Abstract
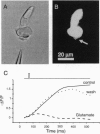
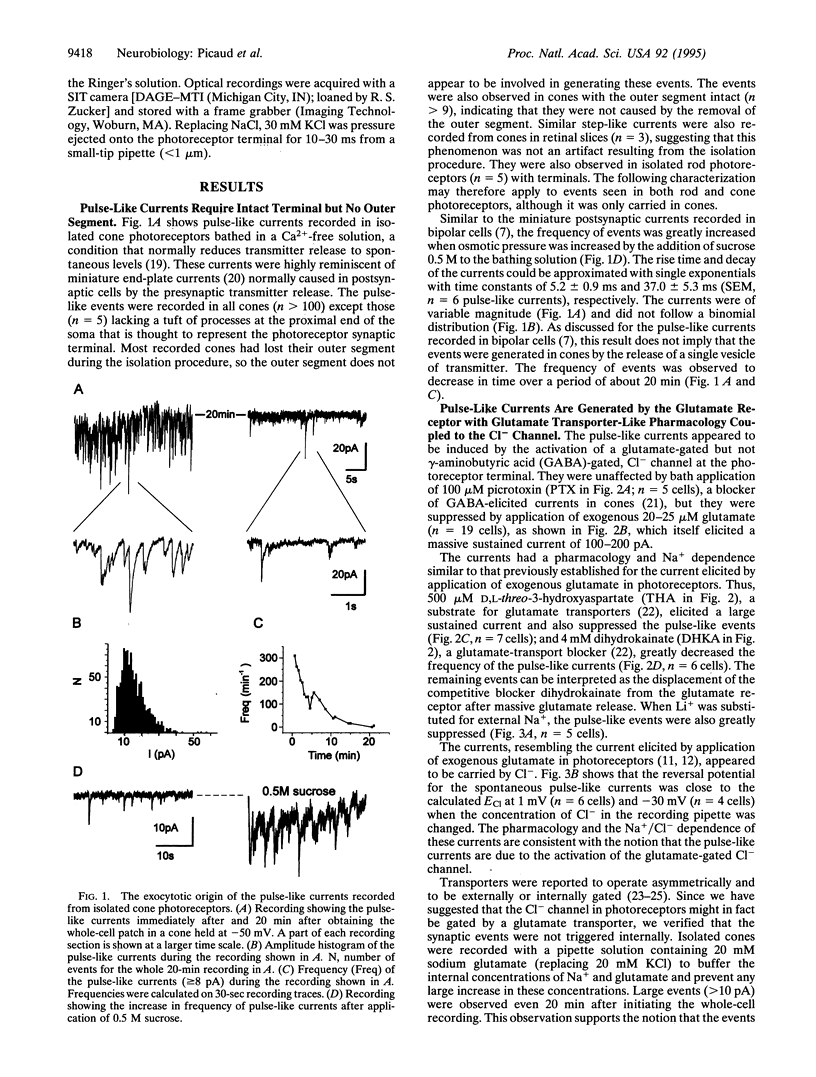
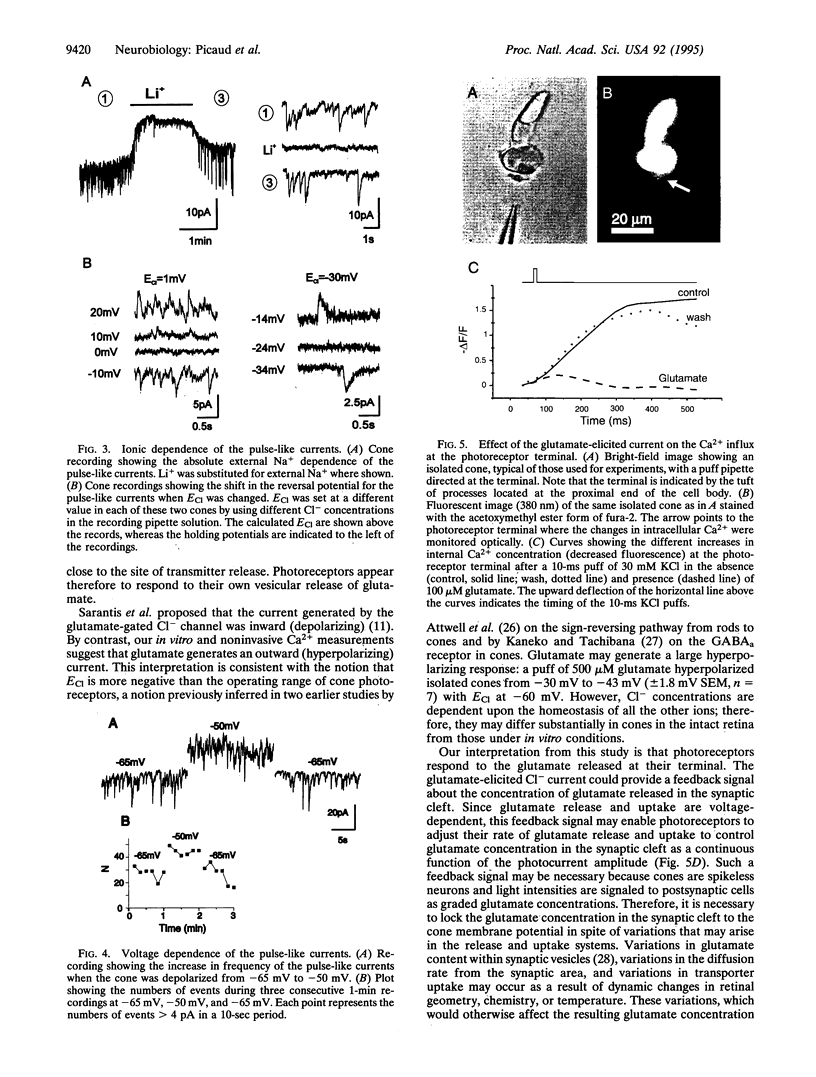
Pulse-like currents resembling miniature postsynaptic currents were recorded in patch-clamped isolated cones from the tiger salamander retina. The events were absent in isolated cones without synaptic terminals. The frequency of events was increased by either raising the osmotic pressure or depolarizing the cell. It was decreased by the application of either glutamate or the glutamate-transport blockers dihydrokainate and D,L-threo-3-hydroxyaspartate. The events required external Na+ for which Li+ could not substitute. The reversal potential of these currents followed the equilibrium potential for Cl- when internal Cl- concentration was changed. Thus, these miniature currents appear to represent the presynaptic activation of the glutamate receptor with glutamate transporter-like pharmacology, caused by the photoreceptor's own vesicular glutamate release. Using a noninvasive method to preserve the intracellular Cl- concentration, we showed that glutamate elicits an outward current in isolated cones. Fluorescence of the membrane-permeable form of fura-2 was used to monitor Ca2+ entry at the cone terminal as a measure of membrane depolarization. The increase in intracellular Ca2+ concentration, elicited by puff application of 30 mM KCl, was completely suppressed in the presence of 100 microM glutamate. Puff application of glutamate alone had no measurable depolarizing effect. These results suggest that the equilibrium potential for Cl-, ECl, was more negative than the activation range for Ca2+ channels and that glutamate elicited an outward current, hyperpolarizing the cones.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Barbour B., Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993 Sep;11(3):401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Attwell D., Werblin F. S., Wilson M. The properties of single cones isolated from the tiger salamander retina. J Physiol. 1982 Jul;328:259–283. doi: 10.1113/jphysiol.1982.sp014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Werblin F. S., Wilson M., Wu S. M. A sign-reversing pathway from rods to double and single cones in the retina of the tiger salamander. J Physiol. 1983 Mar;336:313–333. doi: 10.1113/jphysiol.1983.sp014583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub G. S., Korenbrot J. I., Copenhagen D. R. Release of endogenous glutamate from isolated cone photoreceptors of the lizard. Neurosci Res Suppl. 1989;10:S47–S55. doi: 10.1016/0921-8696(89)90008-x. [DOI] [PubMed] [Google Scholar]
- Barbour B., Brew H., Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991 May;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C., Lam D. M. L-glutamic acid: a neurotransmitter candidate for cone photoreceptors in human and rat retinas. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5117–5121. doi: 10.1073/pnas.80.16.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Cammack J. N., Rakhilin S. V., Schwartz E. A. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994 Oct;13(4):949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Jahr C. E. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989 Oct 12;341(6242):536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Eliasof S., Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J Neurosci. 1993 Jan;13(1):402–411. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986 Apr;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993 Oct;16(10):415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- Maple B. R., Werblin F. S., Wu S. M. Miniature excitatory postsynaptic currents in bipolar cells of the tiger salamander retina. Vision Res. 1994 Sep;34(18):2357–2362. doi: 10.1016/0042-6989(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Lam D. M. Uptake of aspartic and glutamic acid by photoreceptors in goldfish retina. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7185–7189. doi: 10.1073/pnas.78.11.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Schwartz E. A. Evidence for the identification of synaptic transmitters released by photoreceptors of the toad retina. J Physiol. 1983 Jan;334:325–349. doi: 10.1113/jphysiol.1983.sp014497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T., Kanner B. I. Localization of the glutamate transporter GLT-1 in rat and macaque monkey retinae. Neurosci Lett. 1994 Mar 14;169(1-2):137–140. doi: 10.1016/0304-3940(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Sarantis M., Everett K., Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature. 1988 Mar 31;332(6163):451–453. doi: 10.1038/332451a0. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. L-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. gamma-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]