Abstract
The retina signals stimulus contrast via parallel On and Off pathways and sends the information to higher visual centers. Here we study the role of the On pathway using mice that have null mutations in the On-specific GRM6 receptor in the retina (Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, Balannik V, McDearmon EL, Omura C, Lumayag S, Invergo BM, Brandon M, Glawe B, Cantrell DR, Donald R, Inayat S, Olvera MA, Vessey KA, Kirstan A, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS. Vis Neurosci 24: 111–123, 2007; Maddox DM, Vessey KA, Yarbrough GL, Invergo BM, Cantrell DR, Inayat S, Balannik V, Hicks WL, Hawes NL, Byers S, Smith RS, Hurd R, Howell D, Gregg RG, Chang B, Naggert JK, Troy JB, Pinto LH, Nishina PM, McCall MA. J Physiol 586: 4409–4424, 2008). In these “nob” mice, single unit recordings in the primary visual cortex (V1) reveal degraded selectivity for orientations due to an increased response at nonpreferred orientations. Contrast sensitivity in the nob mice is reduced with severe deficits at low contrast, consistent with the phenotype of night blindness in human patients with mutations in Grm6. These cortical deficits can be largely explained by reduced input drive and increased response variability seen in nob V1. Interestingly, increased variability is also observed in the superior colliculus of these mice but does not affect its tuning properties. Further, the increased response variability in the nob mice is traced to the retina, a result phenocopied by acute pharmacological blockade of the On pathway in wild-type retina. Together, our results suggest that the On and Off pathways normally interact to increase response reliability in the retina, which in turn propagates to various central visual targets and affects their functional properties.
Keywords: On/Off pathways, retina, mGluR6, trial-to-trial variability, superior colliculus
information in a visual scene is processed by parallel pathways in the visual system. Many of these parallel pathways originate in the retina, including the On and Off pathways that report the increase and decrease of contrast (Hartline 1938; Kuffler 1953). The On and Off pathways diverge at the cone-bipolar cell synapse. On bipolar cells bear sign-inverting metabotropic glutamate receptor 6 (GRM6) (Nomura et al. 1994; Masu et al. 1995) and depolarize upon light onset, whereas the Off bipolar cells bear sign-preserving ionotropic glutamate receptors and depolarize at light offset (Werblin and Dowling 1969). Along the retino-thalamo-cortical pathway, the On-Off pathways first converge onto simple cells in the primary visual cortex (V1) (Hubel and Wiesel 1959). The On-Off subregions are spatially segregated in a simple cell receptive field, conferring orientation selectivity on these cells (Hubel and Wiesel 1962). In contrast, along the retino-collicular pathway, the On-Off subregions overlap almost completely in the receptive fields of neurons in the superior colliculus (SC) (McIlwain and Buser 1968; Cynader and Berman 1972; Wang et al. 2010a). This On-Off convergence in the SC is believed to be important for detection of object salience, irrespective of contrast (Knudsen 2011). Thus the visual system can derive different functions by combining these parallel On-Off pathways using specific circuit architectures.
Experimental tests of the functions of the On and Off pathways have targeted the On pathway-specific metabotropic GRM6, using its agonist dl-2-amino-4-phosphonobutyric acid (2-APB) or L-AP4 (Slaughter and Miller 1981) or various Grm6 mutant mice (Masu et al. 1995; Pardue et al. 1998; Pinto et al. 2007; Maddox et al. 2008). Such elimination of the On pathway affects eye-specific segregation in the lateral geniculate nucleus (Huberman et al. 2003), the formation of V1 orientation columns in ferrets (Chapman and Godecke 2000) and results in loss of On responses in mouse SC (Masu et al. 1995) and decreased saccade initiation to bright targets in monkeys (Schiller et al. 1986). Yet, how the lack of On pathway in the retina affects responses/functions of single cells in higher visual centers is not completely known. Early studies in cats (Sherk and Horton 1984) and monkeys (Schiller 1982) have reported largely normal orientation selective responses in V1, except for decreased responses to bright edges upon blocking the On responses with 2-APB. However 2-APB has a dose-dependent effect on the Off pathway as well (Arkin and Miller 1987; Jin and Brunken 1996). Moreover, the effect of loss of On responses on contrast sensitivity in higher visual centers has not been assessed. Finally, it is known now that the On and Off pathways are not completely independent but connected through cross inhibition, dominantly from the On to the Off pathway (Zaghloul et al. 2003; Roska et al. 2006). It is thus important to determine whether and how removal of the On pathway, which would lead to a loss of this cross talk between the pathways, influences the remaining responses in higher visual areas. In this study, we used mutant mice that bear point mutations in Grm6 to study the function of On pathway. By recording extracellular responses from single cells in V1, the SC, and the retina of these mutant mice, our data suggest that the On pathway normally enhances the reliability of Off responses, which propagates to higher central visual targets exerting functional effects.
MATERIALS AND METHODS
Animals.
Two lines of mice bearing the null allele of Grm6 receptor were initially derived from a spontaneous mutation (Grm6nob3) (Maddox et al. 2008) and forward mutagenesis (Grm6nob4) (Pinto et al. 2007). Both lines were obtained as homozygous nulls on a C57BL/6J background from the laboratory of Dr. Larry Pinto at Northwestern University. Grm6nob3 and Grm6nob4 mutants both bear point mutations in the Grm6 gene and lack surface expression of the protein as assayed by immunohistochemistry of retinal sections (Pinto et al. 2007; Maddox et al. 2008). We confirmed the mutants by a lack of On bipolar cell responses as monitored by the absence of b-wave in their electroretinogram recordings (data not shown). Since we did not observe any difference between the two lines, we combined the data from both and collectively refer to them as nob (no b-wave) mice. Adult mutant (n = 40) and wild-type (WT) C57BL/6 (n = 57) mice of both sexes, reared in a normal 12-h light/dark cycle, were used in our experiments. All experimental protocols were approved by Northwestern University Institutional Animal Use and Care Committee.
In vivo electrophysiology.
Extracellular, single-unit recordings were made in anesthetized mice in vivo, according to previously published procedures (Wang et al. 2010a,b). Animals were anesthetized with urethane (1.2–1.3 g/kg ip) and supplemented with chlorprothixene (10 mg/kg im). Atropine (0.3 mg/kg) and dexamethasone (2.0 mg/kg) were administered subcutaneously to minimize mucus secretion and edema, respectively. The level of anesthesia was confirmed by the lack of toe pinch reflex, and additional urethane (0.2–0.3 g/kg) was administered as needed. Temperature was monitored with a rectal probe and maintained at 37°C. Silicone oil was applied to both eyes to prevent drying. The animal was maintained in a stable condition by oxygen delivery either through a tracheotomy or through the nose. For cortical recordings, a small craniotomy of ∼2 mm2 was made over V1, 2.8–3.0 mm lateral from the midline suture and 0.5–1.0 mm anterior from the lambda suture in the left hemisphere. Tungsten microelectrodes (5–10 MΩ; FHC, Bowdoinham, ME) were used to record from all layers of the cortex up to a depth of 600 μm, perpendicular to the pial surface. For SC recordings, a craniotomy was made at 0.7–1.5 mm lateral to midline suture and 0.2–0.8 mm anterior to the lambda suture and the electrode was lowered through an intact overlaying cortex, perpendicular to the pial surface, up to a depth of 1–1.5 mm to locate the SC, as described previously (Wang et al. 2009). Recordings were made from the superficial retino-recipient layer, extending up to a depth of 300 μm from the surface of the SC.
Spikes (filtered between 0.5 and 7 kHz and sampled at 25 kHz) were acquired and sorted offline using a System 3 workstation (Tucker Davis Technologies, FL). The animals were euthanized at the end of the experiment by an overdose of pentobarbital solution (150 mg/kg, Euthasol from Virbac).
Visual stimulation.
Visual stimuli were presented to the eye contralateral to the recording hemisphere on a flat panel CRT monitor (40 × 30 cm, 60 Hz, mean luminance ∼35 cd/mm2) placed 25 cm away in front of the animal. Stimuli were generated using custom-written codes in MATLAB (Mathworks) (Niell and Stryker 2008; Wang et al. 2010a; Sarnaik et al. 2013) with the Psychophysics Toolbox extensions (Brainard 1997; Pelli 1997) and presented with gamma correction. Each stimulus set included a blank condition in which the screen was at mean luminance to measure the average spontaneous firing rate that was subtracted from all responses.
On-Off responses in the SC were probed using single bright spots (5° × 5°) flashed transiently (0.5 s On, 0.5 s Off) at each of 13 × 13 or 11×11 or 13 × 7 grid locations on a gray background. They were presented in a pseudorandom sequence and repeated for at least five times at each location. V1 On-Off responses were similarly studied using flashing spot stimuli (4 ×4°, 0.2 or 0.5 s On, 0.3 or 0.5 s Off).
To determine orientation selectivity, drifting sinusoidal gratings at 12 directions (30° steps), 2-Hz temporal frequency, and 100% contrast were presented. Gratings with either four spatial frequencies [0.01, 0.02, 0.04, and 0.08 cycles/degree (cpd) for cortical recordings] or six (0.01, 0.02, 0.04, 0.08, 0.16, and 0.32 cpd for collicular recordings) were used (Niell and Stryker 2008; Wang et al. 2010a). Cortical contrast sensitivity and orientation tuning at different contrasts were examined at the most commonly preferred spatial frequency of 0.04 cpd, using drifting gratings at 12 directions and contrasts varying from 0 to 100% (steps of 20%). Each condition was presented for 1.5 s, with a 0.5-s interstimulus interval, in a pseudorandom order, for four to six repeats.
In vitro retinal recordings.
After at least 30 min of dark adaptation, mice were euthanized by cervical dislocation. Retinas were isolated under infrared illumination and placed into the recording chamber with the ganglion cell layer contacting a 256-channel multielectrode array (256 MEA-200/30iR-ITO; Multichannel Systems, Reutlingen, Germany). The retina was perfused with oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (in mM: 124 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 22 glucose, 26 NaHCO3, and 20 HEPES) and maintained at 33.5°C during the entire recording session. In some experiments, 100 μM 2-APB were added to the perfusion medium. Full field flash stimulation (2 s light on and 2 s light off) was generated on an LCD display (KCD-VDCF-BA Kopin, Taunton, MA) and projected onto the retina to characterize the visual responses of retinal ganglion cells (RGCs). Three repeats, each consisting of 75 trials of the stimulus, were presented. Voltage signals from the MEA were sampled at 25 kHz. Spike waveforms were collected using a 5.5 SD voltage threshold in MCRack (Multichannel Systems, Reutlingen, Germany), sorted into units in the Offline Sorter (Plexon, Dallas, TX). Spike timestamps were exported to MATLAB for further analysis.
Data analysis.
Responses to flash onset and offset were separately analyzed as On and Off responses. For SC and V1 analysis, responsive locations were selected by enforcing a threshold. For a given location, if the spike rate within a certain time window after stimulus onset/offset (50–300 ms in SC and 50–200 ms in V1) exceeded mean + 2SD of the spontaneous firing in at least 40% of the trials, it was qualified as responsive. At the responsive locations, the firing rates were averaged over the entire 0.5 s of On or Off stimulus, and the spontaneous rate was subtracted to obtain On/Off responses. The sum of responses at all such locations was used to compare the total response magnitude.
Responses to drifting gratings were averaged across the 1.5-s duration of presentation. We determined the direction at which the response was the greatest when averaged over all spatial frequencies, and the preferred spatial frequency was the one that yielded the maximum response at this direction. All subsequent analyses were done for the responses to different directions at the preferred spatial frequency. Orientation selectivity index (OSI) was defined as (R′ − Rorth)/(R′ + Rorth), where R′ was the mean response of Rpref (the response at the preferred direction, θpref, i.e., which was the one that evoked maximum response at the preferred spatial frequency) and Rpref+π (the response at the direction opposite to the preferred direction, as θpref and θpref+π represent the same orientation) and Rorth was the mean response to the two directions orthogonal to θpref; Direction selectivity index (DSI) as (Rpref − Rpref+π)/(Rpref + Rpref+π). Orientation selectivity was also quantified by a global OSI (gOSI), defined as 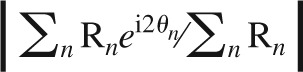 (Swindale 1998; Dragoi et al. 2001; Ringach et al. 2002; Sohya et al. 2007), which ranges from 0 (nonselective) to 1(highly selective). The tuning curves were fitted with a sum of two Gaussians centered at θpref and θpref+π and with the same SD, using a nonlinear least-squares fit in MATLAB (The MathWorks). For the cells that could be reasonably well fitted, the tuning width was calculated as the half-width at half-maximum of the fitted curve above the baseline. Cells were classified into simple and complex based on the discrete Fourier transform of their responses to drifting gratings at the preferred direction and spatial frequency, with an F1/F0 ratio ≥1 denoting simple cells and F1/F0 ratio <1 denoting complex cells.
(Swindale 1998; Dragoi et al. 2001; Ringach et al. 2002; Sohya et al. 2007), which ranges from 0 (nonselective) to 1(highly selective). The tuning curves were fitted with a sum of two Gaussians centered at θpref and θpref+π and with the same SD, using a nonlinear least-squares fit in MATLAB (The MathWorks). For the cells that could be reasonably well fitted, the tuning width was calculated as the half-width at half-maximum of the fitted curve above the baseline. Cells were classified into simple and complex based on the discrete Fourier transform of their responses to drifting gratings at the preferred direction and spatial frequency, with an F1/F0 ratio ≥1 denoting simple cells and F1/F0 ratio <1 denoting complex cells.
The normalized responses to different contrasts (c) were fitted with Naka-Rushton function, Response(c) = A·cn/(c50n + cn) (Naka and Rushton 1966; Albrecht and Hamilton 1982), where A is the maximal response, n describes the shape of the curve, and c50 is the contrast at half maximal response, using a nonlinear least-squares fit in MATLAB (The MathWorks). For contrast response curves that did not saturate, the fitted c50 and n values were inaccurate despite good fits (high R square values) as also reported by others (Grubb and Thompson 2003). Hence we used the fitted curves to calculate the contrast at 50% of maximal response and the slope of the tangent to the curve at 20% of maximal response (Grubb and Thompson 2003).
On and Off responses in the retina were divided into early (0–0.5 s after stimulus) and late (0.5–1.9 s after stimulus), and peak firing rates were calculated using a sliding window of 100 ms. A 100-ms window before flash onset was used to compute the spontaneous firing rate of the cell that was then subtracted from the evoked firing rates. A given cell was considered On/Off responsive if the evoked peak firing rate exceeded 1 spike·100 ms−1·trial−1.
Responses to drifting grating stimuli (SC or V1) or to full-field flash (retina) were also used to analyze spike variability. We used a temporal window of 80 ms for cortical and collicular analyses and 100 ms for retinal analysis. By sliding this window over the spike rasters, we calculated the mean and the variance of the spike counts in each window. Variability was quantified using the Fano factor (variance/mean of the spike count) in the time window at which the peak response occurred. Fano factors were calculated at the preferred orientation and at all three cycles of grating presentations for each cell. We used various window sizes (10, 50, 100, 200, and 500 ms and 1s for V1 and SC; 10, 50, 125, and 250 ms for retina) and confirmed that our results did not depend on the window size. Latency of response was computed for the first spike after stimulus onset/offset and also for the peak response (center of the time window during which it occurred).
Statistical analyses.
Nonparametric tests that do not require any assumptions about the distribution of the data were used in all cases. Data are summarized as means ± SE, which was calculated using a smooth bootstrapping method. Comparison of distributions was done using the two-sample Kolmogorov-Smirnov test (K-S test) and comparisons between medians of datasets were done using two-sample Mann-Whitney U-test or Wilcoxon signed-rank test (for paired data). All statistical tests were evaluated at α = 5% probability of false positives. Two-sided statistical tests were performed, unless mentioned otherwise. Statistical analyses and graphing were done in MATLAB and Prism (GraphPad Software).
RESULTS
Loss of on responses in the superior colliculus and V1 of nob mice.
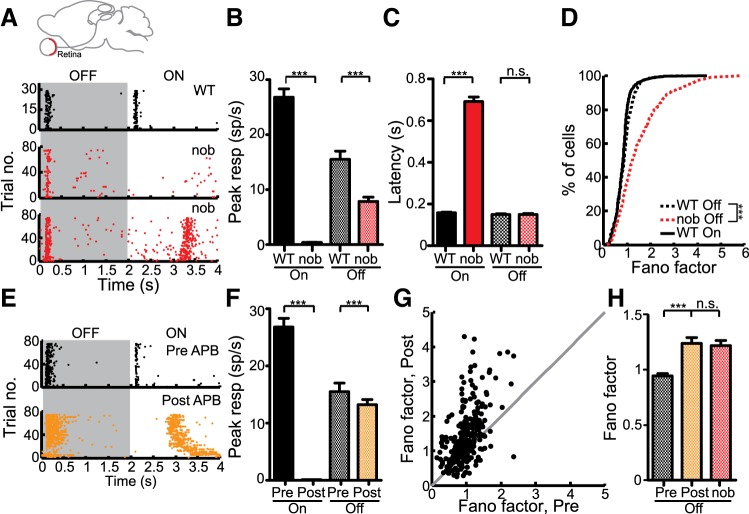
We first assessed the loss of On responses in the nob mice by recording from their SC. The SC is the major retinal target in mice (Hofbauer and Drager 1985), and single cells in the visual SC, with their robust and overlapping On and Off responses, are ideal to assess the loss of On responses in the retinal output of the nob mutants. Flashing spot stimuli that evoked both On and Off responses in WT mice (Fig. 1A, top) failed to evoke any reliable On responses in the nob SC (Fig. 1A, bottom). To quantify this, we used a threshold to select “responsive” regions when single spots were flashed at one of many locations on the screen. The total number of spikes evoked at all these responsive regions conveys both the strength of the response and its spatial extent. Even though our selection criteria (see materials and methods) could include nonresponsive locations simply by chance, the total spikes following stimulus onset were still drastically reduced in the nob mice, while those following stimulus offset did not change (Fig. 1C, means ± SE; On: WT = 381.6 ± 119.5 spikes/s, nob = 2.5 ± 3.7 spikes/s, P < 0.001; Off: WT = 217 ± 83.1 spikes/s, nob = 258.2 ± 71.0 spikes/s, P = 0.94; WT: n = 22, nob: n = 22).
Fig. 1.
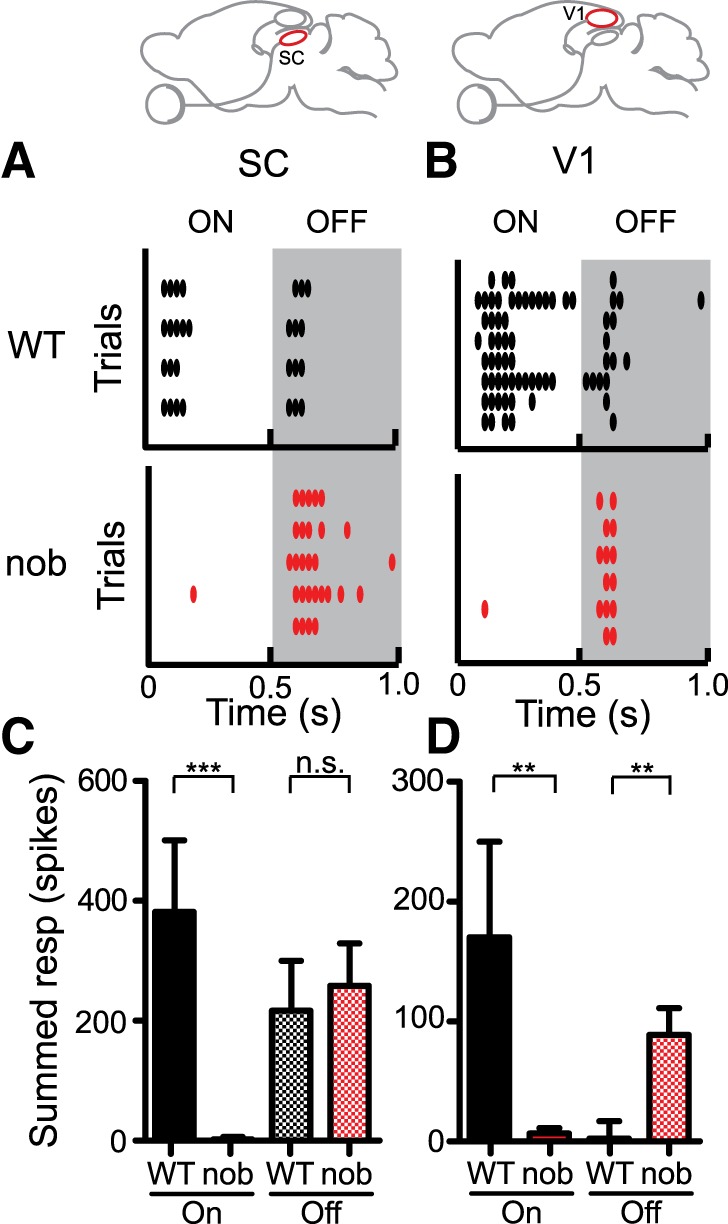
Loss of On responses in the superior colliculus (SC) and V1 of nob mice. A: raster plot of On and Off responses to a flashed bright spot in the SC of a wild-type (WT) mouse (top). Absence of any reliable On responses in the nob SC (bottom). B: examples showing both On and Off responses to a flashed bright spot in the V1 of WT mouse (top) and no On response in a nob V1 cell (bottom). Note that the WT example is not representative of the prominence of Off responses, but chosen to illustrate both On and Off responses. C: summed response (spikes) is pooled over all the responsive stimulus locations in the receptive fields of SC neurons (WT, n = 22, nob, n = 22 cells). Responsive locations were identified using a threshold (see materials and methods). D: summed response is pooled over all responsive stimulus locations in V1 receptive fields (WT, n = 17, nob, n = 17 cells). All bars indicate means + SE. **P < 0.01, and ***P < 0.001.
We similarly analyzed V1 neuron responses to flashing spots. Stimulus-locked and reproducible On responses were not observed in nob mice (Fig. 1B, bottom), unlike in WT mice (Fig. 1B, top, and Fig. 1D; On: WT =170.2 ± 79.8 spikes/s, nob = 6.7 ± 4.4 spikes/s, P = 0.003; WT: n = 17, nob: n = 17). It is important to note that the flashing spot stimulus is not effective in evoking Off responses in WT mouse V1 (Liu et al. 2009; Sarnaik et al. 2013). Accordingly, the increase in Off responses in nob V1 that we observed (Fig. 1D; Off: WT = 2.3 ± 14.5 spikes/s, nob = 88.9 ± 22.4 spikes/s, P = 0.007) could be due to an experimental bias. Since On responses were highly deficient in the nob cells, unless strong Off responses were present, the cell would appear unresponsive and hence discarded. Note that we did not present flashing spot stimuli preceding other visual stimuli described in subsequent sections. This suggests that any bias favoring sampling strong Off cells in nob, if present, would not affect all subsequent datasets.
Weaker orientation selectivity in V1 of nob mice.
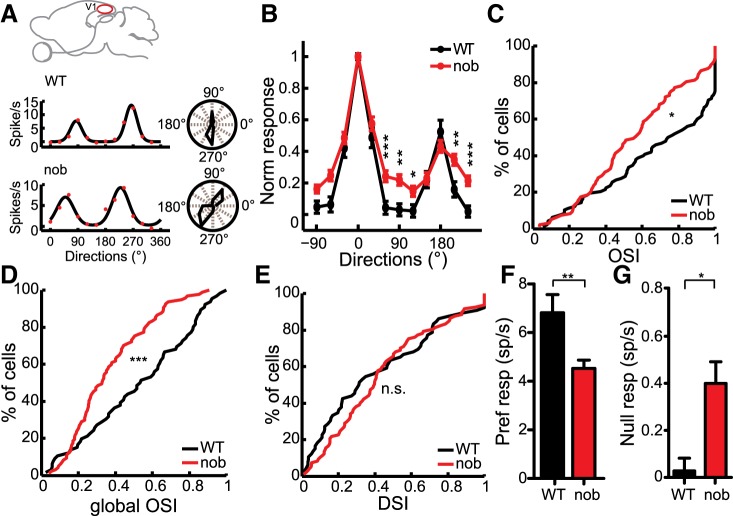
Selectivity for stimulus orientation is a salient property that emerges from the spatial segregation of On and Off visual inputs in the receptive fields of simple cells in V1 (Hubel and Wiesel 1962; Reid and Alonso 1995; Hirsch et al. 1998; Niell and Stryker 2008). We assessed the effect of loss of On inputs on cortical orientation selectivity using drifting sinusoidal gratings. Many cells in the nob mice were clearly orientation selective, displaying strong responses to gratings of opposite directions, i.e., the same orientation (Fig. 2A). However, there were quantitative differences in the degree of orientation selectivity as illustrated in the average tuning curves (Fig. 2B, WT: n = 66, nob: n = 97). The OSI (see materials and methods for details), which compared the firing rates at the preferred and orthogonal orientations, was reduced in the nob mice (Fig. 2C, WT: 0.84 ± 0.07, nob: 0.57 ± 0.04, P = 0.02). Another measure of selectivity is gOSI, which takes into account the responses at all orientations and combines the width and magnitude of tuning in a single measure (see materials and methods). This metric also indicated degraded orientation selectivity in nob mice (Fig. 2D, WT: 0.54 ± 0.06, nob: 0.33 ± 0.03, P < 0.001). On the other hand, we did not observe a difference in direction selectivity index between the WT and nob mice (Fig. 2E, WT: 0.31 ± 0.07, nob: 0.40 ± 0.04, P = 0.21). We also examined the effect of removing On pathway on simple cells (F1/F0 ratio ≥1) and putative layer IV cells (350–550 μm below pial surface; Sarnaik et al. 2013; Liu et al. 2010) and observed a similar decrease in orientation selectivity in both groups (see Table 1).
Fig. 2.
Weaker orientation selectivity in nob V1. A: responses to different stimulus directions of V1 cells in WT (top) and nob (bottom) mice. Polar plots (right) indicate orientation selective responses (radius = magnitude). B: normalized response tuning curves in WT and nob mice (WT, n = 66, nob, n = 97 cells). C: cumulative distribution of orientation selectivity index (OSI) in WT and nob mice. D: orientation selectivity quantified by the global OSI (gOSI). E: a cumulative plot showing similar direction selectivity indexes (DSI) in WT and nob mice. F: response at the preferred orientation. G: increased responses to the null orientation in nob mice. All bars indicate means + SE. *P < 0.05, **P < 0.01, and ***P < 0.001.
Table 1.
Analysis of simple and putative layer IV cells in nob V1
| All Cells |
Simple Cells |
Putative Layer IV Cells |
||||
|---|---|---|---|---|---|---|
| Property | WT | nob | WT | nob | WT | nob |
| OSI | 0.84 ± 0.07 (n = 66) | 0.57 ± 0.04* (n = 97) | 0.90 ± 0.06 (n = 54) | 0.59 ± 0.05‡ (n = 71) | 0.86 ± 0.10 (n = 33) | 0.44 ± 0.06† (n = 46) |
| gOSI | 0.54 ± 0.06 (n = 66) | 0.33 ± 0.03‡ (n = 97) | 0.62 ± 0.06 (n = 54) | 0.34 ± 0.04‡ (n = 71) | 0.62 ± 0.09 (n = 33) | 0.26 ± 0.04‡ (n = 46) |
| Pref response, spikes/s | 6.8 ± 0.8 (n = 66) | 4.5 ± 0.3† (n = 97) | 5.70 ± 0.81 (n = 54) | 4.30 ± 0.34* (n = 71) | 7.50 ± 1.04 (n = 33) | 4.40 ± 0.48* (n = 46) |
| Null response, spikes/s | 0.08 ± 0.05 (n = 66) | 0.40 ± 0.09* (n = 97) | 0.00 ± 0.04 (n = 54) | 0.27 ± 0.08† (n = 71) | 0.02 ± 0.09 (n = 33) | 0.53 ± 0.19* (n = 46) |
| Half-saturation contrast c50, % | 30.2 ± 6.8 (n = 20) | 59.7 ± 4.7‡ (n = 34) | 30.1 ± 7.3 (n = 19) | 56.3 ± 5.9‡ (n = 27) | 28.8 ± 12.1 (n = 11) | 56.3 ± 9.7† (n = 14) |
| Slope of contrast response curve | 2.18 ± 0.22 (n = 20) | 1.33 ± 0.11‡ (n = 34) | 1.84 ± 0.23 (n = 19) | 0.99 ± 0.11‡ (n = 27) | 1.97 ± 0.29 (n = 11) | 1.02 ± 0.15† (n = 14) |
| Fano factor: low contrast | – | – | 0.70 ± 0.06 (n = 70) | 1.33 ± 0.10‡ (n = 66) | 0.63 ± 0.12§ (n = 27) | 1.29 ± 0.20‡§ (n = 26) |
| Fano factor: high contrast | – | – | 0.47 ± 0.05 (n = 70) | 0.88 ± 0.06‡ (n = 66) | 0.40 ± 0.10§ (n = 27) | 0.80 ± 0.10‡§ (n = 26) |
Values are means ± SE. All statistical comparisons are made between wild-type (WT) and nob groups. gOSI, global orientation selectivity index.
P < 0.05;
P < 0.01;
P < 0.001;
Fano factor for layer IV simple cells only.
Next, we determined whether the degraded orientation selectivity was a result of decreased preferred responses or increased null responses. We observed a reduction in the preferred responses elicited in nob mice (Fig. 2F, WT: 6.8 ± 0.8 spikes/s, nob: 4.5 ± 0.3 spikes/s, P = 0.005), which is expected if, presumably, half of the feed-forward inputs (i.e., On) to single V1 cells were removed. In addition, nob mice also displayed an increase in the response to the null orientation (Fig. 2G, WT: 0.08 ± 0.05 spikes/s, nob: 0.4 ± 0.09 spikes/s, P = 0.03). Similar changes were seen when only simple cells or putative layer IV cells were analyzed (see Table 1). There was no significant difference in the spontaneous firing rate (WT: 0 ± 0.04 spikes/s, nob: 0.13 ± 0.04 spikes/s, P = 0.21). Together, a decreased peak response and an increased null response, both seem to reduce orientation selectivity in the nob mice.
Deficit in contrast sensitivity in nob mice.
In addition to giving rise to orientation selectivity, the parallel On and Off pathways are important for heightened sensitivity to changes in contrast (Schiller et al. 1986). In fact, the nob mice have poorer contrast sensitivity on an optomotor test (Pinto et al. 2007; Maddox et al. 2008) and monkeys with acute pharmacological removal of the On pathway fail to discriminate bright target stimuli (Schiller et al. 1986). We tested if V1 neurons show a corresponding deficit by studying their responses to drifting gratings at various contrasts in both WT and nob mice (Fig. 3A). On an average, nob cells showed an almost linear increase in responses with increasing contrasts, whereas WT responses usually saturated at higher contrasts (Fig. 3B, WT: n = 30, nob: n = 50). Quantification of these curves revealed that nob cells reached half maximum responses at much higher contrast values than WT cells (Fig. 3C, WT: 30.2 ± 6.8%, nob: 59.7 ± 4.7%, P < 0.001). The gain or the slope of the curves, quantified at 20% of maximum response, was also decreased in nob (Fig. 3D, WT: 2.18 ± 0.22, nob: 1.33 ± 0.11, P < 0.001). These deficits in contrast responses were also evident when either simple or putative layer IV cells were analyzed separately (see Table 1).
Fig. 3.
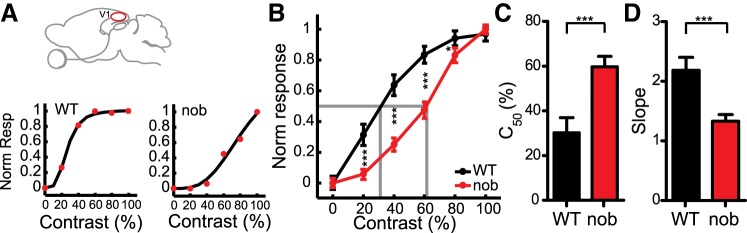
Decreased contrast sensitivity in nob mice. A: responses to 6 different contrasts of example cells in WT and nob mice. Black, solid lines indicate a Naka-Rushton fit. B: average of normalized contrast tuning curves, showing a rightward shift in nob mice (WT, n = 30, nob, n = 50 cells). C: larger half-saturation contrast in nob compared with WT. D: slope of the contrast response curve (determined at 20% of max response) is lower in nob mice. All bars indicate means + SE. ***P < 0.001.
V1 neurons in WT mice exhibit similar orientation tuning at different contrasts, like in cats and monkeys (Sclar and Freeman 1982; Skottun et al. 1987; Troyer et al. 1998; Ferster and Miller 2000; Niell and Stryker 2008). In nob mice, most cells still maintained their tuning at different contrasts, with similar gOSI at low (50 or 60%) and high (100%) contrasts (Fig. 4, A–C, correlation coefficient: WT, r = 0.92, P < 0.001; nob, r = 0.83, P < 0.001). However, a few nob cells showed larger differences in gOSI between the two contrasts (e.g., Fig. 4B), leading to a statistically significant increase when compared with WT (Fig. 4D, WT, gOSI difference: WT = 0.05 ± 0.02; nob = 0.06 ± 0.02, P = 0.03). The increase in nob was very small and arose from larger deviations on both sides of zero (Fig. 4D). Consistently, the tuning width was less invariant at these two contrasts in nob cells (Fig. 4E, WT, r = 0.88, P < 0.001; nob, r = 0.37, P = 0.005; Fig. 4F, inset, tuning width difference: WT = 3.0 ± 0.4°; nob = 6.0 ± 1.4°, P < 0.001).
Fig. 4.
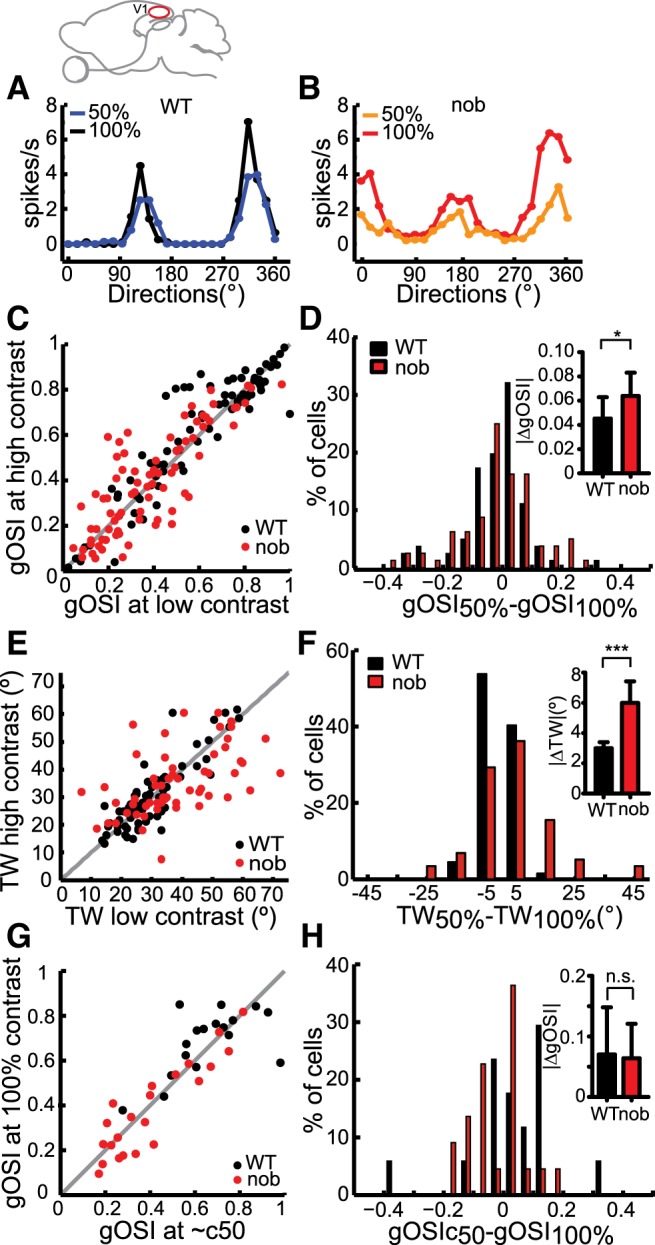
Slight disruption of contrast invariance of orientation tuning in nob V1. A and B: examples of orientation tuning curves at low (50%) and high (100%) contrasts in WT (A) and nob (B) mice. C: scatter plot of gOSI at low (50 or 60%) vs. high (100%) contrast. Black dots: WT, n = 81; red dots: nob, n = 80 cells. Gray line indicates unity. D: distribution of differences in gOSI at the 2 contrasts. Inset: average of absolute differences. E: scatter plot of tuning width (half height at half maximum) at low (50 or 60%) vs. high (100%) contrast. WT, n = 67; nob, n = 58 cells. F: distribution of differences in tuning widths at the 2 contrasts. Inset: average of absolute differences. G and H: gOSI near the half-saturation contrast (c50) of each cell is compared against its gOSI at 100% contrast. WT, n = 20; nob, n = 34 cells. H: average of absolute differences in gOSI. All bars indicate means + SE. *P < 0.05 and ***P < 0.001.
These subtle contrast-dependent changes in nob cells were likely due to the reduced contrast sensitivity in these cells. This was because the 50% contrast was above the half-saturation contrast (c50) of most WT cells but not nob cells (Fig. 3C). Indeed, when comparing the responses at the contrast near c50 (median contrast, WT = 40%; nob = 60%) and those at 100%, gOSI was not more variable in nob than in WT (Fig. 4G, WT: r = 0.61, P = 0.01; nob: r = 0.91, P < 0.001), with similar gOSI differences between the two contrasts in both genotypes (Fig. 4H, WT: 0.07 ± 0.08, n = 17; nob = 0.06 ± 0.06, n = 22, P = 0.49).
Increased response variability in nob V1.
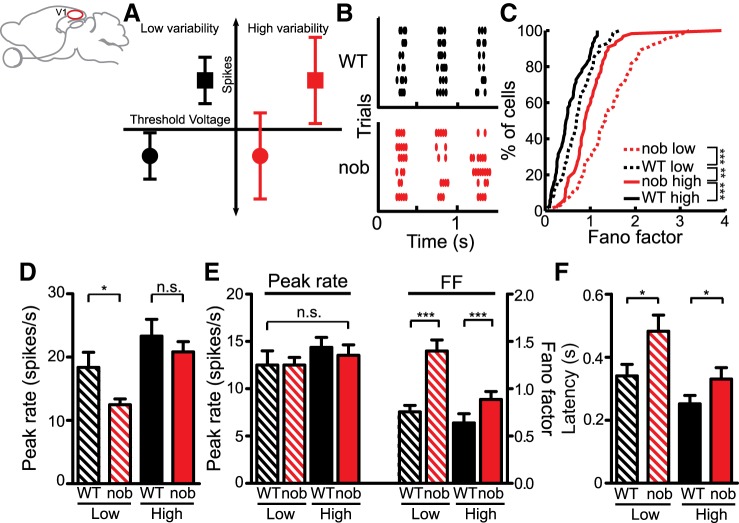
Some of the nob V1 deficits are consistent with reduced input drive due to the loss of the On pathway, such as weaker responses at the preferred orientation and higher c50. Others, however, are not. For example, nob cells showed higher response at the null orientation (Fig. 2G). This could be caused by an increase in trial-to-trial variability of the inputs to V1 cells in nob mice. As shown by the schematic in Fig. 5A, a more variable input is more likely to cross the spiking threshold, even though it is subthreshold on average, such as at the null orientation (Finn et al. 2007). A similar change in variability would not have a similar effect if the mean input were already above threshold, such as when responding to the preferred orientation. To assess whether nob cells showed any alterations in their response reliability, we analyzed the trial-to-trial variability in the spiking responses measured at different contrasts in V1 cells.
Fig. 5.
Increased response variability in nob V1. A: schematic illustrating how an input that is subthreshold on average (circles) can cross threshold and elicit spikes if highly variable. Inputs that are suprathreshold on average (squares) are not similarly affected by changes in their variability. B: raster plots of simple cell responses in WT (top) and nob V1 (bottom). C: distribution of the Fano factor at the peak response at the preferred orientation. D: peak firing rates calculated in a time window of 80 ms at the preferred orientation of each cell. E: similar peak rates of selected (see text) subpopulations of cells (left) and their Fano factors (FF; right). F: latency of the first spike after stimulus onset is also longer in nob mice at both contrasts. All bars indicate means + SE. *P < 0.05 and ***P < 0.001.
We calculated the Fano factor (spike count variance/spike count mean) over a sliding time window (80 ms) for each cycle of the gratings. This was calculated only for simple cells, which display phase-locked firing for each of the stimulus cycles (Fig. 5B, WT: n = 70/81, 86% simple cells, nob: n = 66/80, 83% simple cells). Since we did not observe any difference in the variability among the three cycles (data not shown), we combined data from all cycles. As in cats (Finn et al. 2007; Sadagopan and Ferster 2012), we observed a higher variability, i.e., greater Fano factor, at lower contrasts compared with higher contrasts in WT mice (Fig. 5C, low: 0.70 ± 0.06; high: 0.47 ± 0.05, P = 0.004). This relationship of decreased variability with increased contrasts remained in nob mice (Fig. 5C, low: 1.33 ± 0.10; high: 0.88 ± 0.06, P < 0.001). However, nob mice showed higher variability compared with WT at both contrasts (P < 0.001). The variability in nob mice was so high that it exceeded even the greater variability seen in WT mice at low contrast (P = 0.001). The increased variability in nob mice was also seen at the null orientation and when analyzed with different time windows (data not shown; see materials and methods for details). Analysis of simple cells in putative layer IV also showed increased variability in nob mice (see Table 1).The peak spike rates also decreased in the nob mice (Fig. 5D, low contrast: WT: 18.4 ± 2.36 spikes/s, nob: 12.5 ± 0.90 spikes/s, P = 0.02; high contrast: WT: 23.3 ± 2.65 spikes/s, nob: 20.83 ± 1.62 spikes/s, P = 0.1). We thus performed a mean-matched analysis by selecting a subset of cells with similar magnitudes of peak responses between WT and nob groups (Fig. 5E, low contrast: WT: 12.5 ± 1.51 spikes/s, nob: 12.5 ± 0.81 spikes/s, P = 0.49; high contrast: WT: 14.4 ± 1.06 spikes/s, nob: 13.5 ± 1.10 spikes/s, P = 0.39). Even with similar peak responses, nob mice displayed significantly higher response variability compared with WT mice (Fig. 5E, low contrast: WT: 0.76 ± 0.07, nob: 1.40 ± 0.12, P < 0.001; high contrast: WT: 0.64 ± 0.10, nob: 0.89 ± 0.08, P = 0.004).
Another feature of cortical responses is a decrease in response latency at higher contrasts (Gawne et al. 1996; Reich et al. 2001). This was seen in both WT and nob mice, but the nob mice showed a greater delay, as evident in the timing of the first spike after stimulus onset, at both contrasts (Fig. 5F, low contrast: WT: 0.34 ± 0.04 s, nob: 0.48 ± 0.05 s, P = 0.02; high contrast: WT: 0.25 ± 0.03, nob: 0.33 ± 0.04, P = 0.006).
The observed increase in variability of spike rate responses in nob mice suggests that the input to the cortical cells is more variable under the assumption that the cellular spike threshold of cortical cells is unlikely to be affected in these mutants. This increased variability explains the observed increase in nonoptimal stimulus conditions such as at the null orientations and at low contrasts in some cells. Overall the spiking responses in nob cortical cells are slower, weaker, and more variable than WT.
Increased response variability in the superior colliculus of nob mice.
We next investigated if the increased variability is already apparent in early retinofugal pathways by testing the responses of collicular neurons, which are the major retinal target, situated one synapse away from the retina and also exhibit direction/orientation selectivity (Wang et al. 2010a). We recorded extracellularly from single neurons in the superficial retino-recipient layer of the SC while stimulating with sinusoidal gratings drifting of different directions. We observed a significant increase in spike response variability quantified by the Fano factor in the collicular responses compared with WT (Fig. 6A, WT: 0.53 ± 0.07, nob: 0.67 ± 0.11, P = 0.01). We also saw a decrease in response magnitude (Fig. 6B, WT: 32.5 ± 3.24 spikes/s, nob: 22.1 ± 3.32 spikes/s, P = 0.002) as well as a trend towards an increase in spontaneous firing rates in the nob mice (WT: 0.20 ± 0.09 spikes/s, nob: 0.53 ± 0.21 spikes/s, P = 0.08). There was no significant difference in the latency of the responses to drifting gratings between the two groups (WT: 0.24 ± 0.02 s, nob: 0.24 ± 0.04 s, P = 0.97).
Fig. 6.
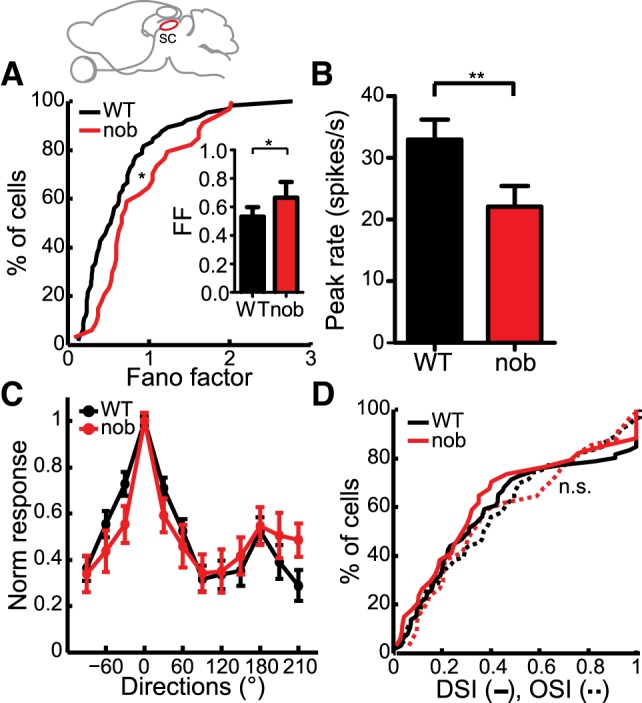
Increased variability in nob SC. A: cumulative distributions of Fano factor in WT and nob SC (WT, n = 66, nob, n = 34 cells). Inset: comparison of the medians. B: decreased peak firing rates in the SC of nob mice. C: normalized tuning curves of responses to various directions are similar in WT and nob mice. D: cumulative plots show no difference in the DSI (solid lines) and OSI (dashed lines) in nob mice. All bars indicate means + SE. **P < 0.01.
Interestingly, the average tuning curves obtained in nob SC neurons were similar to those in WT (Fig. 6C, WT: n = 66, nob: 34). Accordingly, neither direction selectivity nor orientation selectivity was significantly affected in the nob mice (Fig. 6D; DSI, WT: 0.31 ± 0.05, nob: 0.29 ± 0.07, P = 0.61; OSI, WT: 0.37 ± 0.05, nob: 0.33 ± 0.09, P = 0.81). In other words, although the responses in the nob mice were found to be more variable in both V1 and the SC, its impact on response selectivity in these two structures was markedly different, causing a deficit only in the cortical responses. This could be due to the potentially disparate mechanisms of orientation/direction selectivity in these two structures. This result provides the first evidence that direction/orientation selectivity in the colliculus can arise in the absence of the On pathway.
Retinal origin of increased response variability.
Since the increased response variability was seen in both V1 and SC, the retina is likely to be the source of this increase. To test this, we performed in vitro, extracellular recordings from the retina using a multielectrode array. In response to full-field flashes, we sometimes observed a delayed On component in nob mice (Fig. 7A, bottom), as previously reported (Sugihara et al. 1997; Rentería et al. 2006; Pinto et al. 2007; Maddox et al. 2008). The delayed On response occurred only in a subset (265/726 = 36.5%) of nob cells at nearly 700 ms after stimulus onset (Fig. 7C, WT: 158 ± 2.8 ms, nob: 691 ± 21.7 ms, P < 0.001). Since this delayed On was present in only some of the cells (Fig. 7A) and has been shown before to arise by an unknown mechanism intrinsic to the Off pathway (Rentería et al. 2006), we only considered the early part of the response (within 500 ms after stimulus onset or offset) to quantify the peak response. The peak On response in the nob mice was severely reduced (Fig. 7B, WT: 26.8 ± 1.51 spikes/s, nob: 0.4 ± 0.04 spikes/s, P < 0.001). The Off response in nob mice was also decreased in its peak magnitude (Fig. 7B, WT: 15.5 ± 1.51 spikes/s, nob: 7.86 ± 0.78 spikes/s, P < 0.001) but showed no change in its latency from stimulus offset (Fig. 7C, WT: 150 ± 3.4 ms, nob: 150 ± 4.5 ms, P = 0.08). Strikingly, the Off responses in nob mice were much more variable in their trial-to-trial reliability than the WT responses (Fig. 7D, WT: 0.87 ± 0.02, nob: 1.22 ± 0.05, P < 0.001). Thus the increased variability seen in the SC and V1 was already manifested in the retinal responses in nob mice.
Fig. 7.
Retinal origin of increased response variability. A: raster plots of responses to full field flashes of retinal ganglion cells (RGCs) in WT (top) and nob (middle and bottom). In some nob RGCs (bottom) a delayed On response was observed. B: peak firing rates calculated within the first 500 ms following flash onset/offset using a sliding window (100 ms) (Off: WT, n = 591, nob, n = 474; On: WT, n = 709, nob, n = 336 cells). C: latency of the peak response shows a greater delay in the On responses of nob mice. D: distribution of Fano factors at the peak response shows greater values in nob mice. E: example raster plot of a WT RGC before (top) and after treatment with dl-2-amino-4-phosphonobutyric acid (2-APB; bottom). F: decreased On response after 2-APB treatment (Off: n = 576; On: n = 343 cells). G: comparison of the Fano factor before and after 2-APB. Gray line indicates the unity line. H: increase in Fano factor in the Off response after removal of On pathway. All bars indicate means + SE. ***P < 0.001.
To test whether the changes in spiking responses that we observed are a result of acute loss of the On pathway, we used 2-APB (100 μM) to pharmacologically block the GRM6 receptor (Slaughter and Miller 1981). 2-APB is known to block the On pathway and also its inhibition to the Off pathway (Rentería et al. 2006; Manookin et al. 2008). In our recordings, we observed a significant reduction in the peak On responses (Fig. 7, E and F, Pre: 26.82 ± 1.51 spikes/s, Post: 0.13 ± 0.01 spikes/s, P < 0.001) and delayed onset of the remaining On responses (Pre: 167 ± 12.0 ms, Post: 1058 ± 48.7 ms, P < 0.001), similar to the changes seen in nob mice. The Off responses were marginally diminished in magnitude (Fig. 7, E and F, Pre: 15.51 ± 1.51 spikes/s, Post: 13.24 ± 0.89 spikes/s, P < 0.001, Wilcoxon paired test) and showed a slightly larger delay (Pre: 150 ± 5.7 ms, Post: 158 ± 6.0 ms, P = 0.001). Most remarkably, we saw an increased variability in the Off responses in retinas after 2-APB treatment, with most of the post-2-APB Fano factors greater than the pre-2-APB values (Fig. 7, G and H, Off: Pre: 0.95 ± 0.02, Post: 1.24 ± 0.05, P < 0.001). Therefore, the effects we saw in the nob retina could be largely induced due to acute removal of the On pathway, likely involving the disruption of the cross talk between the On and Off pathways.
In summary, the removal of On pathway not only diminished the magnitude of responses but also caused the retinal Off responses to be more variable. These defects in response magnitude and variability seemed to propagate to higher visual centers, compromising selectivity to stimulus orientations and sensitivity to contrasts in individual cortical cells.
DISCUSSION
Parallel channels signaling stimulus onset and offset diverge at the very first synapse in the retina (Werblin and Dowling 1969; Murakami et al. 1975; Slaughter and Miller 1981; Nakajima et al. 1993) and remain largely separate until their convergence onto single cortical cells in V1 (Hubel and Wiesel 1959; Schiller 1982). Here we report that removal of the On pathway within the retina, using mutant nob mice, disrupts two salient properties of cortical cells: orientation selectivity and contrast sensitivity. These deficits were attributed to two effects of loss of On responses, a decrease in response magnitude, and an increase in trial-to-trial variability. Similar deficits of reduced response magnitudes and loss of reliability were also observed in the superior colliculus but did not affect direction/orientation selectivity in those cells, indicating different contributions of the On-Off pathways in these two central visual targets. Reduction of peak responses and increased variability was apparent even in the Off responses of nob RGCs and was phenocopied by acute pharmacological inactivation of the On pathway. Together, these results suggest that normally the On pathway contributes to increase the reliability of the Off responses, presumably via cross talk between the On and Off pathways within the retina. On-Off response properties of the retinal outputs in turn propagate to higher visual targets to critically influence their functions.
Effect of loss of on responses on orientation selectivity in V1.
The segregation of On and Off inputs to cortical simple cells into distinct subregions confers orientation selectivity on these cells (Hubel and Wiesel 1962; Hirsch et al. 1998). After removing one stream of the inputs, we observed reduced orientation selectivity in the nob mice. Besides simply eliminating the On subregions, removal of On input could also affect the structure and maybe development of receptive field subregions in nob V1. For example, genetic removal of the On pathway could alter the structure of retinal waves (Demas et al. 2006), possibly leading to disruption of On-Off anti-correlation (Miller 1994) and formation of less elongated Off-only receptive fields in cortical cells, which would decrease orientation selectivity. Also, unlike in cats, the On and Off subregions in mouse simple cells are greatly overlapped (Liu et al. 2010). Therefore, the loss of On subregions might reveal a much less elongated Off subregion, which would have poorer selectivity. Finally, the increased response variability contributes to the increased responses at nonpreferred orientations, thus decreasing selectivity. Although the relative contribution of all these mechanisms cannot be inferred from our results, it is likely that all of these could act in concert either in the same or different populations of cells in nob mice.
The substantial amount of orientation selectivity observed in nob V1 cells can be attributed to different sources. First, elongated Off subregions that comprise V1 receptive fields can confer orientation selectivity. Second, the absence of the On inputs might unmask the otherwise weak On surrounds of Off centered thalamocortical axons, boosting the selectivity further. We indeed saw occasional On areas in a few receptive fields mapped using reverse-correlation methods, albeit much weaker than the neighboring Off subregions (data not shown). These could also be suggestive of incomplete removal of On inputs (Fig. 1D), which could contribute to the observed orientation selectivity. Third, a homeostatic increase in the remaining cortical Off responses after On removal (Fig. 1D) could boost orientation selectivity. However, the lack of such an increase in retinal Off responses (Fig. 7B) argues that the cortical increase in Off response is more likely an experimental bias of including only strongly responsive Off cells in the dataset. The delayed On responses (∼0.7 s) seen in some nob RGCs unlikely contribute to the residual selectivity. Since we presented drifting gratings at a temporal frequency of 2 Hz for a total time of 1.5 s per trial, the delay between an Off and On stimulus is smaller than the latency of the delayed On response.
Two previous studies used 2-APB to examine the effect of blocking retinal On pathway on V1 responses in cats and monkeys (Schiller 1982; Sherk and Horton 1984). Although there are differences in species, means of removing the On pathway, and visual stimuli, some similarities exist between our and previous studies. Similar to the loss or decrease of light edge/slit-evoked responses observed in cats (Sherk and Horton 1984) and monkeys (Schiller 1982), we also saw a decrease in the response magnitude at preferred orientation in V1 cells. Furthermore, Sherk and Horton (1984) referred to the remaining dark edge responses as being “weak and erratic,” which agrees well with the decreased Off responses we observed in the retina and the increased variability in V1 responses. The residual selectivity as we observed in the nob mice is perhaps why the previous studies concluded that blocking the On pathway did not result in consistent/obvious changes in orientation selectivity, although neither provided any quantification of OSI or tuning width.
The absence of any effect of On removal on direction or orientation selectivity in SC neurons is somewhat surprising, yet not completely unexpected given the On-Off subregion overlap that is observed in collicular cells (Wang et al. 2010b). Our results confirm that collicular selectivity is not dependent on the spatial structure of On-Off response within collicular receptive fields but possibly mediated by precisely timed excitation-inhibition interplays driven by either sign of stimulus contrast.
Role of on and off pathways in contrast sensitivity.
Removal of the On pathway in nob mice drastically reduced responses to bright stimuli in SC and V1, confirming the role of the On pathway in reporting increases in contrast. When presented with stimuli consisting of both bright and dark regions (drifting gratings), the time-averaged net input to single cortical cells would also decrease, possibly causing the responses to fall below threshold at low contrasts and consequently a shift in contrast sensitivity curves towards higher c50 values, as observed in these mice. The effect of the On pathway on contrast sensitivity is further complicated by the fact that these parallel pathways are not completely mutually exclusive due to cross inhibition, most dominantly from the On to the Off pathway (Zaghloul et al. 2003; Liang and Freed 2010). Indeed, the Off responses in nob as well as 2-APB-treated retinas are decreased as would be expected from loss of On-mediated disinhibition. It is known that the On pathway is better than the Off pathway at reporting low contrasts (Zaghloul et al. 2003; Liang and Freed 2010) and via disinhibition of the Off response serves to enhance its contrast gain at higher contrasts (Manookin et al. 2008; Liang and Freed 2010, 2012). These retinal effects might also contribute to the decreased contrast sensitivity, both in terms of the increased c50 and decreased gain that we observed in nob V1. Consistently, the nob mice also exhibit higher contrast thresholds in an optomotor behavior test (Pinto et al. 2007; Maddox et al. 2008), similar to the deficits in human patients suffering from congenital stationary night blindness associated with defects in the GRM6 cascade (Carr 1974; McCall and Gregg 2008).
On pathway and response reliability.
The observation that acute removal of the On pathway using 2-APB increases the trial-to-trial variability of the Off responses strongly suggests a role for the On-Off cross talk in response reliability. It is possible that the normal On pathway mediates the phasic disinhibition of the Off pathway at light offset (Chen and Linsenmeier 1989; Cohen 1998; Zaghloul et al. 2003; Roska et al. 2006), contributing to a sharper rise in the evoked postsynaptic potential in the Off RGCs, which would elicit spiking responses more reliably. Consistent with this hypothesis, we observed a decrease in peak rates of Off RGCs in nob and 2-APB-treated retinas. In nob mice the higher variability in RGC responses is expected to propagate to central visual targets such as SC and V1 because neighboring RGCs show correlated variability (Meister et al. 1995) and they are likely to converge onto the same postsynaptic cells due to retinotopy. Thus their spike variability would be preserved and could manifest as increased variability in different lateral geniculate nucleus neurons that provide input to V1 cells. Therefore, it is possible that the increased trial-to-trial variability observed in nob RGCs is amplified at each synapse along the visual pathway (Kara et al. 2000), thereby increasing the response variability in V1 cells.
In summary, the role of On and Off pathways can be categorized into two broad functions-contrast sensitivity and response reliability. First, the presence of both pathways not only extends the range of contrasts perceived by the visual system (Schiller et al. 1986) but also enhances the sensitivity to changes in contrast. Second, cooperation between these two pathways achieves higher reliability of responses. The contribution of inhibition from the On to the Off pathway in the retina seems to be the first candidate mechanism for future investigations. Since both the variability in V1 responses and their contrast sensitivity are expected to be further transmitted to higher cortical areas, it would be interesting to assess the role of the On pathway in properties such as high velocity tuning observed in certain higher visual areas [e.g., AL in mice (Andermann et al. 2011; Marshel et al. 2011); and MT in primates (Maunsell and Van Essen 1983)].
GRANTS
This work was supported by National Eye Institute Grants EY-018621 and EY-020950 (to J. Cang) and EY-019034 (to X. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.S., X.L., and J.C. conception and design of research; R.S. and H.C. performed experiments; R.S., H.C., and J.C. analyzed data; R.S. and J.C. interpreted results of experiments; R.S. and J.C. prepared figures; R.S. and J.C. drafted manuscript; R.S., H.C., X.L., and J.C. edited and revised manuscript; R.S., H.C., X.L., and J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Larry Pinto and Dr. John Troy for kindly providing us the nob mice. We also thank Bor-Shuen Wang and Xinyu Zhao for helpful discussions.
REFERENCES
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol 48: 217–237, 1982 [DOI] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RÂ. Functional specialization of mouse higher visual cortical areas. Neuron 72: 1025–1039, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkin MS, Miller RF. Subtle actions of 2-amino-4-phosphonobutyrate (APB) on the Off pathway in the mudpuppy retina. Brain Res 426: 142–148, 1987 [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Carr RE. Congenital stationary nightblindness. Trans Am Ophthalmol Soc 72: 448–487, 1974 [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Godecke I. Cortical cell orientation selectivity fails to develop in the absence of on-center retinal ganglion cell activity. J Neurosci 20: 1922–1930, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EP, Linsenmeier RA. Centre components of cone-driven retinal ganglion cells: differential sensitivity to 2-amino-4-phosphonobutyric acid. J Physiol 419: 77–93, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED. Interactions of inhibition and excitation in the light-evoked currents of x type retinal ganglion cells. J Neurophysiol 80: 2975–2990, 1998 [DOI] [PubMed] [Google Scholar]
- Cynader M, Berman N. Receptive-field organization of monkey superior colliculus. J Neurophysiol 35: 187–201, 1972 [DOI] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, Wong RO, Guido W. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron 50: 247–259, 2006 [DOI] [PubMed] [Google Scholar]
- Dragoi V, Rivadulla C, Sur M. Foci of orientation plasticity in visual cortex. Nature 411: 80–86, 2001 [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci 23: 441–471, 2000 [DOI] [PubMed] [Google Scholar]
- Finn IM, Priebe NJ, Ferster D. The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron 54: 137–152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Kjaer TW, Richmond BJ. Latency: another potential code for feature binding in striate cortex. J Neurophysiol 76: 1356–1360, 1996 [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J Neurophysiol 90: 3594–3607, 2003 [DOI] [PubMed] [Google Scholar]
- Hartline HK. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Am J Physiol 121: 400–415, 1938 [Google Scholar]
- Hirsch JA, Alonso JM, Reid RC, Martinez LM. Synaptic integration in striate cortical simple cells. J Neurosci 18: 9517–9528, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer A, Drager UC. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J Comp Neurol 234: 465–474, 1985 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol 148: 574–591, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol 160: 106–154, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wang GY, Liets LC, Collins OA, Chapman B, Chalupa LM. Eye-specific retinogeniculate segregation independent of normal neuronal activity. Science 300: 994–998, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XT, Brunken WJ. A differential effect of APB on ON- and OFF-center ganglion cells in the dark adapted rabbit retina. Brain Res 708: 191–196, 1996 [DOI] [PubMed] [Google Scholar]
- Kara P, Reinagel P, Reid RC. Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron 27: 635–646, 2000 [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci 33: 1961–1972, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16: 37–68, 1953 [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci 30: 5533–5543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Freed MA. Cross inhibition from ON to OFF pathway improves the efficiency of contrast encoding in the mammalian retina. J Neurophysiol 108: 2679–2688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BH, Li P, Li YT, Sun YJ, Yanagawa Y, Obata K, Zhang LI, Tao HW. Visual receptive field structure of cortical inhibitory neurons revealed by two-photon imaging guided recording. J Neurosci 29: 10520–10532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Bh Li P, Sun YJ, Li YT, Zhang LI, Tao HW. Intervening inhibition underlies simple-cell receptive field structure in visual cortex. Nat Neurosci 13: 89–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox DM, Vessey KA, Yarbrough GL, Invergo BM, Cantrell DR, Inayat S, Balannik V, Hicks WL, Hawes NL, Byers S, Smith RS, Hurd R, Howell D, Gregg RG, Chang B, Naggert JK, Troy JB, Pinto LH, Nishina PM, McCall MA. Allelic variance between GRM6 mutants, Grm6nob3 and Grm6nob4, results in differences in retinal ganglion cell visual responses. J Physiol 586: 4409–4424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the off visual pathway in daylight. J Neurosci 28: 4136–4150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, Callaway EM. Functional specialization of seven mouse visual cortical areas. Neuron 72: 1040–1054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa YA, Tomomitsu Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, Takada M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80: 757–765, 1995 [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol 49: 1127–1147, 1983 [DOI] [PubMed] [Google Scholar]
- McCall MA, Gregg RG. Comparisons of structural and functional abnormalities in mouse b-wave mutants. J Physiol 586: 4385–4392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain J, Buser P. Receptive fields of single cells in the cat's superior colliculus. Exp Brain Res 5: 314–325, 1968 [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science 270: 1207–1210, 1995 [DOI] [PubMed] [Google Scholar]
- Miller K. A model for the development of simple cell receptive fields and the ordered arrangement of orientation columns through activity-dependent competition between ON- and OFF-center inputs. J Neurosci 14: 409–441, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ohtsuka T, Shimazaki H. Effects of aspartate and glutamate on the bipolar cells in the carp retina. Vision Res 15: 456–458, 1975 [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185: 536–555, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem 268: 11868–11873, 1993 [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci 28: 7520–7536, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 77: 361–369, 1994 [DOI] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci 39: 2443–2449, 1998 [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Pinto LH, Vitaterna MH, Shimomura K, Siepka SM, Balannik V, McDearmon EL, Omura C, Lumayag S, Invergo BM, Brandon M, Glawe B, Cantrell DR, Donald R, Inayat S, Olvera MA, Vessey KA, Kirstan A, McCall MA, Maddox D, Morgans CW, Young B, Pletcher MT, Mullins RF, Troy JB, Takahashi JS. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci 24: 111–123, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Mechler F, Victor JD. Temporal coding of contrast in primary visual cortex: when, what, and why. J Neurophysiol 85: 1039–1050, 2001 [DOI] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378: 281–284, 1995 [DOI] [PubMed] [Google Scholar]
- Rentería R, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci 26: 11857–11869, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci 22: 5639–5651, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol 95: 3810–3822, 2006 [DOI] [PubMed] [Google Scholar]
- Sadagopan S, Ferster D. Feedforward origins of response variability underlying contrast invariant orientation tuning in cat visual cortex. Neuron 74: 911–923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnaik R, Wang BS, Cang J. Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb Cortex 2013. February 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH. Central connections of the retinal ON and OFF pathways. Nature 297: 580–583, 1982 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. Functions of the ON and OFF channels of the visual system. Nature 322: 824–825, 1986 [DOI] [PubMed] [Google Scholar]
- Sclar G, Freeman RD. Orientation selectivity in the cat's striate cortex is invariant with stimulus contrast. Exp Brain Res 46: 457–461, 1982 [DOI] [PubMed] [Google Scholar]
- Sherk H, Horton J. Receptive field properties in the cat's area 17 in the absence of on- center geniculate input. J Neurosci 4: 381–393, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottun BC, Bradley A, Sclar G, Ohzawa I, Freeman RD. The effects of contrast on visual orientation and spatial frequency discrimination: a comparison of single cells and behavior. J Neurophysiol 57: 773–786, 1987 [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211: 182–185, 1981 [DOI] [PubMed] [Google Scholar]
- Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J Neurosci 27: 2145–2149, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara H, Inoue T, Nakanishi S, Fukuda Y. A late ON response remains in visual response of the mGluR6-deficient mouse. Neurosci Lett 233: 137–140, 1997 [DOI] [PubMed] [Google Scholar]
- Swindale NV. Orientation tuning curves: empirical description and estimation of parameters. Biol Cybern 78: 45–56, 1998 [DOI] [PubMed] [Google Scholar]
- Troyer TW, Krukowski AE, Priebe NJ, Miller KD. Contrast-invariant orientation tuning in cat visual cortex: thalamocortical input tuning and correlation-based intracortical connectivity. J Neurosci 18: 5908–5927, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Sarnaik R, Cang J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 65: 246–256, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci 30: 16573–16584, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rangarajan KV, Lawhn-Heath CA, Sarnaik R, Wang BS, Liu X, Cang J. Direction-specific disruption of subcortical visual behavior and receptive fields in mice lacking the beta subunit of nicotinic acetylcholine receptor. J Neurosci 29: 12909–12918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol 32: 339–355, 1969 [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci 23: 2645–2654, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]