Abstract
Background
Regulatory T cells (Tregs) exhibit functional abnormalities in the context of hepatocellular carcinoma (HCC). The microRNAs (miRNAs) are identified as the key modulators in Tregs. This study was to explore whether the expression profiles of miRNAs of Tregs were different in HCC-activated Tregs and whether Foxp3 had special effects on them.
Methods
We isolated HCC-activated Tregs from mice bearing HCC and compared the expression profiles of miRNAs between HCC-activated Tregs and control Tregs by microarray. RNA interference against Foxp3 was also performed through transfection of synthetic siRNAs to Tregs for analyzing the effect of Foxp3 on the expression of miRNAs. Tregs isolated from HCC patients (n = 12) and healthy controls (n = 7) were used for validation of the differentially expressed miRNAs. Finally, bioinformatic analysis was applied to infer their possible roles.
Results
We found nine specifically altered miRNAs in HCC-activated Tregs from the murine model. After transfection with siRNAs against Foxp3, control Tregs showed obvious reduction of Foxp3 and five miRNAs were significantly changed; HCC-activated Tregs exhibited a slight reduction of Foxp3 with three miRNAs significantly changed. Tregs from HCC patients and healthy controls finally confirmed the up-regulation of four miRNAs (hsa-miR-182-5p, hsa-miR-214-3p, hsa-miR-129-5p and hsa-miR-30b-5p). Following bioinformatic analysis suggested these altered miRNAs would target eight important signaling pathways that could affect the functions of Tregs.
Conclusions
Our studies provided the first evidence that Tregs in HCC had the specifically altered expression of miRNAs, which was affected by Foxp3. These results are useful both in finding new biomarkers and in further exploring the functions of Tregs in HCC patients.
Keywords: Regulatory T cells, Hepatocellular carcinoma, microRNAs array, Foxp3 RNA interference, Bioinformatics
Background
Hepatocellular carcinoma (HCC) is the fifth most common cancer with relatively poor overall survival worldwide [1]. Accumulating evidence implies that CD4+ CD25+ Foxp3+ regulatory T cells (Tregs) are the critical factor affecting the progression and prognosis of HCC [2,3]. HCC patients have increased Tregs in peripheral blood, ascites and tumor tissue [4,5] and this prevalence of Tregs correlates with tumor stage and patients survival [6-8]. Not only the number but also the functional phenotypes of Tregs are found abnormal in HCC. Tregs in peripheral blood of HCC patients preferentially up-regulate CCR6, which facilitates their migration to tumor sites [9]. HCC-activated Tregs also express high levels of glucocorticoid-induced tumor necrosis factor receptor (GITR) and the inducible T cell co-stimulator (ICOS), both of which are key mediators for the suppressive function of Tregs [10]. In addition, the enhanced suppressive function of Tregs is confirmed by different studies in HCC patients [10-12].
Tregs constitute the key components in tumor immune suppression [13]. Recent studies demonstrate that Tregs are widely modulated by the single-stranded microRNAs (miRNAs) [14,15]. Depleting Dicer, a key enzyme in the maturation of miRNAs, causes diminished Tregs and compromises their suppressive function [16]. Following studies demonstrate that miR-21 enhances the expression of Foxp3 while miR-31 suppresses it in human Tregs [17]. miR-155, modulated by Foxp3, guarantees competitive fitness of Tregs to the IL-2 signal by targeting suppressor of cytokine signaling 1 (SOCS1) protein [18,19]. Deficiency of miR-146a, another recently confirmed microRNA in Tregs, leads to increased Tregs with an impaired function [20].
However, it is still unclear at present for the following questions in liver cancer research. Firstly, it is still unknown that whether the expression profiles of miRNAs of Tregs are different between control Tregs and HCC-activated Tregs. Secondly, it is worthy to demonstrate whether Foxp3 has a special role and influences the expression profiles of miRNAs of Tregs in HCC. Thirdly, if there are any specifically altered miRNAs in Tregs in HCC, do they affect the functions of Tregs in HCC? It is also need to be further explored. In this study, we used Tregs from both the murine HCC model and HCC patients to answer these questions. We found nine miRNAs differentially expressed in HCC-activated Tregs and alterations of these miRNAs were specific to HCC-activated Tregs in the murine model. Foxp3 affected the expression of these miRNAs. Tregs isolated from human blood confirmed that four miRNAs up-regulated in HCC patients. To our knowledge, this study was the first attempt to characterize miRNAs perturbations and the consequences in Tregs activated by HCC. Our data provided a systemic view on alterations in HCC-activated Tregs, which was not only useful in finding new biomarkers but also in further exploring the functions of Tregs in HCC patients.
Methods
Human Tregs separation
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient sedimentation using Lymphosep (Biowest, France) lymphocyte separation media. CD4+ CD25+ CD127- Tregs were enriched by human regulatory T cell isolation kit (Miltenyi, German). In brief, non-CD4+ and CD127high cells were first depleted with microbeads and then the pre-enriched CD4+ CD127dim cells went through positive selection for CD25+ T cells. The purity of Tregs was monitored via fluorescence-activated cell sorting (FACS). The participants included HCC patients (n = 12) and matched healthy controls (n = 7). Ethical approval for the use of human subjects was obtained from the Research Ethics Committee of Zhongshan Hospital (Shanghai, PR China), and informed consent was obtained from each participant.
Cell lines and animals
Hepa 1–6 (CRL-1830), the murine HCC cell line, was obtained from American Type Culture Collection (ATCC, USA) and maintained in DMEM (Biowest) supplemented with 10% FBS (Biowest). C57BL/6 J mice (6 to 8 weeks of age) were purchased from the Chinese Academy of Science and housed at the Animal Maintenance Facility of the Shanghai Medical College, Fudan University. All animal experiments were performed in conformity with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Care of Experimental Animals Committee of Fudan University approved all animal protocols (Permit Number: SYXK 2009–0082).
Hepa 1–6 tumor bearing mice were established as following: mice were injected subcutaneously at the left flank with 3 × 106 Hepa 1–6 cells in 200 μL RPMI 1640 (Biowest) or 200 μL RPMI 1640 alone as control. Three groups of tumor bearing mice and control mice were established (12 mice in each group). Two weeks after inoculation, the mice with visible tumor were sacrificed and spleens were collected for isolation of Tregs using mouse regulatory T cell isolation kit (Miltenyi). In brief, CD4+ T cells were enriched by negative selection and then went through positive selection for CD25+ T cells. The purity of Tregs was monitored via FACS.
FACS analysis
For FACS, previously collected Tregs were stained with regulatory T cell Kit (eBioscience, USA). Briefly, cells were first incubated with anti-CD4-FITC and anti-CD25-PE Abs at 4°C for 30 minutes. After washing, cells were treated with fixation/permeabilization buffer and then incubated with anti-Foxp3-PE-Cy5 Abs at 4°C for 30 minutes. Stained cells were subsequently analyzed using an EPICS ALTRA Flow Cytometer (Beckman Coulter, USA).
Transfection of siRNAs
Validated siRNAs against mouse Foxp3 and AllStars negative control siRNAs were obtained from Qiagen company in a FlexiTube format (Qiagen, German). Four species of siRNAs against Foxp3 (SI01005319, SI01005326, SI01005333 and SI01005340) were mixed in equal amount to generate the Foxp3 siRNAs pool. siRNAs were transfected into HCC-activated Tregs and control Tregs using HiPerFect transfection reagent (Qiagen) according to the manufacturer’s protocol. Tregs were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS, 5 μg/mL plate-bounded anti-CD3, 5 μg/mL soluble anti-CD28 antibodies (Biolegend, USA) and 40 ng/mL rm IL-2 (PeproTech, USA) at 37°C in humidified atmosphere containing 5% CO2 in air. Mixture of 100 nM of siRNAs and 6 μL of HiPerFect reagent were used for each transfection. Then Tregs were harvested for further analysis forty-eight hours after transfection. Two-step qRT-PCR and FACS validated the efficiency of Foxp3 RNA interference (RNAi).
miRNAs microarray
miRNAs microarray was assisted by Kangcheng Bioscience Service Company (Shanghai). In brief, total RNA was extracted from Tregs of each group using TRIzol (Invitrogen, USA) and miRNeasy mini kit (Qiagen). RNA quality and quantity was measured by NanoDrop-1000 spectrophotometer (Nanodrop Technologies, USA). miRNAs were labeled by the miRCURY™ Hy3™/Hy5™ Power labeling kit (Exiqon, Denmark) and hybridized on the miRCURYTMLNA Array (v.16.0) (Exiqon). The GenePix 4000B microarray scanner (Molecular Devices, USA) scanned the slides. After normalization, volcano plot was used to identify the differentially expressed miRNAs (fold change ≥ 1.5 and P-value ≤ 0.05 fold change: the ratio of normalized intensities, HCC-activated Tregs vs. control Tregs; P-value: t-test results between groups). Three independent arrays were performed for HCC-activated Tregs and control Tregs. One array was performed for HCC-activated Tregs transfected with siRNAs against Foxp3 or control siRNAs respectively; one array was performed for control Tregs transfected with siRNAs against Foxp3 or control siRNAs respectively.
Real-time PCR
Total RNA was extracted from Tregs using TRIzol (Invitrogen) and miRNeasy mini kit (Qiagen). RNA quality and quantity was measured by NanoDrop-1000 spectrophotometer. miRNAs were transcribed to cDNA by cDNA Synthesis Kit (Epicentre, USA) with specific reverse transcription primers (The primers were listed in Additional file 1: Table S1 ). Then the cDNA was used for real time-PCR by miScript SYBRGreen PCR Kit (Qiagen) with specific primers (The primers were listed in Additional file 2: Table S2). For quantification of Foxp3, the cDNA was first synthesized by cDNA Synthesis Kit with Oligo(dT)21 primer, and the real time-PCR was performed by miScript SYBRGreen PCR Kit with specific primers (Invitrogen, the primers were listed in Additional file 2: Table S2. The expression levels of miRNAs were presented as 2-Δ Δ Ct or 2-Δ Ct relative to U6 levels and the levels of mRNA were presented as 2-Δ Δ Ct relative to GAPDH levels.
Bioinformatic analysis
We predicted target genes using the online algorithm TargetScan (Release 6.2, http://www.targetscan.org) [21]. The network of miRNAs and target genes was constructed in the software Exploratory Gene Association Networks (EGAN) [22]. In brief, the total target genes lists were imported into EGAN and further filtered based on Tregs MeSH term (T-Lymphocytes, regulatory). Target genes with direct relation with Tregs MeSH term were presented in the network and further analyzed for functional enrichment. Pathway enrichment was based on the pathways database Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) through DAVID Bioinformatics Resources 6.7 ( http://david.abcc.ncifcrf.gov/home.jsp) [23].
Statistical analysis
Microarray data of miRNAs was analyzed by volcano plot and the filtering criteria were set at expression fold change ≥ 1.5 and P-value ≤ 0.05. Unsupervised hierarchical clustering was performed by MultiExperiment Viewer software (v4.8.1, USA). qRT-PCR validation data was analyzed by Mann–Whitney test. Statistical significance was set at P-value < 0.05 (*) or P- value < 0.01 (**).
Results
miRNAs were differentially and specifically expressed in HCC-activated Tregs
Tregs were isolated from spleens of mice and the purity of Tregs was examined by fluorescence-activated cell sorting (FACS); only the cells with the purity above 95% (data not shown) were further processed for microarray analysis. Differentially expressed miRNAs in HCC-activated Tregs were selected by volcano plot filtering (fold change ≥ 1.5 and P-value ≤ 0.05). Eleven miRNAs were identified as shown in Figure 1A. There were four up-regulated miRNAs (mmu-miR-709, mmu-miR-467a-3p, mmu-miR-182-5p and mmu-miR-25-5p) and seven down-regulated miRNAs (mmu-miR-615-3p, mmu-miR-409-3p, mmu-miR-680, mmu-miR-129-5p, mmu-miR-151-5p, mmu-miR-142-5p and mmu-miR-30b-5p), as the values presented in Table 1. Then we performed unsupervised hierarchical clustering of the eleven miRNAs. We found these miRNAs clearly discriminated the HCC-activated Tregs from control Tregs, as shown in Figure 1B.
Figure 1.
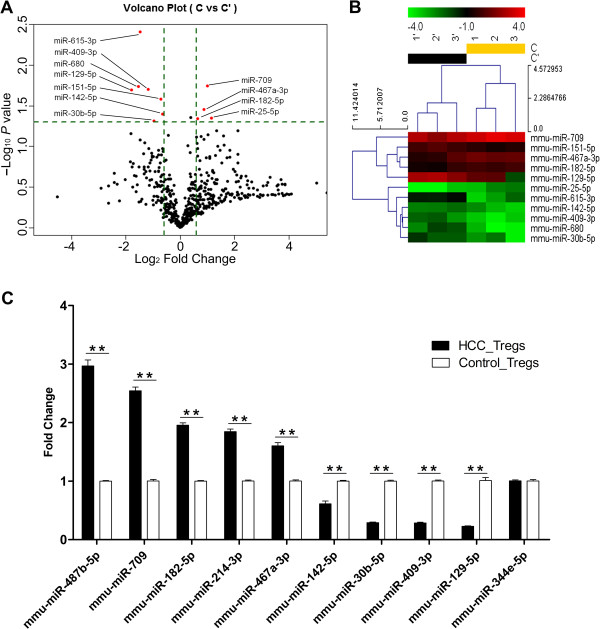
miRNAs differentially expressed in HCC-activated Tregs. (A) Differentially expressed miRNAs identified in the microarray. Eleven miRNAs (red plots) passed the volcano plot filtering. (B) Unsupervised hierarchical clustering of differentially expressed miRNAs. Scale bar: up-regulated (red), down-regulated (green). Log2 transformed data were used. C: HCC-activated Tregs (three replicates: 1, 2, 3); C’: control Tregs (three replicates: 1’, 2’, 3’). (C) Validation of the differentially expressed miRNAs by qRT-PCR. Values were presented as mean ± SEM from three independent experiments performed in triplicates and were analyzed using the two-tailed Mann–Whitney test. **P < 0.01 for indicated comparison.
Table 1.
Differentially expressed miRNAs in HCC-activated Tregs
| Name | Fold change (Tumor vs. Control) | P -value |
|---|---|---|
| mmu-miR-25-5p |
2.21 |
0.04 |
| mmu-miR-709 |
1.98 |
0.02 |
| mmu-miR-467a-3p |
1.82 |
0.04 |
| mmu-miR-182-5p |
1.54 |
0.05 |
| mmu-miR-129-5p |
0.29 |
0.02 |
| mmu-miR-680 |
0.34 |
0.02 |
| mmu-miR-615-3p |
0.36 |
0.00 |
| mmu-miR-409-3p |
0.44 |
0.02 |
| mmu-miR-30b-5p |
0.51 |
0.05 |
| mmu-miR-151-5p |
0.61 |
0.03 |
| mmu-miR-142-5p | 0.63 | 0.04 |
By TargetScan, we found that mmu-miR-25-5p, mmu-miR-615-3p, mmu-miR-151-5p and mmu-miR-680 had few target genes directly relating with Tregs in MeSH database, so we excluded the four miRNAs for further exploration. As mmu-miR-155 and mmu-let-7i have been well documented in T cells [19,24,25], we observed that mmu-miR-487b-5p and mmu-miR-214-3p were classified into the same group with mmu-miR-155 and mmu-let-7i respectively after hierarchical clustering (data not shown). Therefore, we also included these two miRNAs for further validation. To verify the credibility of qRT-PCR validation, we included the miR-344e-5p as a negative control as it did not pass volcano plot filtering (fold change = 1.85, P-value = 0.54) in microarray. Among the ten miRNAs validated by qRT-PCR, we found that mmu-miR-487b-5p, mmu-miR-709, mmu-miR-182-5p, mmu-miR-214-3p and mmu-miR-467a-3p were up-regulated in HCC-activated Tregs, mmu-miR-142-5p, mmu-miR-30b-5p, mmu-miR-409-3p and mmu-miR-129-5p were down-regulated (P < 0.01), while miR-344e-5p did not change significantly, as shown in Figure 1C.
Foxp3 was involved in regulating the miRNAs
As Foxp3 is the master regulator in Tregs, it prompts us to check whether these miRNAs would be specifically affected by Foxp3. We compared the mean fluorescence intensity (MFI) of Foxp3 in Tregs after magnetic sorting. FACS results showed that the sorted Tregs had comparable percentages of Foxp3 positive cells; however, the MFI was higher in HCC-activated Tregs compared with control Tregs (Figure 2A). We transfected Tregs with siRNAs against Foxp3 or negative controls and forty-eight hours we determined the efficiency of silencing. The qRT-PCR data demonstrated that mRNA levels of Foxp3 reduced significantly (34%) in control Tregs, whereas the levels did not change significantly in HCC-activated Tregs (Figure 2B, left). While the FACS results showed the protein levels of Foxp3 reduced in both groups, we observed a more potent decrease of Foxp3 in control Tregs after siRNA silencing (Figure 2B, right). Then we examined the expression levels of the nine miRNAs in Tregs from our microarray data after transfection. In control Tregs, mmu-miR-487b-5p, mmu-miR-214-3p, mmu-miR-30b-5p and mmu-miR-129-5p showed significant down-regulation while mmu-miR-409-3p showed significant up-regulation (Figure 2C, left). Compared with control Tregs, although mmu-miR-487b-5p and mmu-miR-129-5p showed similar down-regulation in HCC-activated Tregs, mmu-miR-409-3p was actually significantly down-regulated; mmu-miR-214-3p and mmu-miR-30b-5p did not exhibit significant changes (Figure 2C, right).
Figure 2.
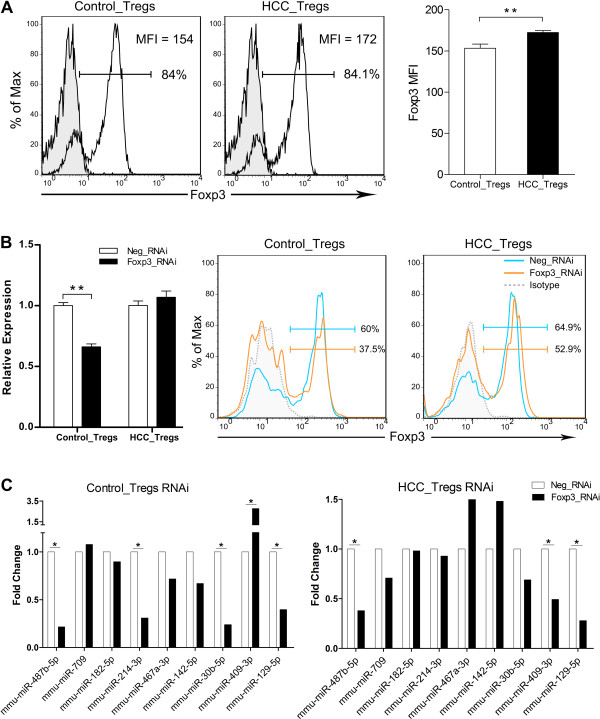
Special modulation of the miRNAs by Foxp3. (A) Representative FACS plots of Foxp3 were shown in control Tregs and HCC-activated Tregs (left). Mean fluorescence intensity (MFI) was included in each of the panels. Summary of the Foxp3 MFI was presented (right) in control Tregs and HCC-activated Tregs. (B) Tregs were transfected with Foxp3-specific siRNAs or negative controls and forty-eight hours later cells were harvested. Expression levels of Foxp3 were determined by qRT-PCR (left) and representative FACS plots were shown in control Tregs and HCC-activated Tregs (right). Results in (A) and (B) were presented as mean ± SEM of three independent experiments performed in triplicates and analyzed by the two-tailed Student’s t-test. **P < 0.01 for indicated comparison. (C) Expression patterns of the nine miRNAs after Foxp3 silencing in control Tregs (left) and HCC-activated Tregs (right). The expression levels of each miRNAs after Foxp3 silencing were shown as fold change relative to the negative control siRNAs. Data were from one microarray. * fold change > 1.5 for indicated comparison. Control_Tregs: Tregs from control mice; HCC_Tregs: Tregs from mice bearing Hepa 1–6; Neg_RNAi: Tregs transfected with negative control siRNAs; Foxp3_RNAi: Tregs transfected with siRNAs against Foxp3.
Expression patterns of the miRNAs in human Tregs
We wondered whether these miRNAs were also differentially expressed in HCC patients. Because miR-487b-5p, miR-709 and miR-467a-3p did not express in human tissue (miRBase 19), we checked the expression levels of the rest six miRNAs in Tregs from peripheral blood samples. Compared with the healthy controls, the expression levels of hsa-miR-182-5p, hsa-miR-214-3p, hsa-miR-129-5p and hsa-miR-30b-5p were significantly up-regulated in Tregs from HCC patients while the hsa-miR-409-3p and hsa-miR-142-5p did not show significant changes (Figure 3).
Figure 3.
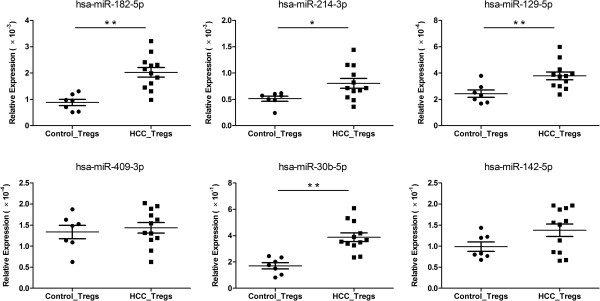
Expression levels of the miRNAs in Tregs from healthy controls and HCC patients. Expression levels of six selected miRNAs were determined by qRT-PCR in Tregs sorted from peripheral blood of healthy controls (n = 7) and HCC patients (n = 12). Expression data were normalized to U6 levels. Horizontal lines represented the mean and error bars represented the SEM. Control_Tregs: Tregs from healthy controls; HCC_Tregs: Tregs from HCC patients. Results were analyzed by the two-tailed Mann–Whitney test. *P < 0.05, **P < 0.01 for indicated comparison.
Possible roles of target genes inferred by bioinformatic analysis
The functions of these four miRNAs (hsa-miR-182-5p, hsa-miR-214-3p, hsa-miR-129-5p and hsa-miR-30b-5p) in human Tregs are not clear. Therefore, we applied bioinformatic methods to explore their roles. Target genes of these four miRNAs were predicted by TargetScan 6.2 and all the genes were imported to software EGAN. The total 109 target genes involved in Tregs functions were enriched based on MeSH database and constructed into the network (Figure 4A). These target genes were further analyzed by functional enrichment based on KEGG pathways via DAVID and eight pathways were enriched with statistical significance (Figure 4B). Among these pathways, two pathways were involved in cytokine signaling (Cytokine-cytokine receptor interaction and Jak-STAT signaling pathway); two pathways were associated with chemotaxis (Chemokine signaling pathway and Cell adhesion molecules (CAMs)); two pathways were related with immune response to graft (Allograft rejection and Graft-versus-host disease). The other two pathways were Intestinal immune network for IgA production and NOD-like receptor signaling pathway.
Figure 4.
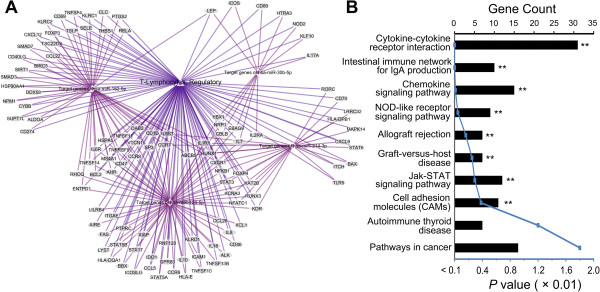
Bioinformatic analysis of target genes of the four miRNAs. (A) The network of target genes of the four miRNAs involved in Tregs functions. Each ellipse represented a target gene predicted by TargetScan. The four rhombuses were miRNAs and the triangle was the MeSH term of Tregs. Purple lines indicated genes involved in Tregs according to MeSH database; magenta lines outlined the miRNAs-target genes relations. (B) Pathway enrichment of target genes. The y-axis represented pathways enriched based on predicted target genes of the four miRNAs. The histogram indicated gene number in each pathway (upper black x-axis); the curve line indicated the P value (lower blue x-axis). **P < 0.01 with the Bonferroni correction.
Discussion
In this study, we found nine differentially expressed miRNAs in Tregs from the murine HCC model, which were modulated by Foxp3, and validated four of them in Tregs from HCC patients. Bioinformatic analysis suggested the four miRNAs had important roles by targeting genes in several pathways affecting Tregs functions.
It has been reported that alterations of miRNAs in CD4+ T cells and Tregs are correlated with certain activation states and diseases [26-28]. Our array data found a group of differentially expressed miRNAs in Tregs from the murine HCC model. Interestingly, the expression patterns of these miRNAs were specific to HCC-activated Tregs. It is supposed that Tregs suppress the immune response in a context dependent way [29-31]. For example, depleting the signal transducer and activator of transcription 3 (STAT3), which is essential for proper development of Th17 cells, results in failure of Tregs to suppress Th17 cell-mediated disease [32]. Assisted by bioinformatic analysis, we selected ten miRNAs for qRT-PCR validation. The validation data were consistent with array results, indicating the up-regulation of five miRNAs and down-regulation of four miRNAs. Because miRNAs can simultaneously regulate a large number of genes, they might be the proper candidate for this context dependent modulation. We proposed that the specific tumor antigen, tumor associated antigen or tumor derived signals might contribute to the unique alterations in HCC-activated Tregs, which was possibly mediated by miRNAs.
Because Foxp3 is the crucial transcription factor in Tregs, we wondered whether these altered miRNAs were affected by Foxp3. As Foxp3 has been reported to be up-regulated in activated Tregs in some reports [33,34], we first compared the MFI of Foxp3 in control Tregs and HCC-activated Tregs. Consisted with previous reports, we found higher MFI in HCC-activated Tregs. After transfection of siRNAs against Foxp3, we determined the mRNA and protein levels of Foxp3. Control Tregs showed significant down-regulation of Foxp3 after silencing at both the mRNA and protein levels; however, HCC-activated Tregs showed slight down-regulation of Foxp3 protein, and no significant changes of Foxp3 mRNA. So the increased expression of Foxp3 in HCC-activated Tregs might involve in the reduced efficacy of Foxp3 RNAi. One report recently demonstrates that under certain inflammatory milieu Foxp3 undergoes phosphorylation, which affects its stability and function [35]. This report and our present data support the hypothesis that both the modification state and the expression level of Foxp3 in HCC-activated Tregs can affect the efficiency of Foxp3 silencing.
We also found five miRNAs showed significant fold changes after Foxp3 RNAi in control Tregs, among which three miRNAs showed significant fold change in HCC-activated Tregs. Two miRNAs (mmu-miR-214-3p and mmu-miR-30b-5p) were significantly changed only in control Tregs. Our findings were consistent with previous studies, which have demonstrated that Foxp3 modulates the expression of miR-155 that maintains the functions of Tregs [18]. Considering the relatively low silencing efficacy of Foxp3 in HCC-activated Tregs, we thought the modulation of these two miRNAs were not so sensitive to Foxp3 levels compared with that of the other three miRNAs.
We further validated the expression levels of these miRNAs in Tregs from HCC patients and healthy controls. Because miR-487b-5p, miR-709 and miR-467a-3p did not express in human tissue (miRBase 19), we validated the expression levels of the rest six miRNAs. Four miRNAs showed significant changes in HCC-activated Tregs compared with healthy controls. Interestingly, compared with data from the murine model, two of the four miRNAs (hsa-miR-182-5p and hsa-miR-214-3p) showed the similar up-regulation while the other two miRNAs (hsa-miR-129-5p and hsa-miR-30b-5p) showed reverse changes. We were not sure whether this discrepancy was due to the differences of species or HCC tumor models. Further experiments were need to clarify this question.
The functions of four up-regulated miRNAs were not reported in Tregs and we performed bioinformatic analysis to infer their possible roles. The target genes with direct relations with Tregs MeSH term were found significantly involved in eight pathways. Two of them were cytokine signaling, including genes IL6ST, IL6R, STAT3 and IL17A which have been reported to facilitate the differentiation of Th17 by inhibiting Tregs induction [36-38]. Up-regulation of these miRNAs might break the balance between Th17 and Tregs and finally accelerate the production of Tregs, which contributes to the abnormal homeostasis of Tregs in HCC [9,39,40]. Another two pathways related with chemotaxis, which has been reported to be critical for the migration and distribution of Tregs in HCC. CC chemokine receptor 6 (CCR6) axis has an important role in recruiting Tregs to tumor sites in HCC [9]; TGF-beta and macrophage-derived chemokine (CCL22) signaling pathways induce aggregation of Tregs at the tumor sites in HCC too [41]. These two pathways included genes in chemotaxis and cell adhesion such as CCR6, CCR7, CXCR1, SELE and ICOS[9,42-44]. These alterations might contribute to the abnormal distribution of Tregs in HCC at the tumor sites. We also found two pathways relating with immune response to allograft (Allograft rejection and Graft-versus-host disease). Although it is well established that Tregs are critical for maintaining the tolerance to allograft [45-47], it is not clear whether the same genes or pathways work similarly in Tregs during the progression of HCC. These new clues needed further exploring. IgA production is essential for the intestinal homeostasis, in which Tregs are indispensable via secretion of TGF-beta [48,49]. It was possible that Tregs applied the same mechanism via TGF-beta in HCC. NOD-like receptor is one of the conserved pattern-recognition receptors (PRRs) which included Toll-like receptors (TLRs) [50]. Previous studies have demonstrated that TLR1, TLR2, TLR4 and TLR7 have important functions in Tregs [51-54] and we proposed that NOD-like receptors were new key PRRs in the context of HCC.
Conclusions
In summary, we confirmed nine differentially expressed miRNAs in Tregs from the HCC murine model. These miRNAs exhibited a specific expression pattern in HCC-activated Tregs and were affected by Foxp3. Four miRNAs were finally found to be up-regulated in HCC patients for the first time. Bioinformatic analysis indicated the four miRNAs (hsa-miR-182-5p, hsa-miR-214-3p, hsa-miR-129-5p and hsa-miR-30b-5p) targeted eight signaling pathways involved in Tregs. These results provided interesting information on the intrinsic functional changes occurred in HCC-activated Tregs, which were worthy of further exploration.
Abbreviations
HCC: Hepatocellular carcinoma; Tregs: Regulatory T cells; miRNAs: microRNAs; RNAi: RNA interference; MFI: Mean fluorescence intensity; FACS: Fluorescence-activated cell sorting.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LC, HM and HH performed the experiments, interpreted the findings and prepared the manuscript. LG, XW and JM assisted in animals’ maintenance. QG prepared blood samples and obtained informed consent from the patients. BL performed the statistical analysis. GZ participated in the design, CL conceived of the study, participated in the design, and assisted with data interpretation and manuscript writing. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Primers for Reverse Transcription.
Primers for Real-time PCR.
Contributor Information
Long Chen, Email: chlong1031@gmail.com.
Huiying Ma, Email: huiyingma89@gmail.com.
Heng Hu, Email: hades_hhg@hotmail.com.
Lingling Gao, Email: 12211010028@fudan.edu.cn.
Xuan Wang, Email: 09301010225@fudan.edu.cn.
Jiaqi Ma, Email: 09301030081@fudan.edu.cn.
Qiang Gao, Email: qgao1981@gmail.com.
Binbin Liu, Email: liu.binbin@zs-hospital.sh.cn.
Guomin Zhou, Email: gmzhou@shmu.edu.cn.
Chunmin Liang, Email: cmliang@fudan.edu.cn.
Acknowledgements
This work was supported by grants from the National Science Foundation of China (No.30871312) and the National Basic Research Program of China (973 program, No.2011CB910700).
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125(7):1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19(2):217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136(11):1745–1754. doi: 10.1007/s00432-010-0833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y, Nakamoto Y, Nakada A, Terashima T, Arihara F, Kitahara M, Kakinoki K, Arai K, Yamashita T, Sakai Y, Mizukoshi E, Kaneko S. Frequency of CD45RO + subset in CD4 + CD25(high) regulatory T cells associated with progression of hepatocellular carcinoma. Cancer Lett. 2011;307(2):165–173. doi: 10.1016/j.canlet.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108(3):804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE. 2011;6(9):e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroza-Gonzalez A, Verhoef C, Ijzermans JN, Peppelenbosch MP, Kwekkeboom J, Verheij J, Janssen HL, Sprengers D. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology. 2013;57(1):183–194. doi: 10.1002/hep.26013. [DOI] [PubMed] [Google Scholar]
- Cao M, Cabrera R, Xu Y, Firpi R, Zhu H, Liu C, Nelson DR. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Invest. 2007;87(6):582–590. doi: 10.1038/labinvest.3700540. [DOI] [PubMed] [Google Scholar]
- Cabrera R, Ararat M, Eksioglu EA, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol. 2010;72(4):293–301. doi: 10.1111/j.1365-3083.2010.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lin F, Su J, Gao Z, Li Y, Yang J, Deng Z, Liu B, Tsun A, Li B. Molecular mechanisms underlying the regulation and functional plasticity of FOXP3(+) regulatory T cells. Genes Immun. 2012;13(1):1–13. doi: 10.1038/gene.2011.77. [DOI] [PubMed] [Google Scholar]
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136(1):26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205(9):1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothe F, Simion A, Akl H, Mourtada M, El Rifai M, Burny A, Romero P, Martiat P, Badran B. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol. 2009;39(6):1608–1618. doi: 10.1002/eji.200838509. [DOI] [PubMed] [Google Scholar]
- Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30(1):80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol. 2009;182(5):2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142(6):914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Paquette J, Tokuyasu T. EGAN: exploratory gene association networks. Bioinformatics. 2009;26(2):285–286. doi: 10.1093/bioinformatics/btp656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Jindra PT, Bagley J, Godwin JG, Iacomini J. Costimulation-dependent expression of microRNA-214 increases the ability of T cells to proliferate by targeting Pten. J Immunol. 2010;185(2):990–997. doi: 10.4049/jimmunol.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divekar AA, Dubey S, Gangalum PR, Singh RR. Dicer insufficiency and microRNA-155 overexpression in lupus regulatory T cells: an apparent paradox in the setting of an inflammatory milieu. J Immunol. 2011;186(2):924–930. doi: 10.4049/jimmunol.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang Y, Fu X, Liu H, Lu L, Wu Y. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(3):R81. doi: 10.1186/ar3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis G, Ferracin M, Biondani A, Caniatti L, Rosaria Tola M, Castellazzi M, Zagatti B, Battistini L, Borsellino G, Fainardi E, Gavioli R, Negrini M, Furlan R, Granieri E. Altered miRNA expression in T regulatory cells in course of multiple sclerosis. J Neuroimmunol. 2010;226(1–2):165–171. doi: 10.1016/j.jneuroim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Bronevetsky Y, Villarino AV, Eisley CJ, Barbeau R, Barczak AJ, Heinz GA, Kremmer E, Heissmeyer V, McManus MT, Erle DJ, Rao A, Ansel KM. T cell activation induces proteasomal degradation of Argonaute and rapid remodeling of the microRNA repertoire. J Exp Med. 2013;210(2):417–432. doi: 10.1084/jem.20111717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N, Sakaguchi S. Treg cells acquire new directions, cytokines navigate. Immunity. 2012;37(3):443–444. doi: 10.1016/j.immuni.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, de Roos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458(7236):351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37(3):511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Yi S. Foxp3 is critical for human natural CD4 + CD25+ regulatory T cells to suppress alloimmune response. Transpl Immunol. 2012;26(2–3):71–80. doi: 10.1016/j.trim.2011.10.005. [DOI] [PubMed] [Google Scholar]
- van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YYJ, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2009;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19(3):322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Huang MC, Goetzl EJ. VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol. 2011;267(2):124–132. doi: 10.1016/j.cellimm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17 F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31(4):587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45(2):254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Zabala M, Lasarte JJ, Perret C, Sola J, Berraondo P, Alfaro M, Larrea E, Prieto J, Kramer MG. Induction of immunosuppressive molecules and regulatory T cells counteracts the antitumor effect of interleukin-12-based gene therapy in a transgenic mouse model of liver cancer. J Hepatol. 2007;47(6):807–815. doi: 10.1016/j.jhep.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, Wang HY, Cheng SQ, Xie D, Wang XF. TGF-beta-miR-34a-CCL22 Signaling-Induced Treg Cell Recruitment Promotes Venous Metastases of HBV-Positive Hepatocellular Carcinoma. Cancer Cell. 2012;22(3):291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueha S, Yoneyama H, Hontsu S, Kurachi M, Kitabatake M, Abe J, Yoshie O, Shibayama S, Sugiyama T, Matsushima K. CCR7 mediates the migration of Foxp3+ regulatory T cells to the paracortical areas of peripheral lymph nodes through high endothelial venules. J Leukoc Biol. 2007;82(5):1230–1238. doi: 10.1189/jlb.0906574. [DOI] [PubMed] [Google Scholar]
- Eikawa S, Ohue Y, Kitaoka K, Aji T, Uenaka A, Oka M, Nakayama E. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6- and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6. J Immunol. 2010;185(11):6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S, Saban D, Lee H, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O, Santolaria T, Calise D, Saati TA, Hudrisier D, Romagnoli P, van Meerwijk JPM. Prevention of acute and chronic allograft rejection with CD4 + CD25 + Foxp3+ regulatory T lymphocytes. Nat Med. 2007;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, Yun SH, Toxavidis V, Strom TB, Lin CP, Koulmanda M. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16(6):718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106(46):19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsutzky S, Cazac BB, Roes J, Guzman CA. TGF-beta receptor signaling is critical for mucosal IgA responses. J Immunol. 2004;173(5):3305–3309. doi: 10.4049/jimmunol.173.5.3305. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Powrie FM. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol. 2013;5(7):a018341. doi: 10.1101/cshperspect.a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu KS, Mizoguchi E, Cotoner CA, Nguyen DD, Mingle B, Iweala OI, McBee ME, Stefka AT, Prioult G, Haigis KM, Bhan AK, Snapper SB, Murakami H, Schauer DB, Reinecker HC, Mizoguchi A, Nagler CR. Toll-like receptor 4-mediated regulation of spontaneous helicobacter-dependent colitis in IL-10–deficient mice. Gastroenterology. 2009;137(4):1380–1390. doi: 10.1053/j.gastro.2009.07.004. e1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Signaling through TLR7 enhances the immunosuppressive activity of murine CD4 + CD25+ T regulatory cells. J Leukoc Biol. 2009;87(1):117–125. doi: 10.1189/jlb.0908559. [DOI] [PubMed] [Google Scholar]
- Nyirenda MH, Sanvito L, Darlington PJ, O’Brien K, Zhang GX, Constantinescu CS, Bar-Or A, Gran B. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187(5):2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Luo F, Cai Y, Liu N, Wang L, Xu D, Chu Y. TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol. 2011;186(4):1963–1969. doi: 10.4049/jimmunol.1002320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for Reverse Transcription.
Primers for Real-time PCR.


