Abstract
Pseudomonas aeruginosa produces a spectrum of exoproducts many of which have been implicated in the pathogenesis of human infection. Expression of some of these factors requires cell-cell communication involving the interaction of a small diffusible molecule, an "autoinducer," with a positive transcriptional activator. In P. aeruginosa PAO1, LasI directs the synthesis of the autoinducer N-(3-oxododecanoyl)-L-homoserine lactone (OdDHL), which activates the positive transcriptional activator, LasR. Recently, we have discovered a second signaling molecule-based modulon in PAO1, termed vsm, which contains the genes vsmR and vsmI. Using HPLC, mass spectrometry, and NMR spectroscopy we now establish that in Escherichia coli, VsmI directs the synthesis of N-butanoyl-L-homoserine lactone (BHL) and N-hexanoyl-L-homoserine lactone (HHL). These compounds are present in the spent culture supernatants of P. aeruginosa in a molar ratio of approximately 15:1 and their structures were unequivocally confirmed by chemical synthesis. Addition of either BHL or HHL to PAN067, a pleiotropic P. aeruginosa mutant unable to synthesize either of these autoinducers, restored elastase, chitinase, and cyanide production. In E. coli carrying a vsmR/vsmI'::lux transcriptional fusion, BHL and HHL activated VsmR to a similar extent. Analogues of these N-acyl-L-homoserine lactones in which the N-acyl side chain has been extended and/or oxidized at the C-3 position exhibit substantially lower activity (e.g., OdDHL) or no activity (e.g., dDHL) in this lux reporter assay. These data indicate that multiple families of quorum sensing modulons interactively regulate gene expression in P. aeruginosa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton N. J., Bycroft B. W., Chhabra S. R., Stead P., Gledhill L., Hill P. J., Rees C. E., Winson M. K., Salmond G. P., Stewart G. S. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992 Jul 1;116(1):87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- Bainton N. J., Stead P., Chhabra S. R., Bycroft B. W., Salmond G. P., Stewart G. S., Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992 Dec 15;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- Castric K. F., Castric P. A. Method for rapid detection of cyanogenic bacteria. Appl Environ Microbiol. 1983 Feb;45(2):701–702. doi: 10.1128/aem.45.2.701-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra S. R., Stead P., Bainton N. J., Salmond G. P., Stewart G. S., Williams P., Bycroft B. W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone. J Antibiot (Tokyo) 1993 Mar;46(3):441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- Cubo M. T., Economou A., Murphy G., Johnston A. W., Downie J. A. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J Bacteriol. 1992 Jun;174(12):4026–4035. doi: 10.1128/jb.174.12.4026-4035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Fuqua W. C., Winans S. C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994 May;176(10):2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua W. C., Winans S. C., Greenberg E. P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994 Jan;176(2):269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Kaye S., Iglewski B. H. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun. 1993 Apr;61(4):1180–1184. doi: 10.1128/iai.61.4.1180-1184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. M., Passador L., Iglewski B. H., Greenberg E. P. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J Bacteriol. 1994 May;176(10):3076–3080. doi: 10.1128/jb.176.10.3076-3080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Cook D. M., Farrand S. K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995 Jan;177(2):449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Li P. L., Zhang L., Piper K. R., Cook D. M., Tate M. E., Farrand S. K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Yu B., Bainton N. J., Birdsall M., Bycroft B. W., Chhabra S. R., Cox A. J., Golby P., Reeves P. J., Stephens S. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993 Jun;12(6):2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A., Blough N. V., Dunlap P. V. Multiple N-acyl-L-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994 Dec;176(24):7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan S., Sebaihia M., Jones S., Yu B., Bainton N., Chan P. F., Bycroft B., Stewart G. S., Williams P., Salmond G. P. Carbapenem antibiotic production in Erwinia carotovora is regulated by CarR, a homologue of the LuxR transcriptional activator. Microbiology. 1995 Mar;141(Pt 3):541–550. doi: 10.1099/13500872-141-3-541. [DOI] [PubMed] [Google Scholar]
- Meighen E. A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991 Mar;55(1):123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner U. A., Koch A. K., Fiechter A., Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994 Apr;176(7):2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
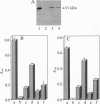
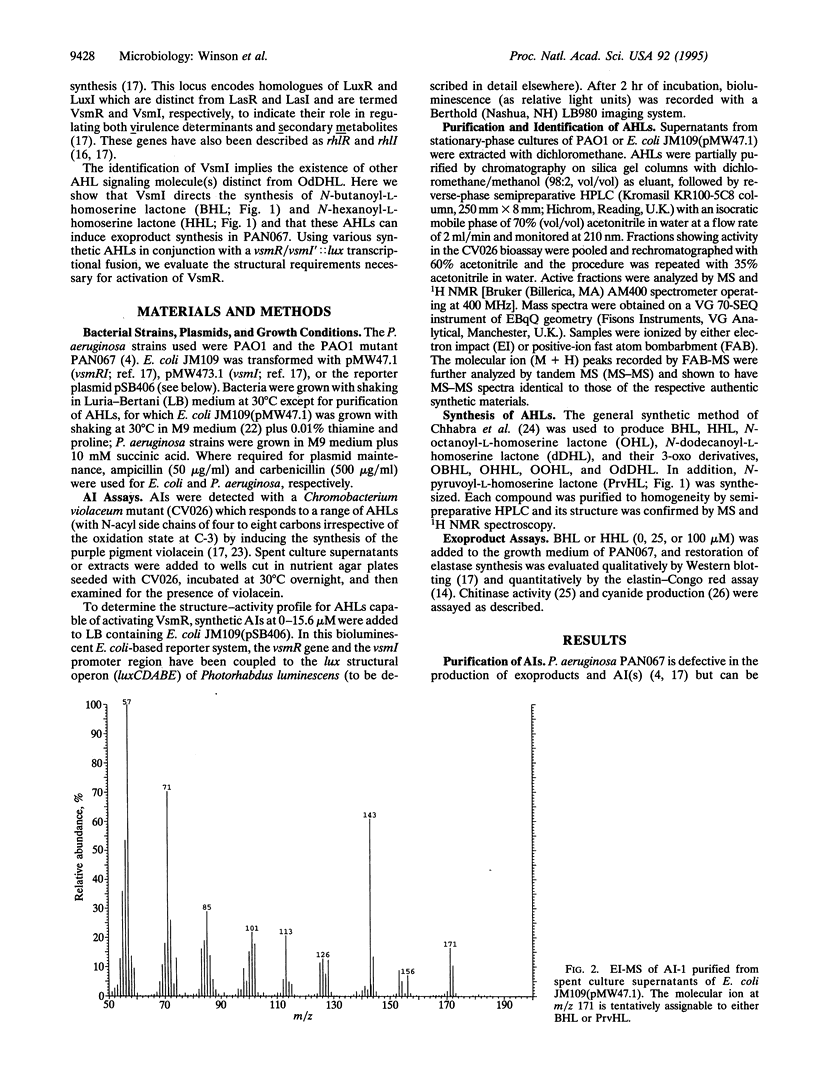
- Passador L., Cook J. M., Gambello M. J., Rust L., Iglewski B. H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993 May 21;260(5111):1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
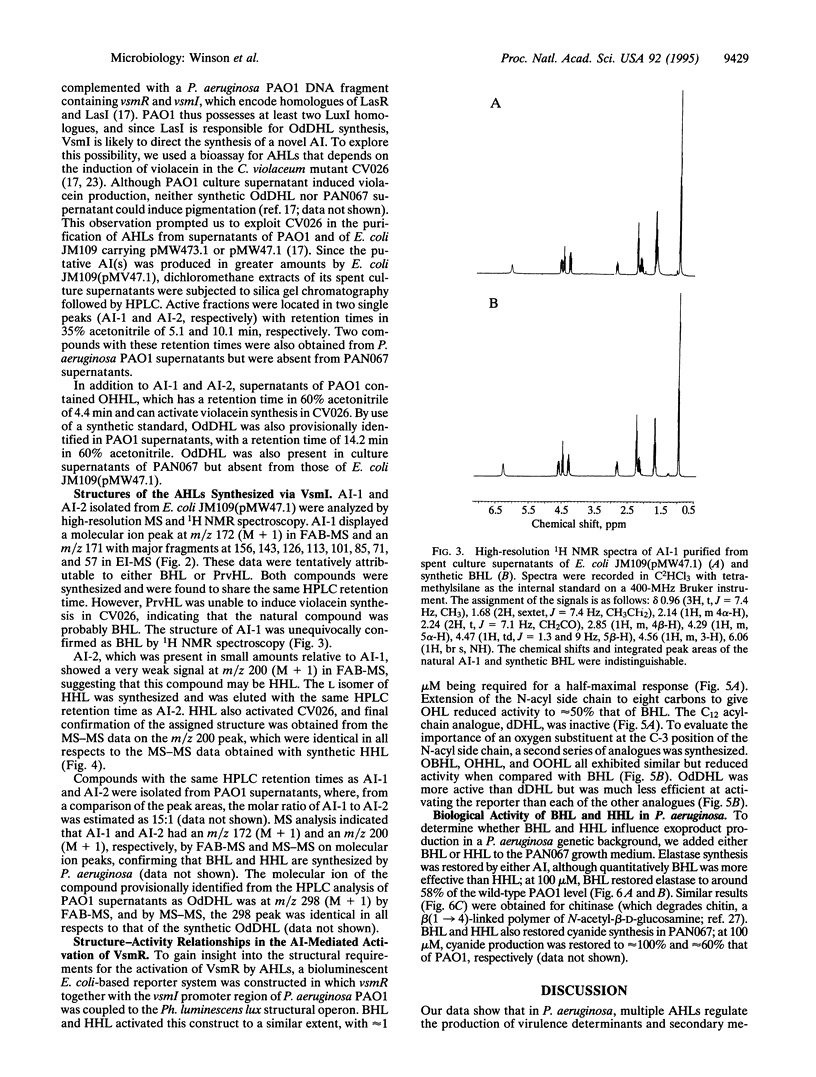
- Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., Greenberg E. P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
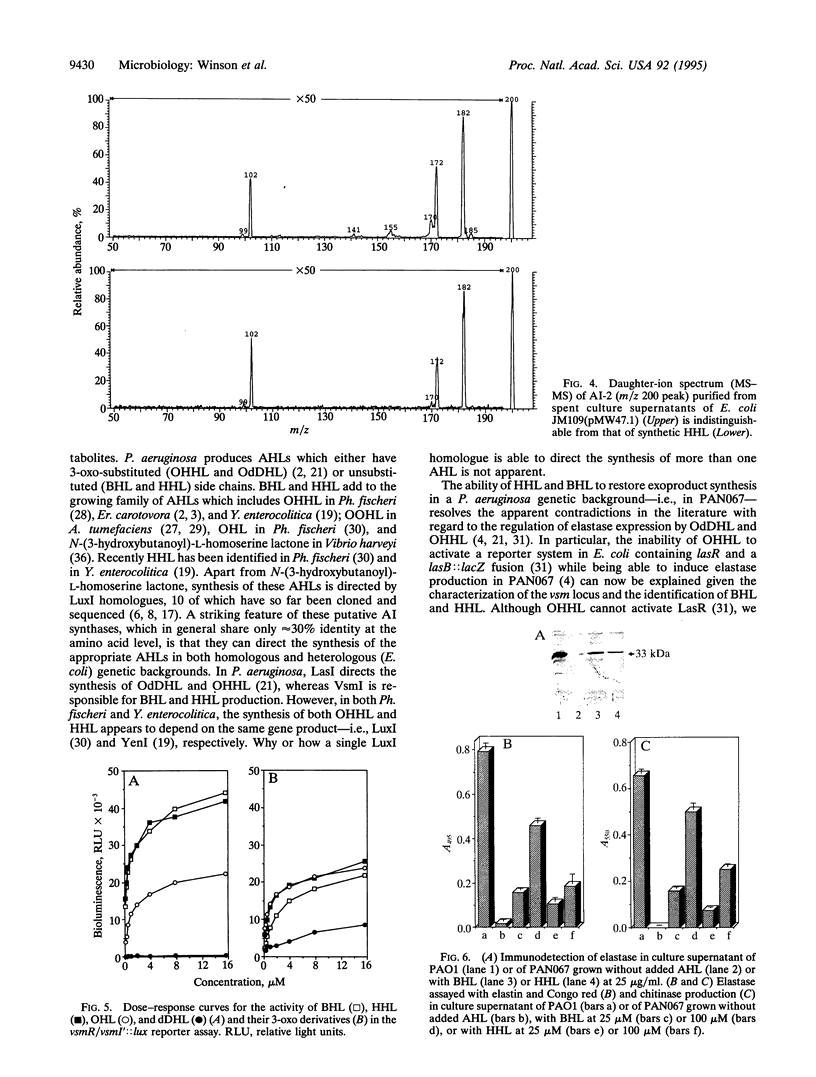
- Pearson J. P., Passador L., Iglewski B. H., Greenberg E. P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson L. S., 3rd, Keppenne V. D., Wood D. W. Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J Bacteriol. 1994 Jul;176(13):3966–3974. doi: 10.1128/jb.176.13.3966-3974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper K. R., Beck von Bodman S., Farrand S. K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993 Apr 1;362(6419):448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- Pirhonen M., Flego D., Heikinheimo R., Palva E. T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993 Jun;12(6):2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond G. P., Bycroft B. W., Stewart G. S., Williams P. The bacterial 'enigma': cracking the code of cell-cell communication. Mol Microbiol. 1995 May;16(4):615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Swift S., Bainton N. J., Winson M. K. Gram-negative bacterial communication by N-acyl homoserine lactones: a universal language? Trends Microbiol. 1994 Jun;2(6):193–198. doi: 10.1016/0966-842x(94)90110-q. [DOI] [PubMed] [Google Scholar]
- Swift S., Winson M. K., Chan P. F., Bainton N. J., Birdsall M., Reeves P. J., Rees C. E., Chhabra S. R., Hill P. J., Throup J. P. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol Microbiol. 1993 Nov;10(3):511–520. doi: 10.1111/j.1365-2958.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Murphy P. J., Kerr A., Tate M. E. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993 Apr 1;362(6419):446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]