ABSTRACT
BACKGROUND
The relationship between practice intensity and the quality and outcomes of care has not been studied.
OBJECTIVE
To examine the relationship between primary care physicians’ costliness both for defined episodes of care and for defined patients and the quality and outcomes of care delivered to Medicare beneficiaries.
STUDY DESIGN
Cross sectional analysis of physician survey data linked to Medicare claims. Physician costliness measures were calculated by comparing the episode specific and overall costs of care for their patients with the care delivered by other physicians.
PARTICIPANTS
We studied physicians participating in the 2004–2005 Community Tracking Study Physician Survey linked with administrative claims from the Medicare program for the years 2004–2006.
MAIN MEASURES
Proportion of eligible beneficiaries receiving each of seven preventive services and rates of preventable admissions for acute and chronic conditions.
KEY RESULTS
The 2,211 primary care physician respondents included 937 internists and 1,274 family or general physicians who were linked to more than 250,000 Medicare enrollees. Patients treated by more costly physicians (whether measured by the overall costliness index or the episode-level index) were more likely to receive recommended preventive services, but were also more likely to experience preventable admissions. For instance, physicians in the lowest quartile of costliness performed appropriate monitoring for hemoglobin A1C for diabetics 72.8 % of the time, as compared with 81.9 % for physicians in the highest quartile of costliness (p < 0.01). In contrast, patients treated by the physicians in the lowest quartile of episode costliness were admitted at a rate of 1.8/100 for both acute and chronic Prevention Quality Indicators (PQIs), as compared with 2.9/100 for both acute and chronic PQIs for those treated by physicians in the highest quartile of costliness (p < 0.001).
CONCLUSIONS
Physician practice patterns are associated with the quality of preventive services delivered to Medicare patients. Ongoing efforts to influence physician practice patterns may have differential effects on different aspects of quality.
KEY WORDS: quality of care/patient safety, incentives in health care, payment systems, Medicare
INTRODUCTION
The continuing national debate over federal spending, deficits, and the debt underscores the need to find ways to address problems related to escalating costs and inadequate quality of care in the US health care system.1,2 Changing physician practice incentives, through payment reforms such as global payments, pay-for-performance, or shared savings arrangements, is a primary focus. Ideally, a reformed payment system would promote the delivery of high value care, meaning care that is relatively high quality and low cost. Some reforms, such as accountable care organizations (ACOs), explicitly set up rewards for both reducing costs and improving quality. Many are concerned, however, that efforts to reduce costs will have deleterious effects on quality, either indirectly by shifting care from higher quality to lower quality physicians (who happen to be less costly), or directly by leading to underprovision of beneficial care.
Under ACOs and other global payment arrangements, physicians and physician organizations will begin to scrutinize their practice patterns for specific clinical conditions that could be treated more efficiently. Although US patients and physicians have been practicing under a “more is better” approach, providing more services for specific clinical conditions might not necessarily equate with providing higher quality care overall. In many cases, additional services delivered at the margin might be considered low or indeterminate value, such that economizing on care might not be detrimental to quality.3 Since physicians who deliver more services have more opportunities to deliver high quality care, costly physicians might deliver care of higher quality as measured by standard process measures. Alternatively, physicians with systems in place to manage and coordinate care, such as those required to succeed under global payments, might deliver less costly care that is also higher quality. Although some prior research demonstrates that areas of the country that are more costly or have more physicians generally don’t provide higher quality care, such cross sectional observational studies conducted at the area level are sensitive to confounding.4–6 Some recent studies conducted at the individual level that address the potential endogeneity (i.e., reverse causality) of the relationship between cost and quality find that more costly care results in better outcomes, but these studies have mostly examined hospitals.7–9
In this study, we examine the relationship between physician practice patterns as measured by their relative costs per episode of care and per patient and the quality of care delivered to Medicare beneficiaries by analyzing data from a large, nationally representative sample of physicians, the Community Tracking Study (CTS) Physician Survey, linked to claims data from Medicare.10 We create two summary measures of physician costliness, and examine how these measures relate to quality of care. We focus both on process measures for routine and preventive care that Medicare beneficiaries should receive, as well as outcomes related to hospitalization for ambulatory care sensitive medical conditions that should be reduced with adequate primary care.
METHODS
Data on Physicians
The Community Tracking Study Physician Survey, conducted by the Center for Studying Health System Change, was a periodic telephone survey of a nationally representative sample of non-federal physicians who had completed residency training and spent at least 20 h per week in direct patient care. The fourth CTS survey, conducted in 2004–2005, sampled physicians drawn from 60 local health care markets that together are representative of the continental United States. The 2004–2005 survey had 6,628 respondents (weighted response rate of 52 %). Details of the survey are available at www.hschange.org/index.cgi?data=04. Our study included 2,211 primary care physicians (PCPs) who treated Medicare patients during the 2004–2006 period. We defined PCPs as those with a primary specialty of family practice, general practice, geriatrics, or general internal medicine.
Data on Medicare Patients
We obtained data from the Medicare program on elderly (age > 65), non-end stage renal disease (ESRD) Medicare beneficiaries who were enrolled in the traditional fee-for-service Medicare program and for whom surveyed physicians submitted at least one claim during the 3-year period 2004–2006. For each patient identified in this manner, we obtained a complete history of all claims submitted by all Medicare providers for the entire time period. Since claims data are not available for patients enrolled in a Medicare Advantage health plan, patients are only included for full-year periods when they were enrolled in traditional Medicare. CTS survey data and Medicare claims were linked by obtaining Medicare’s Unique Physician Identifier Number (UPIN) from the American Medical Association for CTS respondents and matching it to the UPIN recorded on the Medicare claims. Because beneficiaries were indirectly sampled through contact with a CTS physician respondent, they are not nationally representative for two reasons: physicians had different likelihoods of being included in the CTS sample and because patients seeing a greater number of unique physicians had a greater likelihood of being included in the beneficiary sample. We constructed beneficiary weights that were based on the weight assigned to the physician respondent through which they entered the beneficiary sample, divided by the number of unique physicians seen in 2004–2006. Weighted beneficiary characteristics closely matched those obtained from administrative data for elderly, non-ESRD patients.
Assigning Patients to PCPs
We assigned beneficiaries to a primary care physician using an algorithm that matched the beneficiary to the primary care physician who provided the plurality of his/her evaluation and management (E&M) visits over the entire 2004–2006 period. The assignment was based on all care over the time period, and assigns each beneficiary to the single PCP who had the most contact with the patient. Thus, if the plurality PCP was not included in our survey sample, that patient would not be included in subsequent analyses.
Measuring Physician Costliness and Intensity of Care
We created two measures of physician relative costliness: relative resource intensity of care for specific clinical episodes (relative resource intensity per episode) and relative risk-adjusted total costs (relative resource intensity per patient).
In order to derive costliness measures that reflect differences in utilization rather than payment rates, we first calculated standardized costs for all Part A and Part B services received during the study period. Standardized costs were used to remove geographic adjustments to Medicare payments. Standardized cost differs from actual Medicare payment in two important ways. First, standardized cost incorporates the full allowed reimbursement from all payment sources (Medicare, patient cost sharing, and other insurers). Second, standardized cost eliminates the effects of various adjustments Medicare makes in setting local payment rates, such as geographic payment differences for local input price variations and differential payments across classes of providers (e.g., disproportionate share (DSH) and Graduate Medical Education (GME) payments; cost-based reimbursement of critical access hospitals vs. Diagnosis Related Group (DRG)-based prospective payment for most other short-term hospitals). All costs were then adjusted to reflect calendar year (CY) 2006 reimbursement rates.
We calculated the relative resource intensity per episode using Symmetry Episode Treatment Groups (ETG), version 6.0 (Ingenix, Eden Prairie, Minnesota), which is in widespread use nationally by insurers to profile physician costliness. Each episode groups clinically related services delivered to a patient with a specific condition over a defined period of time into one of about 600 different episode types, which reflect treatment for both chronic diseases and acute conditions. Episodes for chronic conditions are defined as calendar years. Episodes were attributed to primary care physicians if the physician provided the plurality of PCP evaluation and management services for the episode, subject to a minimum of 15 % of the total evaluation and management costs for that episode. A total of 901,135 episodes were assigned to one of the PCP respondents to the survey.
To calculate the relative resource intensity per episode, we first calculated the total observed cost for each patient by summing the standardized costs of all services assigned to an episode. We adjusted the data to eliminate extreme values for each episode type by setting all costs below one-third of the 25th percentile to that value and above three times the 75th percentile to that value. For each episode, we then calculated the ratio of the observed cost to the average cost of all episodes of that type within our data. We then aggregated the ratio of observed-to-expected costs across all episodes assigned to each PCP, to calculate the episode costliness index for each physician.
Similar to our calculation of episode costs, we summed all person-level standardized costs for each beneficiary for each year when calculating the relative resource intensity per patient measure. To control for health status predictive of spending, we used the Hierarchical Condition Categories (HCC) risk-adjustment model calculated using the same year of data.11 We then divided the observed costs for each patient by the predicted costs as estimated using the HCC model based on diagnoses from the prior year. These were then averaged across patients to yield a physician level measure of relative resource use per patient; this final operation justifies the truncation of extreme values—if we had not done that, the physician-level summary could be dominated by an extreme and likely errant value from one patient. Finally, in calculating both indices, we required that physician have at least 15 eligible episodes or patients in order to be included.
Although correlated (r = 0.24, p < 0.001), the relative cost indices appear to measure related but distinct constructs.
Quality Measures
Quality of Preventive Services
We investigated Medicare claims to measure beneficiaries’ receipt of recommended tests for diabetes monitoring (hemoglobin A1C monitoring, retinal eye exams, cholesterol screen, and nephropathy screen), cancer screening (mammography, colonoscopy/sigmoidoscopy), and receipt of a pneumococcal vaccination. The measures have been used in previous studies and are ascertainable by claims.12 Except for pneumococcal vaccination and colon cancer screening, these services should be delivered annually. We included all available years of quality measures in our models, but the results were not substantively different when we limited to the most recent year available for each measure. Fecal occult blood testing was excluded because it is not adequately captured in claims and because colonoscopy has become the most prevalent method for screening for colorectal cancer. Influenza vaccination was also excluded, because it is often administered in settings not captured in claims.
Prevention Quality Indicators
We also examined the full set of Prevention Quality Indicators (PQIs) developed with the support of the Agency for Healthcare Research and Quality (AHRQ).1 PQIs can be used to assess the quality of care for ambulatory care sensitive conditions for which good outpatient care can potentially prevent the need for hospitalization, or for which early intervention can prevent complications or more severe disease. Because these types of admissions are relatively infrequent, we stratified PQIs into acute and chronic categories, and created composite measures in both of these domains consisting of any acute or any chronic PQI, and allowed these to accrue over the entire time period available for each beneficiary.13
Patient (and physician) Control Variables
Patient control variables were derived from the Medicare beneficiary summary file and included age, race/ethnicity (categorized as white, black, or other), sex, and Medicaid coverage, an indicator of low socioeconomic status.
Physician control variables derived from the CTS survey included primary care specialty (general internal medicine versus family and general practice), age, sex, race, years in practice (less than 5 years, 5–10 years, or more than 10 years), foreign medical graduate status, board certification, and the percentages of practice revenue from Medicare or Medicaid (categorized in terciles). We did not include practice type as a control variable, because practice type contributes to practice patterns.
Statistical Analyses
We first present descriptive information on the primary care physicians included in the study and their associated patient populations. Comparisons of the included sample of Medicare patients linked to CTS PCPs to the entire Medicare population are reported elsewhere.14
For all analyses, the two relative resource intensity indices were divided into quartiles, and we compared physicians in the highest and middle two quartiles with each other and with those in the lowest quartile. We examined the unadjusted proportion of times each individual quality measures and the PQI composites were met for each of these groups of physicians.
We next estimated a series of logistic regression models assessing the association between the physician relative resource intensity and the individual quality measures. The predictors included quartiles of relative resource intensity as described above (with physicians in the lowest quartile serving as the omitted comparison group), as well as patient-level control variables, and physician level control variables. These models were estimated using proc genmod to control for the nesting of patients within physicians, and included a fixed effect for the 60 CTS sites to control for any time-invariant local market effects. For the PQI models, we also included a control variable for the number of years over which the PQI admissions accrued.
Our study was approved by the Centers for Medicare and Medicaid Services Privacy Board and by the Institutional Review Board at Harvard Medical School.
RESULTS
The 2,211 PCP respondents included 937 general internists who were linked to more than 123,000 Medicare patients who they treated at least once between 2004 and 2006, and 1,274 family or general physicians linked to over 129,000 Medicare patients (Table 1). Most physicians (62 %) had been in practice for 11 or more years and 87 % were board certified. About one-third were in a solo or two-person practice and one-quarter were in hospital-based practices. Seventy percent derived at least 20 % of practice revenue from Medicare. The Medicare beneficiaries linked to these physicians, either for specific episodes of care (∼300,000) and/or as their principal provider (∼250,000), were slightly younger than the general Medicare population (45 % ages 65–74 versus 38 %, p < 0.01), but were otherwise similar with 61 % being female and 90 % White (data not shown).
Table 1.
Description of PCPs and Beneficiaries Linked to PCPs
| PCP respondents (Physicians) | Beneficiaries linked to a PCPa | ||||
|---|---|---|---|---|---|
| N | Percent | N | Percent | ||
| Specialty | General internal medicine | 937 | 42.38 | 123,284 | 48.82 |
| Family/general practice | 1,274 | 57.62 | 129,268 | 51.18 | |
| Years in practice | 0–5 | 336 | 15.20 | 20,506 | 8.12 |
| 6–10 | 497 | 22.48 | 50,922 | 20.16 | |
| 11+ | 1,378 | 62.32 | 181,124 | 71.72 | |
| Board certification | Yes | 1,923 | 86.97 | 222,832 | 88.23 |
| No | 288 | 13.03 | 29,720 | 11.77 | |
| Location of medical school | U.S./Canadian | 1,685 | 76.21 | 204,577 | 81.00 |
| Elsewhere | 526 | 23.79 | 47,975 | 19.00 | |
| Practice type | Solo/2-person | 842 | 38.08 | 106,548 | 42.19 |
| Small group, 3–10 | 336 | 15.20 | 49,507 | 19.60 | |
| Medium group, 11–50 | 176 | 7.96 | 23,094 | 9.14 | |
| Large group, >50 | 94 | 4.25 | 11,486 | 4.55 | |
| Medical school | 123 | 5.56 | 6,800 | 2.69 | |
| Hospital practice/other | 554 | 25.06 | 50,777 | 20.11 | |
| Group/staff HMO | 86 | 3.89 | 4,340 | 1.72 | |
| Practice revenue derived from medicaid (terciles, %) | 0–5 | 958 | 43.33 | 126,975 | 50.28 |
| 6–20 | 803 | 36.32 | 97,238 | 38.50 | |
| 21+ | 450 | 20.35 | 28,339 | 11.22 | |
| Practice revenue derived from medicare (terciles, %) | 0–20 | 662 | 29.94 | 45,736 | 18.11 |
| 21–40 | 822 | 37.18 | 100,770 | 39.90 | |
| 41+ | 727 | 32.88 | 106,046 | 41.99 | |
| Practice revenue derived from managed care (terciles, %) | 0–25 | 702 | 31.75 | 87,841 | 34.78 |
| 26–50 | 697 | 31.52 | 91,247 | 36.13 | |
| 51+ | 812 | 36.73 | 73,464 | 29.09 | |
| Practice revenue prepaid, capitated (%) | 0 | 959 | 43.37 | 127,820 | 50.61 |
| 1–34 | 840 | 37.99 | 94,508 | 37.42 | |
| 35–100 | 412 | 18.63 | 30,224 | 11.97 | |
aBeneficiaries include those linked to PCPs as their usual primary care physician
Physician Relative Resource Intensity and Delivery of Preventive Services and Rates of PQI Admissions
For the delivery of preventive services, there was generally a positive relationship between relative resource intensity per episode and per patient and the quality measures. Physicians in the highest quartile of both measures delivered higher rates of preventive services to their patients (Table 2). For instance, physicians in the lowest quartile of relative resource use per episode performed appropriate monitoring for hemoglobin A1C for diabetics 75.8 % of the time, as compared with 82.3 % for physicians in the highest quartile of costliness (p < 0.01). Similar results were obtained for the relative resource use per patient measure (72.8 % for physicians in the lowest quartile compared with 81.9 % for physicians in the highest quartile, p < 0.01). For the most part, performance of physicians in the middle two and highest quartiles were similar.
Table 2.
The Unadjusted Relationship Between Quality of Care and Relative Resource Use per Patient and per Episode
| Hemoglobin A1C monitoring | Eye exam for diabetics | Cholesterol screen for diabetics | Nephro-pathy screen for diabetics | Colon cancer screening | Breast cancer screening | Pneumonia vaccine | Acute PQI composite | Chronic PQI composite | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Episode level costliness+ | Lower 25 %§ | 75.8 | 38.7 | 73.0 | 20.5 | 6.6 | 40.1 | 5.3 | 1.8 | 1.8 |
| Middle 50 % | 82.4** | 39.0 | 74.8 | 19.8 | 8.1*** | 45.2* | 6.5*** | 2.3*** | 2.3*** | |
| Upper 25 % | 82.3** | 39.9 | 80.2***++ | 22.9 | 8.1*** | 47.0 | 6.1 | 2.9***+ | 2.9***+ | |
| Beneficiary level costliess# | Lower 25 %§ | 72.8 | 36.2 | 74.9 | 15.0 | 5.5 | 33.3 | 5.5 | 1.6 | 2.0 |
| Middle 50 % | 82.7** | 39.1* | 76.2 | 21.7* | 7.7*** | 46.0*** | 6.4*** | 2.2*** | 2.1 | |
| Upper 25 % | 81.9** | 40.9* | 72.9 | 20.6 | 8.8*** | 45.8*** | 6.0 | 2.8***++ | 2.8** | |
*p < 0.05, **p < 0.01, ***p < 0.001 for comparing the middle 50 % and the upper 25 % to the lower 25 %
+p < 0.05, ++p < 0.01, +++p < 0.001 for comparing the middle 50 % to the upper 25 %
§reference group
In contrast, more resource intensive physicians also had the highest rates of both chronic and acute PQI admissions. For instance, patients treated by the physicians in the lowest quartile of relative resource use per episode were admitted at a rate of 1.7 per 100 for both acute and chronic PQIs, as compared with 2.9 per 100 for both acute and chronic PQIs for those treated by physicians in the highest quartile of relative resource use per episode (p < 0.001). In unadjusted analyses, those in the middle two quartiles were intermediate between the lowest and highest quartiles.
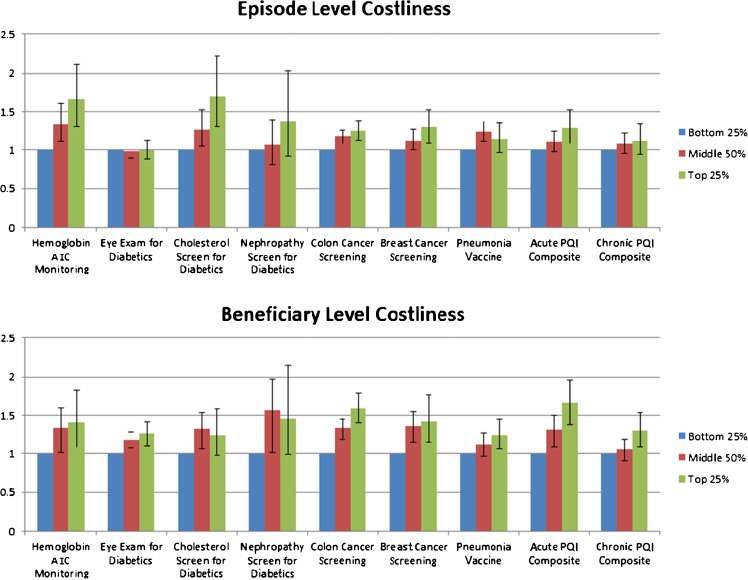
Adjusted results for both relative resource indices showed consistent results (Fig. 1). The adjusted results show the adjusted odds of meeting a quality measure (or having a PQI admission) for the bottom, middle two, and top quartiles of the episode-level and patient-level intensity measures. For instance, compared to the physicians in the lowest quartile of beneficiary costliness, the odds of an acute PQI admission for a physician in the middle two and highest quartiles were 1.30 (95 % C.I., 1.11, 1.52) and 1.64 (1.39, 1.96) respectively. Of note, for most results, the confidence intervals for the upper and middle two quartiles are overlapping, although the point estimates are consistent with a “dose–response” relationship. We also note that for one of the nine measures of per-episode costliness and two of the nine measures of per-beneficiary costliness, the point estimates for the middle group were larger than those of the upper quartile.
Figure 1.
Relationships between quality of care and relative resource use per patient and per episode. + Beneficiary level costliness is defined by calculating the mean of the ratio of actual to risk adjusted (using HCC scores) predicted total spending for each individual assigned to a PCP, and then assigning PCPs to a quartile of costliness based upon this score. # Episode level costliness is defined by calculating the mean of the ratio of actual to the average risk adjusted costs for similar episodes of care, and then assigning PCPs to a quartile of costliness based upon this score.
Discussion
The relationship between physician practice patterns and the quality and outcomes of care has rarely been studied.15–18 Prior studies at the area level generally show no or inverse relationships between costs and quality.4,5,19 No study that we are aware of, however, has examined the relationship between measures of the costliness of individual physicians and quality. Our study has several notable findings. First, we find that more costly physicians, whether measured by relative resource intensity per episode or per patient, generally had higher rates of delivery of preventive services, although rates were similar for higher and medium cost physicians. Our findings related to measured practice patterns and outcomes of care as measured by preventable hospital admissions, however, are in the opposite direction. The most costly physicians as measured using these two different methods consistently had higher rates of preventable hospital admissions for both acute and chronic conditions when compared to the least costly physicians.
Studies show that patients with more chronic medical conditions have higher rates of receipt of preventive services.20–22 This is thought to be because such patients have more opportunities (e.g., visits) for receiving such services. Thus, because costlier physicians might see their patients more frequently or more frequently refer them to other physicians for care, their patients will have more opportunities to receive preventive services. Thus, it is not surprising that physicians in higher quartiles of both episode-level and overall costliness had higher rates of delivery of preventive services.
In contrast, patients being cared for by more costly physicians also have higher rates of admissions for ambulatory care sensitive conditions when compared to the lowest quartile of physicians. Notably, these findings persist when the PQI admissions are not included when calculating either of the relative resource use indices. Thus, these physicians perform better on specific measures of prevention, but simultaneously have higher rates of preventable admissions as measured by the PQIs. One possible explanation is that patients cared for by more costly physicians might experience more fragmented and less coordinated care, thus resulting in more frequent admissions that could have been prevented. A contrasting explanation, however, is that such physicians might have a lower threshold for hospitalizing patients or using the emergency room, thus resulting in both more costly care and more frequent admissions overall. A similar threshold-based phenomena has been posited in the context of avoidable hospitalization of nursing home residents.23 Finally, for both relative resource use measures, unobserved differences in patient health could confound relationships.
On the surface, our findings related to the delivery of preventive services appear to be at odds with studies that find higher spending areas of the country also deliver care of similar or lower quality when compared to lower spending areas of the country.4,5 In contrast, our findings are similar to recent studies that demonstrated lower mortality rates for higher spending hospitals.7–9 Most studies of geographic variation are ecological in the sense that individuals are grouped by area. The degree of heterogeneity within areas, however, far outweighs differences between areas, suggesting that although quality, in general, might be lower in low spending areas, patients being cared for by higher cost physicians could still have higher rates of delivery of preventive services within these areas.24 Since we control for geographic area in our analyses, our findings are consistent with this hypothesis. Moreover, our observation of worse outcomes (e.g., higher rates of preventable admissions) for higher spending physicians is consistent with the notion that higher spending does not necessarily produce better outcomes for patients.
Our results should be considered in light of several limitations. First, we were unable to assess screening prior to the baseline year, likely leading to underestimation of population rates for some measures. This issue is particularly relevant for colorectal cancer screening and pneumococcal vaccination, where beneficiaries may not require repeat testing for 10 years after receipt of a service. However, because we control for both patient and physician-level factors, we have no reason to think that rates of previous screening should have differed across physicians. Second, we studied patients enrolled in the traditional Medicare program wherein physician services are reimbursed through standard fee-for-service payments. Thus, our relative resource intensity measures may not be reflective of the entirety of a physician’s practice. Moreover, we were only able to include patients in the traditional Medicare program for calculating the indices. Fourth, there are inherent limitations to episode groupers and these may have influenced our findings.25,26 The fact that our findings were consistent across both intensity measures, however, allays this concern. Fifth, our sample was constructed based on respondents to the 2004–2005 CTS physician survey. Although the sample is large and nationally representative, it might not be reflective of all US primary care physicians. Finally, although point estimates for the middle two and upper quartiles are consistent with a dose response effect, these two groups, in general, were statistically indistinguishable.
In conclusion, in this large nationally representative study of the relationship between primary care physician practice patterns and quality of care and outcomes for their Medicare patients, we find that costly physicians tended to have higher rates of delivery of preventive services, but also more frequently had patients admitted for ambulatory care sensitive conditions. Our findings suggest that it will be important to continue monitoring quality of care for physicians entering global payment arrangements.
Acknowledgements
Contributors
The authors thank Cynthia Saiontz-Martinez for expert statistical programming and Johan Hong for editorial assistance.
Funders
This work was supported by a grant from the National Institutes of Aging (1R01AG027312).
Prior Presentations
None.
Conflict of Interest
The authors have no pertinent conflicts of interest to disclose.
REFERENCES
- 1.Davies S, McDonald KM, Schmidt E, Schultz E, Geppert J, Romano PS. Expanding the uses of AHRQ’s prevention quality indicators: validity from the clinician perspective. Med Care. 2011;49:679–85. doi: 10.1097/MLR.0b013e3182159e65. [DOI] [PubMed] [Google Scholar]
- 2.Chernew ME, Sabik L, Chandra A, Newhouse JP. Ensuring the fiscal sustainability of health care reform. N Engl J Med. 2010;362:1–3. doi: 10.1056/NEJMp0910194. [DOI] [PubMed] [Google Scholar]
- 3.Choosing Wisely. 2013. (Accessed 18 December, 2013, at http://www.choosingwisely.org/.)
- 4.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–99. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–88. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 6.Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Aff (Millwood). 2004; Suppl Web Exclusives:W4-184-97. [DOI] [PubMed]
- 7.Hadley J, Reschovsky JD. Medicare spending, mortality rates, and quality of care. Int J Health Care Finance Econ. 2012;12:87–105. doi: 10.1007/s10754-012-9107-0. [DOI] [PubMed] [Google Scholar]
- 8.Kaestner R, Silber JH. Evidence on the efficacy of inpatient spending on Medicare patients. Milkbank Q. 2010;88:560–94. doi: 10.1111/j.1468-0009.2010.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stukel TA, Fisher ES, Alter DA, et al. Association of hospital spending intensity with mortality and readmission rates in Ontario hospitals. J Am Med Assoc. 2012;307:1037–45. doi: 10.1001/jama.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CTS Physician Surveys and the HSC 2008 Health Tracking Physician Survey. 2013. (Accessed 18 December, 2013, at http://www.hschange.org/index.cgi?data=04.)
- 11.Risk Adjustment. 2013. (Accessed 18 December, 2013, at http://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors.html.)
- 12.Pham HH, Schrag D, Hargraves JL, Bach PB. Delivery of preventive services to older adults by primary care physicians. J Am Med Assoc. 2005;294:473–81. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. AHRQ Quality Indicators Quality Indicator User Guide: Prevention Quality Indicators (PQI) Composite Measures Version 4.3: Agency for Healthcare Research and Quality; 2011 August.
- 14.Landon BE, Reschovsky JD, O’Malley AJ, Pham HH, Hadley J. The relationship between physician compensation strategies and the intensity of care delivered to Medicare beneficiaries. Health Serv Res. 2011;46:1863–82. doi: 10.1111/j.1475-6773.2011.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis P, Sandy LG, Larson AJ, Stevens SL. Wide variation in episode costs within a commercially insured population highlights potential to improve the efficiency of care. Health Aff (Millwood) 2012;31:2084–93. doi: 10.1377/hlthaff.2012.0361. [DOI] [PubMed] [Google Scholar]
- 16.Jha AK, Orav EJ, Dobson A, Book RA, Epstein AM. Measuring efficiency: the association of hospital costs and quality of care. Health Aff (Millwood) 2009;28:897–906. doi: 10.1377/hlthaff.28.3.897. [DOI] [PubMed] [Google Scholar]
- 17.Mangione CM, Gerzoff RB, Williamson DF, et al. The association between quality of care and the intensity of diabetes disease management programs. Ann Intern Med. 2006;145:107–16. doi: 10.7326/0003-4819-145-2-200607180-00008. [DOI] [PubMed] [Google Scholar]
- 18.Yasaitis L, Fisher ES, Skinner JS, Chandra A. Hospital quality and intensity of spending: is there an association? Health Aff (Millwood) 2009;28:w566–72. doi: 10.1377/hlthaff.28.4.w566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Aff. 2004;Jan-Jun:W4-184-97. [DOI] [PubMed]
- 20.Werner RM, Greenfield S, Fung C, Turner BJ. Measuring quality of care in patients with multiple clinical conditions: summary of a conference conducted by the Society of General Internal Medicine. J Gen Intern Med. 2007;22:1206–11. doi: 10.1007/s11606-007-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45:480–8. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 22.Min LC, Reuben DB, MacLean CH, et al. Predictors of overall quality of care provided to vulnerable older people. J Am Geriatr Soc. 2005;53:1705–11. doi: 10.1111/j.1532-5415.2005.53520.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Malley AJ, Marcantonio ER, Murkofsky RL, Caudry DJ, Buchanan JL. Deriving a model of the necessity to hospitalize nursing home residents. Res Aging. 2007;29:606–25. doi: 10.1177/0164027507305731. [DOI] [Google Scholar]
- 24.Newhouse JP, Garber AM. Geographic variation in health care spending in the United States insights from an institute of medicine reportgeographic variation in US health care spendingviewpoint. JAMA. 2013;310:1227–8. doi: 10.1001/jama.2013.278139. [DOI] [PubMed] [Google Scholar]
- 25.Rosen A, Liebman E, Aizcorbe A, Cutler D. Comparing commercial systems for characterizing episodes of care: Bureau of Economic Analysis. 2012. [Google Scholar]
- 26.MaCurdy T, Kerwin J, Gibbs J, et al. Evaluating the functionality of the symmetry ETG and Medstat MEG software in forming episodes of care using medicare data. Burlingame: Acumen, LLC; 2008. [Google Scholar]