Abstract
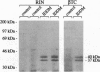
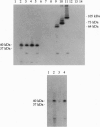
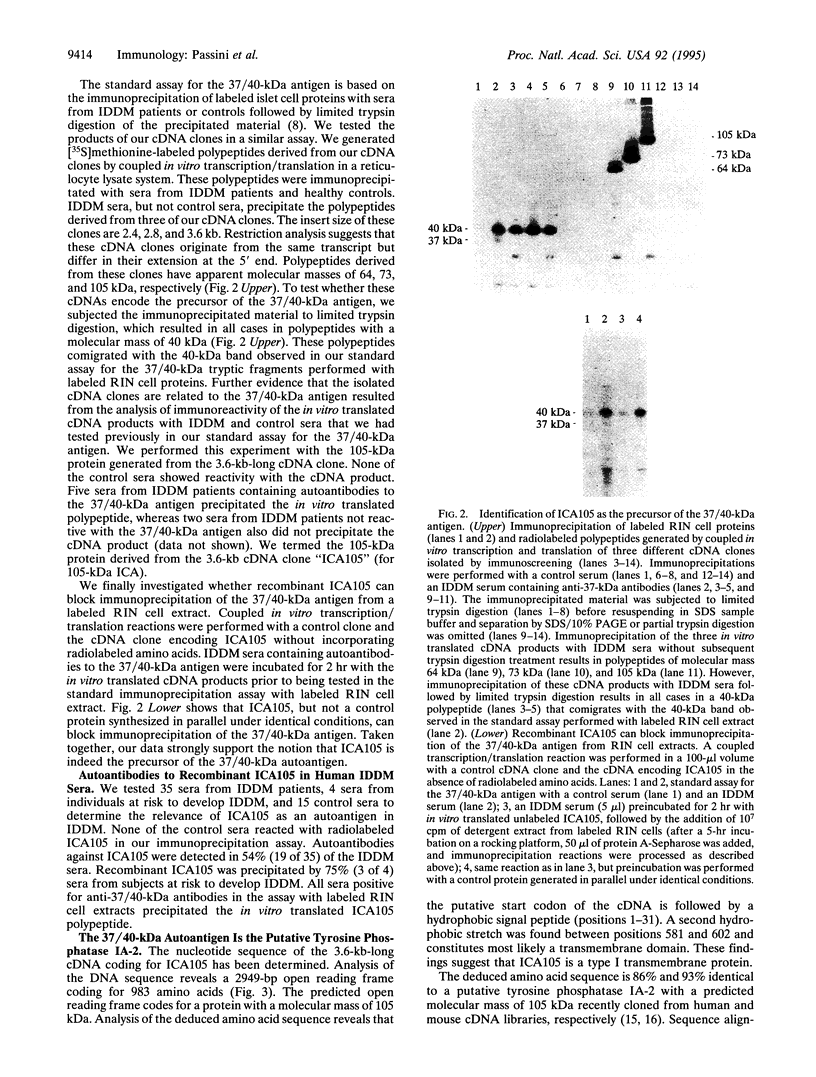
Major targets for autoantibodies associated with the development of insulin-dependent diabetes mellitus (IDDM) include tryptic fragments with a molecular mass of 37 kDa and/or 40 kDa of a pancreatic islet cell antigen of unknown identity. The assay identifying autoantibodies against the 37/40-kDa antigen in human sera is based on the immunoprecipitation of 35S-labeled rat insulinoma cell proteins with sera from IDDM patients, followed by limited trypsin digestion of the immunoprecipitated material. To identify cDNA clones coding for the 37/40-kDa antigen, we have screened a cDNA expression library from rat insulinoma cells with a serum from an IDDM patient that precipitated the 37/40-kDa antigen in our assay. Among the cDNA products that reacted with the IDDM serum, we identified one cDNA clone whose open reading frame encodes a protein with a predicted mass of 105 kDa that we termed "ICA105" for 105-kDa islet cell antibody. The deduced amino acid sequence has high homology to a recently cloned putative tyrosine phosphatase IA-2 from human and mouse cDNA libraries. Translation of the cDNA in vitro results in a polypeptide with the expected molecular mass of 105 kDa. The evidence that ICA105 is indeed the precursor of the 37/40-kDa tryptic fragments is based on the following three results: (i) Sera from IDDM patients containing autoantibodies to the 37/40-kDa antigen precipitate the in vitro translated polypeptide, whereas sera from healthy subjects as well as sera from IDDM patients not reactive with the 37/40-kDa antigen do not precipitate the cDNA product. (ii) Immunoprecipitation of the in vitro translated protein with sera containing autoantibodies to the 37/40-kDa antigen followed by limited trypsin digestion of the precipitated proteins results in a 40-kDa polypeptide. (iii) The protein derived from our cDNA but not from an unrelated control cDNA clone can block immunoprecipitation of the 37/40-kDa antigen from a labeled rat insulinoma cell extract. The availability of the cloned 37/40-kDa antigen should facilitate the identification of individuals at risk of IDDM with increased accuracy. Furthermore, the identification of the 37/40-kDa antigen as the putative tyrosine phosphatase IA-2 is of relevance in elucidating the role of this antigen in the development of IDDM.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Kaufman D. L., Campbell L., Gibbs K. A., Shah S. C., Bu D. F., Erlander M. G., Tobin A. J., Maclaren N. K. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992 Feb 22;339(8791):458–459. doi: 10.1016/0140-6736(92)91061-c. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bingley P. J., Bonifacio E., Gale E. A. Can we really predict IDDM? Diabetes. 1993 Feb;42(2):213–220. doi: 10.2337/diab.42.2.213. [DOI] [PubMed] [Google Scholar]
- Bingley P. J., Christie M. R., Bonifacio E., Bonfanti R., Shattock M., Fonte M. T., Bottazzo G. F., Gale E. A. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994 Nov;43(11):1304–1310. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- Bonifacio E., Bingley P. J., Shattock M., Dean B. M., Dunger D., Gale E. A., Bottazzo G. F. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990 Jan 20;335(8682):147–149. doi: 10.1016/0140-6736(90)90013-u. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2(7892):1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Genovese S., Cassidy D., Bosi E., Brown T. J., Lai M., Bonifacio E., Bottazzo G. F. Antibodies to islet 37k antigen, but not to glutamate decarboxylase, discriminate rapid progression to IDDM in endocrine autoimmunity. Diabetes. 1994 Oct;43(10):1254–1259. doi: 10.2337/diab.43.10.1254. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Hollands J. A., Brown T. J., Michelsen B. K., Delovitch T. L. Detection of pancreatic islet 64,000 M(r) autoantigens in insulin-dependent diabetes distinct from glutamate decarboxylase. J Clin Invest. 1993 Jul;92(1):240–248. doi: 10.1172/JCI116556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. R., Tun R. Y., Lo S. S., Cassidy D., Brown T. J., Hollands J., Shattock M., Bottazzo G. F., Leslie R. D. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992 Jul;41(7):782–787. doi: 10.2337/diab.41.7.782. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Vohra G., Champagne P., Daneman D., Delovitch T. L. Distinct antibody specificities to a 64-kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med. 1990 Sep 1;172(3):789–794. doi: 10.1084/jem.172.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese S., Bingley P. J., Bonifacio E., Christie M. R., Shattock M., Bonfanti R., Foxon R., Gale E. A., Bottazzo G. F. Combined analysis of IDDM-related autoantibodies in healthy schoolchildren. Lancet. 1994 Sep 10;344(8924):756–756. doi: 10.1016/s0140-6736(94)92248-9. [DOI] [PubMed] [Google Scholar]
- Lan M. S., Lu J., Goto Y., Notkins A. L. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994 May;13(5):505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Lu J., Notkins A. L., Lan M. S. Isolation, sequence and expression of a novel mouse brain cDNA, mIA-2, and its relatedness to members of the protein tyrosine phosphatase family. Biochem Biophys Res Commun. 1994 Oct 28;204(2):930–936. doi: 10.1006/bbrc.1994.2549. [DOI] [PubMed] [Google Scholar]
- Rabin D. U., Pleasic S. M., Shapiro J. A., Yoo-Warren H., Oles J., Hicks J. M., Goldstein D. E., Rae P. M. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994 Mar 15;152(6):3183–3188. [PubMed] [Google Scholar]
- Radvanyi F., Christgau S., Baekkeskov S., Jolicoeur C., Hanahan D. Pancreatic beta cells cultured from individual preneoplastic foci in a multistage tumorigenesis pathway: a potentially general technique for isolating physiologically representative cell lines. Mol Cell Biol. 1993 Jul;13(7):4223–4232. doi: 10.1128/mcb.13.7.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]