index of anthropomorphizing, we calculated the difference between the summed percentage of words in the Affect and Social Processes categories and the percentage of words in the Movement category [(Affect + Social) - Movement].
Control Tasks. To ensure that any abnormalities in SM's description did not result from a nonspecific abnormality in her use of words, or a global impairment in social perception, we used two control tasks.
Questionnaire items analysis. We adapted two indices from Klin's (9) SAT method to code subjects' answers to questions about the stimulus film: the Person Index and the Problem-Solving Index. The Person Index is based on subjects' answers to the questions “What kind of person is X?” where X is, in turn, each of the three objects in the movie. This index measures the extent to which subjects derive stable personality features from the objects' actions in the movie, with scores ranging from 0 (only physical properties attributed) through 6 (multiple attributions of psychological traits; see Klin (9) for details). Because we had also included the questions “What is X like?” for all three objects, we calculated a Person Index separately for each set of questions. The Problem-Solving Index counts the number of nine explicit event-related questions (e.g., “Why did the big triangle break the house?”) answered normally by each subject. Both indices were calculated for the original nine normal controls and for SM's first testing session. Good Dog, Carl task. We also examined SM's narration of a children's picture book, Good Dog, Carl (31). This book has words only on the first and last page, the rest of its 40 pages depicting the adventures of a baby with the dog entrusted to look after it. This book differs from the target stimulus in that the characters depicted are overtly animate creatures. The narrations of this book given by SM and by five matched controls were tape-recorded, transcribed, and analyzed in the same way as their descriptions of the target film.
Results
Target Task. Every control subject described the movie as an emotionally and socially significant interaction between the characters (see Table 1 for a typical example). In contrast, SM produced descriptions of normal length (Table 2), but essentially devoid of social attributions (Table 1). The contrast between her response and the affective/social biases of the control subjects is evident in the percentages of words used in the three categories of interest: SM used no Affect words and almost no Social words, but a very high percentage of Movement words whereas controls used consistently more Affect and Social words, but fewer Movement words (Table 2). Strikingly, this pattern was evident both in her initial description of the film and in her final, post-questionnaire description.
Table 1. Transcripts of spontaneous descriptions given by a normal control and by subject SM.
| Normal control |
| “The bigger triangle was in control, or trying to take control of the smaller triangle and the circle, the rectangular shaped place was similar to like a room with a closed door that um, if you went in there you were safe until that triangle came in. The small triangle and the circle were trying to escape from the large triangle and when they did, the large triangle became very furious and destroyed things.” |
| SM (initial description, first testing session) |
| “OK, so, a rectangle, two triangles, and a small circle. Let's see, the triangle and the circle went inside the rectangle, and then the other triangle went in, and then the triangle and the circle went out and took off, left one triangle there. And then the two (pause) parts of the rectangle made like a [sic] upside-down V, and that was it.” |
Table 2. Comparisons of liwc percentages: SM's description of the Heider and Simmel movie, on two testing occasions separated by 18 months.
| liwc category | Matched NC mean (SD) | SM046 (occasion 1) | Z-score (C.I.) | SM046 (occasion 2) | Z-score (C.I.) | OFC mean (SD) |
|---|---|---|---|---|---|---|
| Initial description | ||||||
| Total no. of words | 97.11 (50.4) | 70 | −0.54 (−2.62, 1.54) | 68 | −0.58 (−2.66, 1.50) | 145.63 (95.1) |
| Affect | 3.23 (2.9) | 0 | −1.12 (−3.24, 1.01) | 0 | −1.12 (−3.24, 1.01) | 1.44 (1.4) |
| Positive emotion | 0.95 (1.7) | 0 | −0.57 (−2.65, 1.51) | 0 | −0.57 (−2.65, 1.51) | 0.42 (0.43) |
| Negative emotion | 2.28 (1.6) | 0 | −1.44 (−3.60, 0.72) | 0 | −1.44 (−3.60, 0.72) | 1.02 (1.25) |
| Social processes | 3.28 (3.0) | 1.43 | −0.62 (−2.70, 1.47) | 0 | −1.09 (−3.21, 1.03) | 3.56 (2.4) |
| Movement | 1.41 (1.5) | 5.71 | 2.97 (0.53, 5.42) | 8.82 | 5.12 (2.07, 8.18) | 3.61 (2.13) |
| Final description | ||||||
| Total no. of words | 54.89 (35.6) | 141 | 2.42 (0.10, 4.74) | 67 | 0.34 (−1.73, 2.41) | 73.00 (46.0) |
| Affect | 2.18 (1.4) | 0 | −1.52 (−3.69, 0.65) | 1.49 | −0.48 (−2.56, 1.60) | 2.82 (2.9) |
| Positive emotion | 0 (0) | 0 | - | 0 | - | 1.67 (2.64) |
| Negative emotion | 2.18 (1.4) | 0 | −1.52 (−3.69, 0.65) | 1.49 | −0.48 (−2.56, 1.60) | 1.15 (1.59) |
| Social processes | 4.69 (5.4) | 0.71 | −0.74 (−2.83, 1.35) | 2.99 | −0.32 (−2.39, 1.75) | 11.78 (9.7) |
| Movement | 0.99 (1.4) | 7.8 | 4.71 (1.79, 7.63) | 4.48 | 2.41 (0.09, 4.73) | 1.03 (1.5) |
| Mean of initial and final descriptions | ||||||
| Total no. of words | 76 (32.5) | 105.5 | 0.91 (−1.20, 3.01) | 67.5 | −0.26 (−2.33, 1.81) | 119.25 (75.6) |
| Affect | 2.70 (1.5) | 0 | −1.81 (−4.02, 0.41) | 0.75 | −1.31 (−3.45, 0.84) | 2.03 (1.34) |
| Positive emotion | 0.48 (0.8) | 0 | −0.57 (−2.65, 1.51) | 0 | −0.57 (−2.65, 1.51) | 0.96 (1.13) |
| Negative emotion | 2.23 (1.0) | 0 | −2.31 (−4.61, −0.01) | 0.75 | −1.54 (−3.71, 0.64) | 1.07 (1.17) |
| Social processes | 3.98 (2.6) | 1.07 | −1.13 (−3.26, 0.99) | 1.5 | −0.97 (−3.08, 1.14) | 6.99 (5.5) |
| Movement | 1.20 (0.8) | 6.755 | 6.67 (3.09, 10.25) | 6.65 | 6.55 (3.01, 10.08) | 2.53 (1.7) |
liwc, linguistic inquiry and word count. C.I., confidence interval. NC, normal control.
Because the patterns of word use percentages were similar for SM's initial and final descriptions, we used their average in the statistical analyses given below. These patterns were nearly identical on SM's second testing occasion 18 months later; we report data from both occasions.
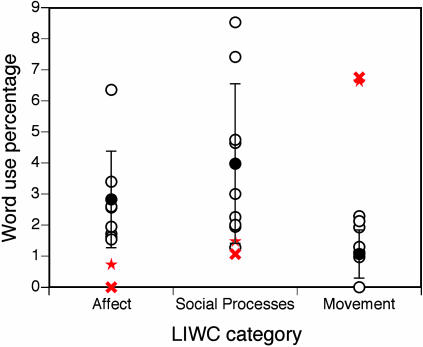
SM used fewer Affect words (first occasion, Z = -1.81; second occasion, Z = -1.31; see Table 2 and Fig. 3), with the effect most marked for her use of negative emotion words (first occasion, Z = -2.31; second occasion, Z = -1.54), because of lower variance in this category for the controls. She also tended to use fewer Social Process words overall but used significantly more words describing Movement (first occasion, Z = 6.67; second occasion, Z = 6.55). This latter measure quantifies the intuitive feeling, upon reading her description, that she described the events of the film in physical but not affective terms. SM's difference score between Affect/Social Processes and Movement word percentages was lower than that of any individual control, and significantly lower than the mean of the control group (Z = -2.99, P < 0.05; Fig. 3) This finding was replicated on the second testing occasion (Z = -2.65, P < 0.05).
Fig. 3.
Comparison of word counts in descriptions given by SM and by normal controls. Percentages of words used in the three categories of interest: Affect, Social Processes, and Movement (see text). SM's word use percentages are indicated for the first (X) and second (star) testing sessions, and normal controls by circles (their mean and SD are also shown). liwc, linguistic inquiry and word count.
Comparisons with OFC-Lesioned Subjects. In contrast to SM, subjects with OFC damage used Affect words in similar percentages to normal controls [mean (SD): 2.03 (1.34); see Table 2]. On average, they actually used a somewhat higher percentage of Social Process words than did normal controls [6.99 (5.53)] and a comparable number of Movement words [2.53 (1.67)]. Z-scores comparing SM with the OFC-lesioned group show a pattern very similar to that comparing SM with the normal controls: lower Affect word use (first occasion, Z =-1.52; second occasion, Z = -0.96), lower Social Process word use (first occasion, Z =-1.07; second occasion, Z = -0.99), and higher Movement word use (first occasion, Z = 2.54; second occasion, Z = 2.48). If we compare the difference score between the summed Affect and Social Processes percentages and the Movement word percentages, SM was lower than that of any individual OFC-lesioned subject, and significantly lower than the mean of the OFC group (Z = -2.10 for the first occasion, and -1.87 for the second occasion).
Control Tasks. In contrast to her spontaneous descriptions, SM's answers to specific questionnaire items were all within 1 SD of the normal control means. In response to the questions, “What was X like?” for each shape in turn, SM's Person Index was 3 [control mean (SD), 3.6 (0.9)]. In response to the questions, “What kind of person was X?” her Person Index was 2 [control mean(SD), 2.8 (1.8)]. SM's Problem-Solving Index, measuring her ability to answer specific socially relevant questions about the actions of the three objects, was 0.33, somewhat lower than that of the controls [mean (SD), 0.49 (0.17)].
In contrast to the pattern observed in her descriptions of the Heider and Simmel film, SM's description of the control picture book contained an entirely normal percentage of words in all three categories: Affect, Social Processes, and Movement (Table 3).
Table 3. Comparisons of liwc percentages: SM's description of the control picture book.
| liwc category | Matched NC mean (SD) | SM | Z-score (C.I.) |
|---|---|---|---|
| Total no. of words | 694 (277) | 233 | −1.66 (−3.60, 0.95) |
| Affect | 1.67 (0.82) | 3.43 | 2.15 (−0.64, 4.07) |
| Positive emotion | 1.20 (0.62) | 2.15 | 1.53 (−1.03, 3.48) |
| Negative emotion | 0.44 (0.26) | 1.29 | 3.27 (0, 5.21) |
| Social processes | 10.65 (2.77) | 12.88 | 0.81 (−1.54, 2.82) |
| Movement | 2.95 (1.96) | 4.29 | 0.68 (−1.62, 2.71) |
liwc, linguistic inquiry and word count. C.I., confidence interval. NC, normal control.
Discussion
Anthropomorphizing does not occur when we attribute emotions and mental content to actual social stimuli (e.g., other people); rather, it occurs when we attribute social meanings to stimuli that are not social, such as computers or clouds, presumably based on cues that signal the presence of agency or emotion. That we do so universally and automatically is a hallmark of human cognition, yet essentially nothing is known about the neural basis of this process. Subject SM, who has bilateral amygdala damage, gave an abnormally inanimate description of a film that was described in anthropomorphic terms by all nine demographically matched healthy controls and all eight OFC-damaged subjects. This lack of anthropomorphizing was quantified by an extremely low proportion of affect and social content words. Because she nevertheless accurately recounted the events of the movie, demonstrating intact memory for the stimulus, her description contained an abnormally high number of movement words. Her impairment was not a nonspecific result of brain damage in general, or of damage to emotion-processing brain structures, because the OFC subjects' descriptions were comparable to those given by the healthy controls. Our control tasks demonstrated that SM's failure to anthropomorphize cannot be due to impairment of basic perception or language, nor does it seem to be due to deficits in declarative social knowledge.
SM does not fail to perceive people, or explicit descriptions of them, as social stimuli, either in real life or in extensive laboratory tasks (27). We believe that she gave abnormal descriptions of the film stimulus we used here because it is not explicitly social; taken at face value, it indeed shows the geometric movements of inanimate shapes. Although her answers to some explicit questions about the film's social content were relatively normal (e.g., “What was the big triangle like?” yielded “A bully.”), she often gave answers that were socially unsophisticated and failed to take account of the overall social narrative of the stimulus (e.g., “Why did the big triangle break the house?” yielded “Because it wanted to, I guess.”). SM thus not only fails completely to provide a social narrative when making the spontaneous description, but also does not develop a normally rich narrative when explicitly cued with questions. An emotional response to the stimulus film may normally activate a social schema (32), which is then reflected both in a richly emotional and social description of the stimulus and in the presence of narrative elements in answers to specific questions about the events of the film. This interpretation is consistent with other findings regarding the amygdala's role in processing social information, including functional imaging studies showing amygdala activity in response to similar animated videos in normal subjects (33, 34).
It remains puzzling that SM is nonetheless able to make some social attributions to specific questioning, evidence that she is not entirely unable to generate social attributions in response to the same stimulus on which she fails in the spontaneous description. Our explanation of this residual ability rests on the hypothesis that social attributions generally arise from at least two distinct sets of processes: those that rely on automatic emotional reactions to the stimulus (which SM presumably lacks), and those that rely on deliberative retrieval of appropriate pieces of declarative knowledge (which SM has). In support of this interpretation of the impairment is SM's documented pattern of impairments on tasks of emotion judgments from faces. She fails to show conditioned emotional responses, fails to recognize fear in facial expressions, and does not recognize the level of emotional arousal in multiple emotions, yet is able to provide entirely normal descriptions of situations and behaviors associated with fear when asked to do so (27, 35). When considered with respect to the present set of results, the former set of impairments in recognizing fear may rely on the ability to trigger an emotional response to the stimulus whereas the latter involves deliberate retrieval from semantic memory in response to an explicit question.
A final question concerns the possibility of a developmental component to SM's impairment. Whereas the age at which she acquired her lesion remains uncertain (27, 35), it was possibly sustained early in life. If the impairment were to depend on a developmental amygdala lesion, the most plausible mechanism explaining the impairment would be that social knowledge was never acquired normally in the first place during development, and hence cannot be generated on our task. However, this explanation evidently does not fit our findings: SM does show clear evidence of having a store of social knowledge. What she lacks is (at least) one specific mechanism for automatically triggering its retrieval.
Acknowledgments
We are grateful to David Kemmerer for the video and for discussions regarding its use, to Daniel Tranel, Josh Greene, Martha Farah, Lesley Fellows, Luiz Pessoa, Susan Carey, and two anonymous reviewers for comments on earlier versions of this manuscript, to Tony Buchanan and Benjamin Lewis for help in collecting data on the picture book task, to Jamie Pennebaker for help with the liwc analyses, and to Kodi Scheer, Matt Karafin, Jocelyn Spoon, and Jennifer Shultz for coding of specific questionnaire items. This work was supported by National Institute of Neurological Disorders and Stroke Program Project Grant NS19632. A.S.H. was supported by National Institutes of Health Grant T32-NS07413 at the Children's Hospital of Philadelphia during preparation of the final manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: OFC, orbitofrontal cortex.
References
- 1.Johnson, S. C. (2003) Philos. Trans. R. Soc. London B Biol. Sci. 358, 549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholl, B. J. & Tremoulet, P. D. (2000) Trends Cognit. Sci. 4, 299-309. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie, S. E. (1995) Faces in the Clouds: A New Theory of Religion (Oxford Univ. Press, Oxford).
- 4.Heider, F. & Simmel, M. (1944) Am. J. Psychol. 57, 243-259. [Google Scholar]
- 5.Hashimoto, H. (1966) J. Child Dev. 2, 3-26. [Google Scholar]
- 6.Marek, J. (1966) Hum. Relat. 19, 353-380. [Google Scholar]
- 7.Berry, D. S. & Springer, K. (1993) Ecol. Psychol. 5, 273-283. [Google Scholar]
- 8.Bowler, D. M. & Thommen, E. (2000) Autism 4, 147-171. [Google Scholar]
- 9.Klin, A. (2000) J. Child Psychol. Psychiatry 41, 831-846. [PubMed] [Google Scholar]
- 10.Abell, F., Happé, F. & Frith, U. (2000) Cognit. Dev. 15, 1-16. [Google Scholar]
- 11.Adolphs, R. (2003) Nat. Rev. Neurosci. 4, 165-178. [DOI] [PubMed] [Google Scholar]
- 12.Cacioppo, J. T., Berntson, G. G., Adolphs, R., Carter, C. S., Davidson, R. J., McClintock, M. K., McEwen, B. S., Meaney, M. J., Schacter, D. L., Sternberg, E. M., et al. (2001) (MIT Press, Cambridge, MA).
- 13.Ochsner, K. N. & Lieberman, M. D. (2001) Am. Psychol. 56, 717-734. [PubMed] [Google Scholar]
- 14.Saxe, R., Carey, S. & Kanwisher, N. (2004) Annu. Rev. Psychol. 55, 87-124. [DOI] [PubMed] [Google Scholar]
- 15.Adolphs, R., Tranel, D., Hamann, S., Young, A. W., Calder, A. J., Phelps, E. A., Anderson, A., Lee, G. P. & Damasio, A. R. (1999) Neuropsychologia 37, 1111-1117. [DOI] [PubMed] [Google Scholar]
- 16.Calder, A. J., Young, A. W., Perrett, D. I., Hodges, J. R. & Etcoff, N. L. (1996) Cognit. Neuropsychol. 13, 699-745. [Google Scholar]
- 17.Breiter, H. C., Etcoff, N. L., Whalen, P. J., Kennedy, W. A., Rauch, S. L., Buckner, R. L., Strauss, M. M., Hyman, S. E. & Rosen, B. R. (1996) Neuron 17, 875-887. [DOI] [PubMed] [Google Scholar]
- 18.Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J. & Dolan, R. J. (1996) Nature 383, 812-815. [DOI] [PubMed] [Google Scholar]
- 19.Morris, J. S., Friston, K. J., Buchel, C., Frith, C. D., Young, A. W., Calder, A. J. & Dolan, R. J. (1998) Brain 121, 47-57. [DOI] [PubMed] [Google Scholar]
- 20.Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B. & Jenike, M. A. (1998) J. Neurosci. 18, 411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vuilleumier, P., Armony, J. L., Driver, J. & Dolan, R. J. (2001) Neuron 30, 829-841. [DOI] [PubMed] [Google Scholar]
- 22.Pessoa, L., McKenna, M., Gutierrez, E. & Ungerleider, L. G. (2002) Proc. Natl. Acad. Sci. USA 99, 11458-11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oya, H., Kawasaki, H., Howard, M. A., 3rd & Adolphs, R. (2002) J. Neurosci. 22, 9502-9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone, V. E., Baron-Cohen, S., Calder, A., Keane, J. & Young, A. (2003) Neuropsychologia 41, 209-220. [DOI] [PubMed] [Google Scholar]
- 25.Adolphs, R., Tranel, D. & Damasio, A. R. (1998) Nature 393, 470-474. [DOI] [PubMed] [Google Scholar]
- 26.Winston, J. S., Strange, B. A., O'Doherty, J. & Dolan, R. J. (2002) Nat. Neurosci. 5, 277-283. [DOI] [PubMed] [Google Scholar]
- 27.Adolphs, R. & Tranel, D. (2000) in The Amygdala: A Functional Analysis, ed. Aggleton, J. P. (Oxford Univ. Press, New York), pp. 587-630.
- 28.Hornak, J., Rolls, E. T. & Wade, D. (1996) Neuropsychologia 34, 247-261. [DOI] [PubMed] [Google Scholar]
- 29.Marinkovic, K., Trebon, P., Chauvel, P. & Halgren, E. (2000) Cognit. Neuropsychol. 17, 187-199. [DOI] [PubMed] [Google Scholar]
- 30.Pennebaker, J. W., Francis, M. E. & Booth, R. J. (2001) (Erlbaum, Mahwah, NJ).
- 31.Day, A. (1991) Good Dog, Carl (Simon & Schuster, New York).
- 32.Kassin, S. M. (1982) in Review of Personality and Social Psychology, ed. Wheeler, L. (Sage Publications, Beverly Hills, CA), Vol. 3, pp. 145-169. [Google Scholar]
- 33.Schultz, R. T., Grelotti, D. J., Klin, A., Kleinman, J., Van der Gaag, C., Marois, R. & Skudlarski, P. (2003) Philos. Trans. R. Soc. London B Biol. Sci. 358, 415-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, A. & Weisberg, J. (2003) Cognit. Neuropsychol. 20, 575-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adolphs, R., Tranel, D., Damasio, H. & Damasio, A. R. (1995) J. Neurosci. 15, 5879-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]