Abstract
The purpose of the present study was to evaluate aceclofenac eye drop through excised goat cornea. Raising pH of the formulation from 6.0 to 8.0, effect of different preservatives or effect of viscosity enhancer decreases apparent permeability coefficient. Topical ophthalmic NSAID are used to treat ocular surface and anterior segment inflammation as well as post operative management of pain and inflammation. Aceclofenac’s unique chemical structure makes it both a potent anti inflammatory drug and lipophilic molecule that penetrates ocular tissue, ensuring relief of pain in cataract and refractive surgery and corneal abrasion. The octanol/water partition coefficient of aceclofenac drug is 1.86 ± 0.75. Permeation characteristics of the drug were evaluated by putting 1 ml formulation on freshly excised goat cornea fixed between donor and receptor compartments of an all-glass modified Franz diffusion cell and measuring the drug permeated in the receptor by spectrophotometry at 275 nm, after 120 min. The results suggest that aceclofenac ophthalmic solution (pH 7) containing BAC provides increased in vitro ocular availability through goat corneas. The combination of methyl paraben and propyl paraben MP–PP preservative in aceclofenac ophthalmic eye drop 0.1% formulated in phosphate buffer increases transcorneal permeation. The developed formulations were evaluated for their pharmacodynamics in albino rabbits, by measuring in-vivo study and were compared to a marketed voltrane ophthalmic solution.
Keywords: Cornea, Permeation, Aceclofenac, Transcorneal
1. Introduction
The cornea is a transparent tissue in the eye that is dependable for the refraction of the incoming light and is a multilayered tissue made up of three major cell layers: the epithelium, the stroma, and the endothelium. A corneal epithelium is a stratified cell membrane and its apical tight junctions between surface epithelial cells are considered to be the most prominent barrier for corneal absorption. A corneal stroma is a hydrated fibrous tissue with dispersed cells and it has a hydrophilic environment limiting the penetration of highly lipophilic compounds. A corneal endothelium is a monolayer of cells with large intercellular junctions, which presents a leaky lipophilic barrier. Due to the dual nature of the cornea, with a lipophilic epithelium and a hydrophilic stroma, the epithelium appears to be rate limiting to the movement of hydrophilic compounds, whereas for lipophilic compounds, the stroma is rate limiting (Ghate et al., 2006) Topical delivery into the conjuctival cul-de sac is by far the most common route of ocular drug delivery. Absorption from this site may be corneal or non corneal. The corneal absorption represents the major mechanism of absorption for most therapeutic entities. The cornea is a trilaminate structure consisting of three major diffusional barriers, the epithelium, stroma and endothelium (Prausnitz and Noonan, 1998; Mitra, 2003). There are two general processes of drug absorption across barrier membranes (Grass and Robinson, 1988; Grass et al., 2006). The first type is called ‘‘the transcellular transport process,’’ where drug molecules have to go through the barrier cells to reach the circulation. Transcellular transport is typically a two step process, starting with drug uptake into the cells, and ending with drug efflux out of the cells. The second type is called ‘‘the paracellular transport process,’’ where the drug molecules travel between the cells (or in the gaps) to reach the circulation (Prausnitz and Noonan, 1998). The mechanism of transcellular transport includes simple diffusion, facilitated diffusion, active transport, endocytosis and exocytosis (Shivali et al., 2011).
Aceclofenac possesses better analgesic, antipyretic and anti-inflammatory efficacy than diclofenac and other NSAIDs which are frequently used for clinical therapy of arthritis, pain and inflammation. Aceclofenac possesses better gastric tolerance than naproxen, diclofenac and indomethacin. Due to the lipophilic nature of the drug it will be better absorbed by the cornea and tissue and will show better action. Voltaren eye drop is used to treat pain, swelling, and sensitivity to light caused by certain eye surgeries.
NSAID (aceclofenac) is an excellent non steroidal anti-inflammatory, analgesic and antipyretic drug. Eye solutions 0.1% w/v of aceclofenac are available to treat ocular inflammatory conditions. NSAID is recommended for treatment of various pains, inflammatory conditions of eye, osteoarthritis, rheumatoid arthritis etc. It acts by blocking the cyclo-oxygenase pathway. For treating ocular diseases, eye drops may be used, but require frequent instillation of highly concentrated solutions due to rapid precorneal loss from the eye. So, a pharmaceutical with prolonged action may be recommended. Aceclofenac eye drop is not available in the market so in this we use a novel approach to formulate aceclofenac eye drop to reduce eye inflammation. Currently these drugs are used topically very widely in the inhibition of intra-operative miosis, management of post-operative inflammation, treatment of seasonal allergic conjunctivitis, prevention and treatment of cystoid macular edema and in the control of pain after photo refractive keratectomy. NSAIDs have also been found to be useful in decreasing bacterial colonization of contact lenses and prevent bacterial adhesion to human corneal epithelial cells (Munish et al., 2008).
2. Material and methods
Aceclofenac, was obtained from Lupin Pune Research Park Hydroxypropylmethylcellulose (HPMC 15 cps), polyvinyl alcohol PVA were obtained from SD Fine chemicals, Mumbai, India. Methylcellulose and chitosan were obtained from Merck India. All preservatives were purchased from Nic Chemicals, Cochin India. All the reagents and solvents used were of analytical grade. Eye ball of goat was transported from a local butcher shop to the laboratory in cold (4 °C) normal saline within 1 h of slaughtering the animal. The cornea was carefully excised along with 2–4 mm of the surrounding scleral tissue and was washed with cold normal saline till the washing was free from proteins. The method of dissection of the cornea and the apparatus was a modified version of the Franz diffusion cell as used by Malhotra and Majumdar. The receptor cell had an internal volume of 10 ml and side arm allowed sampling of receptor fluid. The donor cell was clamped onto the top of the receptor cell. Water at 37 °C was circulated through the water jacket surrounding the receptor cell and Teflon coated magnetic stir bar kept at the bottom of the receptor cell created a homogeneous receptor volume (Malhotra and Majumdar, 1997).
2.1. Animals
Approval for the use of animals in the study was obtained from the Banasthali University Animal Ethics Committee (Banasthali University, Rajasthan, India, Ref. no. BU/BT/184/11-12). New Zealand rabbits of either sex weighing 2.8–4.1 kg were used to measure the in vivo ocular irritation test in the eye. All animals were managed in accordance with the rules of the Animal Experiment Committee in Banasthali University.
2.1.1. Measurement of surface tension
Formulation containing surfactant can emulsify corneal epithelium and help in quicker partitioning of the drug in the epithelium. To discover any possible relationship between the surface tension of formulation and corneal penetration, the surface tension of each aceclofenac ophthalmic solution containing preservatives was measured by a stalagmometer.
2.1.2. Measurement of partition coefficient (log P)
10 mg aceclofenac drug was added to 50 ml of n-octanol (pre saturated with water) and was shaken and then 50 ml of distilled water (pre saturated with n-octanol) was added and the process is continued in a mechanical shaker for 24 h. After 24 h both phases are separated. Absorbance of both the phases was taken and the concentration in each phase was calculated i.e. log {P = [aceclofenac]oct/[aceclofenac]H2O}. The concentration of the base dissolved in n-octanol was obtained by extrapolation from a calibration curve (0–20 μg/ml) of the base in n-octanol at 275 nm (λmax) (Ghosh et al., 2007).
2.1.3. In-vitro transcorneal permeation studies
The whole eye ball of goat was transported from a local butcher shop to the laboratory in cold (4 °C) normal saline within 1 h of slaughtering the animal. The cornea was carefully excised along with 2–4 mm of the surrounding scleral tissue and was washed with cold normal saline till the washing was free from proteins as shown in (Fig. 1). The cornea was mounted by sandwiching the surrounding scleral tissue between clamped donor and receptor compartments of an all glass modified Franz diffusion cell in such a way that its epithelial surface faced the donor compartment. The area of diffusion cell was 0.85 cm2. The receptor compartment was filled with 11 ml of freshly prepared buffer solution. An aliquot (1 ml) of test formulation was placed on the cornea and opening of the donor compartment was sealed with a cover slip. The receptor fluid was maintained at 35 °C with constant stirring, using Teflon coated magnetic stir bead. Three ml sample was withdrawn from receptor compartment at various time intervals up to 120 min and was analyzed spectrophotometrically at 275 nm. Each sample withdrawn was replaced with an equal volume of buffer. Withdrawn samples were analyzed spectrophotometrically (Varian-Cary 5000 UV–VIS-NIR) by measuring absorbance at λmax of 275 nm. Each experiment was continued for about 2.0 h and was performed at least in triplicate. At the end of experiments each cornea (freed from sclera) was weighed, soaked in 1-ml methanol, dried overnight at 90 °C and reweighed. From the difference in weights corneal hydration was calculated.
Figure 1.

Goat cornea.
Calculation of apparent permeability coefficient.
Apparent permeability coefficient was calculated using the following equation
where, ΔQ/Δt (μg/min) is the flux across the corneal tissue. A is the area of diffusion (cm2) C0 is the initial concentration of drug in the donor compartment, and 60 is taken as the factor to convert minute into second. The flux across the cornea was obtained from the slope of the regression line obtained from the linear part of the curve between the amount permeated (Q) and time (t) plot.
2.1.4. In vivo study
Twelve healthy rabbits weighing 2.5–3 kg were selected. Rabbits were kept under anesthesia during in vivo study using 1:1 Ketamine hydrochloride (30 mg/kg) and Xylazine hydrochloride (10 mg/kg) injected through IM route. Six rabbits were given aceclofenac ophthalmic drops 0.1% and other six rabbits marketed eye drop (voltaren ophthalmic®). Aqueous humor was drawn after 5 min, 0.5, 1, 2, 3, 4, 5, 6, 7 and 8 h with the help of a 26 gauge needle, extra care was exercised to avoid any irritation or lacrimation. Samples were analyzed on HPLC (1200 series Quaternary LC, Agilent tech., USA) and results were recorded in duplicate (Deepika et al., 2007; Martin et al., 1991; Faruk et al., 2000).
2.1.4.1. Comparison in experimental postoperative inflammation
Twelve healthy albino rabbits weighing 2.5–3.5 kg were selected. The treatment was started with aceclofenac ophthalmic solution after the appearance of postoperative inflammation following cataract surgery, prevention and treatment of cystoid macular edema (CME) following cataract surgery, ocular discomfort and pain following refractive surgery and intraoperative miosis during cataract surgery. Six rabbits were treated with aceclofenac ophthalmic drops (0.1 w/v) and other six were treated with the given diclofenac sodium ophthalmic solution 0.1% (voltaren ophthalmic®). Based on data from in vivo study, aceclofenac drops were administered two drops every two hrs (from 8 am-8 pm) for the first two days and then two drops every four hrs (from 8 am-8 pm) for further three days. Two drops of the given diclofenac sodium ophthalmic solution 0.1% (voltaren ophthalmic®) were administered every six hrs (8 am-8 pm) for the first two days and then two drops every twelve hours (8 am, 8 pm) for further three days. Results of the therapy were recorded by observing signs of cataract surgery, ocular discomfort and pain recovery of rabbits from disease. All in vivo experiments were in accordance with guidelines of NIH animal laboratory regulations and conducted after approval from the ethics committee.
3. Results and discussion
3.1. Formulation of aceclofenac ophthalmic solution of different pH
Aceclofenac drug solution of 0.02% (w/v, pH 6.0) 0.05% (w/v, pH 6.5) and 0.1% (w/v, pH 7.0, 7.4 and 8.0) concentrations was made in isotonic phosphate buffer according to USP. The required quantity of aceclofenac was dissolved in 100 ml of isotonic phosphate buffer pH 7.4 to have 0.05, 0.1 and 0.15% (w/v) concentrations as shown in (Table 1).
Table 1.
Effect of pH on aceclofenac transcorneal permeation by goat cornea.
| Formulation pH | Papp cm/s * 106 | Relative Papp | Corneal hydration % |
|---|---|---|---|
| pH 6 | 3.969 ± 1.20 | 1.31 | 81.2 ± 0.45 |
| pH 6.5 | 9.212 ± 0.75 | 0.33 | 80.0 ± 0.26 |
| pH 7 | 13.81 ± 0.21 | 0.64 | 80.6 ± 0.34 |
| pH 7.4 | 8.99 ± 0.32 | 0.74 | 79.4 ± 0.21 |
| pH 8 | 7.89 ± 1.22 | 0.28 | 80.8 ± 0.76 |
Value are mean ± SD (N = 3).
3.2. Formulation of aceclofenac ophthalmic solutions 0.1 (w/v, pH 7) containing preservative
Aceclofenac 0.1% (w/v) solution in isotonic phosphate buffer pH 7 containing either benzalkonium chloride (BAC 0.01% w/v), phenyl mercuric nitrate (PMN 0.001% w/v), phenyl mercuric acetate (PMA 0.001% w/v), or benzyl alcohol (BA 0. 5% v/v) was prepared as shown in (Table 2).
Table 2.
Effect of preservative on aceclofenac transcorneal permeation by goat cornea.
| Formulation pH | Papp cm/s * 106 | Relative Papp | Corneal hydration % | Surface tension (dyne/cm) |
|---|---|---|---|---|
| Normal control | 9.998 ± 0.86 | 0.96 | 79.21 ± 0.84 | 71.02 |
| SA | 7.35 ± 0.31 | 0.86 | 78.25 ± 0.64 | 54.61 |
| BAC | 14.53 ± 045 | 0.21 | 79.64 ± 0.45 | 51.34 |
| PMN | 14.211 ± 1.64 | 0.54 | 79.25 ± 0.15 | 65.32 |
| PMA | 6.454 ± 0.65 | 0.12 | 80.64 ± 0.78 | 65.45 |
| THM | 5.23 ± 0.45 | 0.64 | 80.14 ± 0.45 | 64.38 |
| EDTA | 5.511 ± 0.81 | 0.25 | 81.20 ± 0.72 | 89.31 |
| MP-PP | 15.09 ± 0.64 | 0.64 | 80.31 ± 0.51 | 74.31 |
Value are mean ± SD (N = 3).
SA-sorbic acid, BAC-benzalkonium chloride, PMN-phenyl mercuric nitrate, PMA-phenyl mercuric acetate, THM-thiomersal, EDTA-disodium edetate, MM-PP-methyl paraben and propyl paraben.
3.3. Formulation of aceclofenac ophthalmic solutions (0.1, w/v) containing viscosity enhancer
The drug aceclofenac 0.1% (w/v) solutions in isotonic phosphate buffer (pH 7), hydroxypropylmethylcellulose (HPMC), polyvinyl alcohol (PVA), methyl cellulose (MC), chitosan were used. (Table 3) shows the viscolizing effect on transcorneal preparation of aceclofenac. The corneal permeation of aceclofenac through goat cornea is shown in (Fig. 2) and (Table 1), the aceclofenac formulation from pH 6–8 results in a significant decrease in apparent permeability coefficient. Diclofenac ophthalmic solution is commercially available as 0.1% (w/v) solution. For aceclofenac opthalmic solution higher pH may decrease the fraction unionized and apparent permeability coefficient or by cornea contains both positively and negatively charged groups whose magnitude and polarity depend on the degree of protonation. At pH above the iso-electric point (pI = 3.2), the cornea carries a net negative charge and thereby becomes less permeable to negatively charged species or anion (Rojanasakul and Robinson, 1989). However the concentration of the drug in donor at lower pH reduced the cumulative permeation. Topical application of diclofenac produces annoying sensations like burning and intense stinging (Quintana-Hau et al., 2005). Formulations having a lower pH will further increase the ocular irritation potential of aceclofenac. Permeation of drug from formulations of pH 6.0 and 8.0 had a lag phase of 30 min.
Table 3.
Effect of viscosity enhancer on aceclofenac transcorneal permeation by goat cornea.
| Formulation pH | Papp cm/s * 106 | Relative Papp | Corneal hydration % |
|---|---|---|---|
| Normal control | 12.67 ± 0.41 | 0.97 | 79.25 ± 0.46 |
| HPMC | 11.170 ± 0.85 | 0.54 | 78.25 ± 0.51 |
| MC | 5.66 ± 0.43 | 0.64 | 78.63 ± 0.25 |
| CHITOSAN | 8.716 ± 0.75 | 0.45 | 78.15 ± 0.73 |
| PVA | 5.040 ± 0.64 | 0.82 | 78.64 ± 0.61 |
Value are mean ± SD (N = 3).
Figure 2.
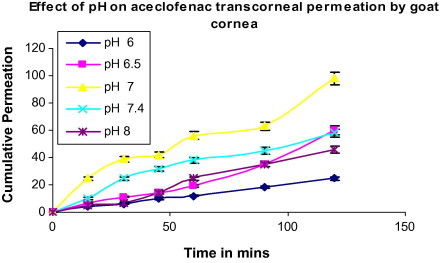
Effect of pH on aceclofenac transcorneal permeation by goat cornea. Values are mean ± S.D. (n = 3)
A significant difference was observed with preservative on corneal permeation of aceclofenac. The results shown in (Table 2) and (Fig. 3), reveal that there was a significant increase (p < 0.05) in apparent permeability coefficient of the drug with the use of the combination of methyl paraben and propyl paraben MP-PP, while there was a significant decrease (p < 0.05) with the use of sorbic acid (SA). The combination of methyl paraben and propyl paraben MP-PP has been reported to enhance the corneal permeation of insulin, a peptide (Sasaki et al., 1994). The corneal hydration is between 78% and 81%. BAC enhanced the corneal permeation. It has earlier been reported that anionic drugs like ibuprofen, flurbiprofen and ketorolac enhance the corneal permeation. There was no significant change in ETDA, PMA THM and SA. The surface tension of the formulation varied between 51.34 and 89.31 dynes/cm, indicating the presence of a surfactant as shown in (Table 2). There was a decrease in the surface tension of the optimized formulation, indicating the increase in corneal permeation by corneal disruption or by emulsification of corneal epithelium due to the presence of benzalkonium chloride BAC. Partition coefficient (log P) of aceclofenac was found to be 1.86 ± 0.75. (Table 3) and (Fig. 4) show the effect of viscosity enhancer on corneal permeation of aceclofenac. The results show that there was a significant decrease in apparent permeability coefficient with the use of MC and PVA as the viscosity enhancer while the use of HPMC showed least reduction in apparent permeability coefficient compared with normal controls. Chitosan gave lesser permeability than HPMC. The use of viscosity enhancer was associated with the increase in lag times because of slower diffusion of the drug from viscous solutions. It is generally believed that the inclusion of a viscosity-increasing agent in an ophthalmic solution will increase ocular bioavailability by prolonging contact time of the drug in the eye. In all the formulations the corneal hydration is between 75% and 80%, indicating no corneal damage.
Figure 3.
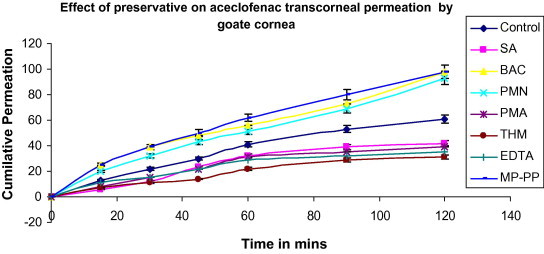
Effect of preservative on aceclofenac transcorneal permeation by goat cornea. Values are mean ± S.D. (n = 3).
Figure 4.
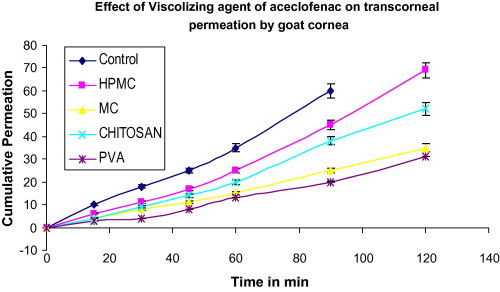
Effect of viscosity enhancer of aceclofenac on transcorneal permeation by goat cornea.
3.3.1. In vivo study
Both groups of rabbits showed the same pattern of recovery from postoperative inflammation following cataract surgery, ocular discomfort and pain. Aceclofenac opthalmic eye drop preparation showed comparatively better cure to signs and symptoms of treatment of postoperative inflammation following cataract surgery, ocular discomfort and pain with less frequent administration thus reducing toxic accumulation of the drug which can be expected from commercial diclofenac sodium ophthalmic solution 0.1% (voltaren ophthalmic®) thus enhancing compliance by less frequent administration. As shown in (Fig. 5), after treatment aqueous humor was collected using a 26-gauge syringe and incubated on agar to see the presence of any organism and the colony count was normal when compared to incubated aqueous humor of another healthy albino rabbit. This shows that both groups received appropriate treatment and no further infection was found. The rabbits were monitored after the treatment by aceclofenac eye drop to check any signs of irritancy or inflammation i.e. redness or increased tear production for 6 months. There were no signs of irritancy or damage to the cornea of rabbit’s eyes. It can be predicted that aceclofena eye drop is not an irritant to the eye but still needs further studies to determine the minimum toxic concentration of this new formulation. So the result shows that the aceclofenac ophthalmic drop 0.1% with BAC is better than market voltaren ophthalmic drop.
Figure 5.
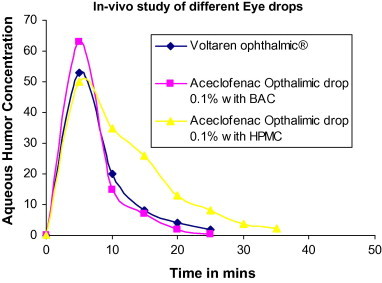
In-vivo study of eye drop.
4. Conclusion
It can be concluded from the present studies that an increase in the concentration of aceclofenac ophthalmic drop showed comparatively better cure to signs and symptoms of postoperative inflammation following cataract surgery increase in transcorneal permeation. Increasing the ph of the formulation from 6.0 to 8.0 and addition of viscosity enhancer to aceclofenac ophthalmic eye drop formulated in phosphate buffer, reduce in apparent permeability coefficient of drug through goat cornea. As a viscosity enhancer HPMC showed least reduction in an apparent permeability coefficient compared with normal controls. Chitosan gave lesser permeability than HPMC. The combination of methyl paraben and propyl paraben MM-PP preservative and benzalkonium chloride BAC in aceclofenac ophthalmic eye drop formulated in phosphate buffer increases transcorneal permeation. In-vivo study result shows that the aceclofenac ophthalmic drop 0.1% with BAC is better than market voltaren ophthalmic drop. Such formulations offer a more intensive treatment of pain and inflammation, a decrease in the number of applications per day and a better patient compliance.
Footnotes
Peer review under responsibility of King Saud University.
Reference
- Deepika A., Dhananjay P., Ashim K.M., Indu P.K. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation by microdialysis sampling of aqueous humour. Int. J. Pharm. 2007;338:21–26. doi: 10.1016/j.ijpharm.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Faruk O., Emin K., Umit U.I., Selim K., Süleyman S.I., Nursabah E.B., Atila B. Penetration of topical and oral ofloxacin into the aqueous and vitreous humor of inflamed rabbit eyes. Int. J. Pharm. 2000;204:91–95. doi: 10.1016/s0378-5173(00)00482-8. [DOI] [PubMed] [Google Scholar]
- Ghate D., Edelhauser H.F. Ocular drug delivery. Exp. Opin. Drug Deliv. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Nayak U.K., Roy P. Development, evaluation and method selection for the Preparation of lamivudine microspheres. Int. J. Pharm. 2007;9:67–71. [Google Scholar]
- Grass G.M., Cooper E.R., Robinson J.R. Mechanisms of corneal drug penetration III: modeling of molecular transport. J. Pharm. Sci. 2006;77:124–126. doi: 10.1002/jps.2600770105. [DOI] [PubMed] [Google Scholar]
- Grass G.M., Robinson J.R. Mechanism of corneal drug penetration I: in vivo and in vitro kinetics. J. Pharm. Sci. 1988;77:3–14. doi: 10.1002/jps.2600770103. [DOI] [PubMed] [Google Scholar]
- Malhotra M., Majumdar D.K. In vitro transcorneal permeation of ketorolac tromethamine from buffered and unbuffered aqueous ocular drops. Ind. J. Exp. Biol. 1997;35:941–947. [PubMed] [Google Scholar]
- Martin M., Awn R., Assumpta M., Mary M., Michael H.M. Pharmacokinetics of amikacin and chloramphenicol in the aqueous humor of rabbits. Antimicrob. Agents Chemother. 1991;22:1791–1798. doi: 10.1128/aac.35.9.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A.K. 2nd ed. Marcel Dekker Inc.; New York: 2003. Ophthalmic Drug Delivery Systems. [Google Scholar]
- Munish Ahuja, Dhake Avinash S., Sharma Surendra K., Majumdar Dipak K. Ocular delivery of NSAIDs. AAPS J. 2008;10(2) doi: 10.1208/s12248-008-9024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz M.R., Noonan J.S. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- Quintana-Hau J.D., Cruz-Olmos E., Lopez-Sanchez M.I., Sanchez-Castellanos V., Baiza-Duran L., Gonzalez J.R., Tornero-Montano, Mondragon-Flores R., Hernandez-Santoyo A. Characterization of the novel ophthalmic drug carrier Sophisen in two of its derivatives: 3A Ofteno and Modusik-A Ofteno. Drug Dev. Ind. Pharm. 2005;31:263–269. doi: 10.1081/ddc-52058. [DOI] [PubMed] [Google Scholar]
- Rojanasakul Y., Robinson J.R. Transport mechanisms of the cornea: characterization of barrier permselectivity. Int. J. Pharmacol. 1989;55:237–246. [Google Scholar]
- Sasaki H., Tei C., Yamamura K., Nishida K., Namamura J. J. Pharm. Pharmacol. 1994;46:871–875. doi: 10.1111/j.2042-7158.1994.tb05705.x. [DOI] [PubMed] [Google Scholar]
- Singla Shivali, Majumdar D.K., Goyal Sachin, Khilnani Gurudas. Evidence of carrier mediated transport of ascorbic acid through mammalian cornea. Saudi Pharmaceut. J. 2011;19:165–170. doi: 10.1016/j.jsps.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]



