Abstract
Mammals exhibit poor recovery after injury to the spinal cord, where the loss of neurons and neuronal connections can be functionally devastating. In contrast, it has long been appreciated that many non-mammalian vertebrate species exhibit significant spontaneous functional recovery after spinal cord injury (SCI). Identifying the biological responses that support an organism's inability or ability to recover function after SCI is an important scientific and medical question. While recent advances have been made in understanding the responses to SCI in mammals, we remain without an effective clinical therapy for SCI. A comparative biological approach to understanding responses to SCI in non-mammalian vertebrates will yield important insights into mechanisms that promote recovery after SCI. Presently, mechanistic studies aimed at elucidating responses, both intrinsic and extrinsic to neurons, that result in different regenerative capacities after SCI across vertebrates are just in their early stages. There are several inhibitory mechanisms proposed to impede recovery from SCI in mammals, including reactive gliosis and scarring, myelin associated proteins, and a suboptimal immune response. One hypothesis to explain the robust regenerative capacity of several non-mammalian vertebrates is a lack of some or all of these inhibitory signals. This review presents the current knowledge of immune responses to SCI in several non-mammalian species that achieve anatomical and functional recovery after SCI. This subject is of growing interest, as studies increasingly show both beneficial and detrimental roles of the immune response following SCI in mammals. A long-term goal of biomedical research in all experimental models of SCI is to understand how to promote functional recovery after SCI in humans. Therefore, understanding immune responses to SCI in non-mammalian vertebrates that achieve functional recovery spontaneously may identify novel strategies to modulate immune responses in less regenerative species and promote recovery after SCI.
Keywords: spinal cord injury, regeneration, immune system, inflammation
INTRODUCTION
SCI was once thought to be incurable, because of a lack of plasticity and regeneration in the adult mammalian central nervous system (CNS). However, studies performed over the past two decades have demonstrated both neurogenesis and considerable plasticity of the adult vertebrate CNS, stimulating studies of biological responses to SCI (Thuret et al., 2006). Based on the premise that neuronal function relies on fundamental pathways conserved across species, neurobiology has a long tradition of addressing basic biological questions by exploiting the advantageous experimental or natural traits of a variety of organisms including sea slug, squid, frog, chicken, and songbirds (Alvarez-Buylla and Nottebohm, 1988; Castellucci et al., 1980; Cohen et al., 1954; Fatt and Katz, 1951; Hodgkin and Huxley, 1939; Kupfermann and Kandel, 1969; Marder, 2002). Similarly, the ability to promote successful recovery after SCI will likely derive from identification of biological processes that determine both success and failure to regenerate axons, in species where regenerative capacity ranges from limited to robust.
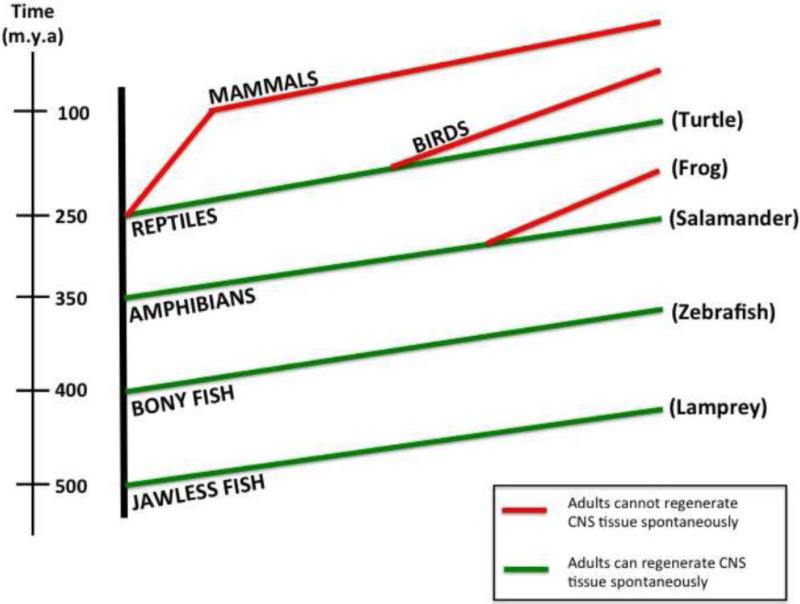
Studies in mammals, which exhibit poor spontaneous recovery after SCI, have focused primarily on identifying factors both intrinsic and extrinsic to neurons that inhibit axon regeneration, e.g. (Buchli and Schwab, 2005; Giger et al., 2010; Gonzenbach and Schwab, 2008). These inhibitory mechanisms include reactive gliosis and a glial scar that contains chondroitin sulfate proteoglycans (CSPGs) and the presence of myelin and myelin-associated proteins (Filbin, 2003; Pernet and Schwab, 2012; Silver and Miller, 2004). Another extrinsic factor studied in the context of mammalian SCI is the immune response, as inflammation has been widely shown to exacerbate neuronal loss and induce secondary damage acutely after SCI (Bartholdi and Schwab, 1997; Klusman and Schwab, 1997; Schnell et al., 1997; Streit et al., 1998). One of the long-considered hypotheses to explain the differential regenerative capacity among vertebrates is that species able to accomplish spontaneous recovery after SCI have less inhibitory extrinsic factors present near the injury zone, including an acute immune response that is weaker or distinct from that observed in mammals (Tanaka and Ferretti, 2009). Increasingly, elements of the immune response are also recognized to benefit axonal regeneration after SCI in mammals, reviewed in (Benowitz and Popovich, 2011; Gensel et al., 2012). Therefore, studies of immune responses to SCI in regenerating vertebrates will enhance the ability to promote an optimal immune response in mammals, in order to prevent loss of neurons and promote recovery. This approach is clinically relevant, as the immune response is routinely manipulated in a variety of clinical settings. For example, FDA-approved antibodies or drugs that target pro-inflammatory cytokines are routinely used in patients with rheumatoid arthritis, Crohn's disease, diabetes (types I and II) and multiple sclerosis (Dinarello et al., 2012). In mouse models of SCI, treatment with three different FDA-approved TNF inhibitors improved biochemical, histological and functional outcomes after SCI (Genovese et al., 2006; Genovese et al., 2008a; Genovese et al., 2008b). Based on the clinical success of monoclonal antibodies and other biologic therapies, a pipeline of therapeutic small molecules targeting pro-inflammatory mediators is in development (Dinarello et al., 2012; Kopf et al., 2010). Therefore, understanding beneficial and detrimental aspects of the immune response to SCI across a variety of species may offer clinically relevant insights. Here, I review data from a growing number of studies that investigated immune responses to SCI in non-mammalian model organisms including the jawless (lamprey) and jawed vertebrates, including teleosts (zebrafish), amphibians (salamander and frog), and reptiles (turtle) (Figure 1).
Figure 1.
Phylogenetic Tree illustrates evolutionary relationships of multiple vertebrate species and their differential capacity to regenerate CNS tissue after injury. The dimensions of the timeline are not drawn to scale.
Immune responses to SCI in Mammals
In mammals, immune responses are thought to contribute to deleterious outcomes, including neuronal death, inhibition of axon regeneration, and poor functional recovery of motor and sensory systems. Microglia, the resident immune cells of the CNS, are among the first cells to respond in mammalian experimental models of SCI (Adrian et al., 1978; David and Kroner, 2011; Popovich et al., 1993; Spitzbarth et al., 2011). Within minutes to days, microglia, together with neutrophils, macrophages and lymphocytes recruited from the periphery, are activated and accumulate at the lesion site (Carlson et al., 1998; Detloff et al., 2008; Fitch et al., 1999; Fitch and Silver, 1997; Gensel et al., 2011; Horn et al., 2008; Kigerl and Popovich, 2009; Popovich et al., 2002; Popovich and Hickey, 2001; Popovich et al., 1993; Popovich et al., 2003; Popovich et al., 1997; Schnell et al., 1997). Resident and invading immune cells release pro-inflammatory mediators, including cytokines that rapidly amplify the local immune response (Alexander and Popovich, 2009; Bartholdi and Schwab, 1995, 1997; Bethea et al., 1998; Brambilla et al., 2005; Fitch et al., 1999; Herbomel et al., 2001; Kigerl and Popovich, 2009; Klusman and Schwab, 1997; Schnell et al., 1999; Streit et al., 1998). Astrocytes promote scarring at the lesion site and synthesize CSPGs, which are inhibitory to neuronal regeneration (Bradbury and Carter, 2010; Bradbury et al., 2002; Garcia-Alias et al., 2009; Rudge and Silver, 1990; Rudge et al., 1989; Silver and Miller, 2004; Smith et al., 1986; Smith et al., 1990; Snow et al., 1990). At the lesion site, the molecular interactions between astrocytes and infiltrating immune cells are not well understood. After SCI in mice, reactive astrocytes expressing the transcription factor STAT3+ confined inflammatory cells at the lesion epicenter, while deletion of STAT3 from astrocytes was pro-inflammatory, resulting in a broader distribution of inflammatory cells around the injury site and decreased neuronal viability (Herrmann et al., 2008; Okada et al., 2006; Wanner et al., 2013). In vitro co-culturing of macrophages with resting STAT3+ astrocytes activated the astrocytes to reorient their processes and surround the inflammatory cells (Wanner et al., 2013). A subpopulation of neural stem cell-derived astrocytes recruited to the injury was shown to restrict the size of the lesion, by being neuroprotective (Goritz et al., 2011; Wanner et al., 2013). Surprisingly, removal of these ependymal-derived astrocytes decreased the numbers of immune cells in the injured spinal cord (Sabelstrom et al., 2013). These studies suggest complex and heterogeneous interactions between neuronal, immune and other non-neuronal cells at the lesion site, that together contribute to recovery after SCI in mammals.
Additional interactions of the nervous and immune systems may also be relevant in mammalian SCI. For example, the vagus nerve, which is part of the autonomic nervous system, has efferent connections to immune organs, which regulate immune functions, such as cytokine production (Olofsson et al., 2012). Autonomic dysreflexia, which occurs when the autonomic nervous system is interrupted and can be life-threatening in SCI patients, caused immune depression in mice and in a human SCI subject (Zhang et al., 2013b) (and see Zhang et al, this issue). At the molecular level, classic immune molecules, such as major histocompatibility complex class I (MHCI), are now known to play a role in normal CNS synapse remodeling and plasticity (Corriveau et al., 1998), while the complement cascade participates in synaptic pruning (Stephan et al., 2012; Stevens et al., 2007) (see also Peterson and Anderson, this issue). Studies in mammals are just beginning to examine if these pathways are activated after CNS injury and if they influence outcomes after injury (Elmer and McAllister, 2012). Whether these systems are equally present and participate in regeneration in non-mammalian vertebrates is unknown, but the complement cascade has been implicated in limb regeneration in salamanders and is upregulated after tail amputation in frog tadpole (Xenopus laevis) (Kimura et al., 2003; Tazaki et al., 2005).
Intraspinal inflammation in humans can persist for years, where it has been proposed to inhibit functional recovery and contribute to the complications of chronic SCI, e.g. (Bao et al., 2010; Davies et al., 2007; Fleming et al., 2006; Hayes et al., 2002; Kanyilmaz et al., 2012; Kwon et al., 2010; Stein et al., 2013). Therefore, inflammation has long been considered a therapeutic target in SCI (Gensel et al., 2011). The 2013 Clinical Practice Guideline for Early Acute Management of SCI in Adults discourages the use of anti-inflammatory steroids or any other pharmacological agent, based on controversial findings from three national clinical trials of methylprednisolone (Medicine, 2013). Thus, there is a need to identify new anti-inflammatory and/or pro-regenerative therapies for SCI. Many molecular aspects of innate immunity are conserved across species, e.g. sea urchin, flies and human (Becker and Becker, 2007; Murphy et al., 2008), and understanding elements of the immune response that coexist with successful functional recovery from SCI in non-mammalian vertebrates may shed light on desired aspects of the immune response in mammals.
Lamprey
Lamprey is the most extant basal vertebrate and to date, is the only experimental model that has been demonstrated to satisfy all NIH criteria for functional regeneration after complete spinal cord transection (Cohen et al., 1988; Guth et al., 1980; Smith et al., 2011). In most studies of SCI in lamprey, the injury model is a complete transection. Full functional recovery is achieved over a stereotypical time course of 12 weeks, which corresponds to regeneration of reticulospinal axons through the lesion site (Cohen et al., 1989; Cohen et al., 1986; Rovainen, 1967, 1976; Selzer, 1978; Wood and Cohen, 1979, 1981; Yin and Selzer, 1984). The lamprey spinal cord is non-myelinated and contains descending axons from reticulospinal neurons, as well as intraspinal motor neurons, sensory neurons and interneurons (Grillner and Jessell, 2009). Interestingly, regeneration does not occur equally in all reticulospinal axons, but instead, is accomplished by a predictable subset of descending giant reticulospinal neurons whose cell bodies are located in stereotypical locations in the brain (Busch and Morgan, 2012; Jacobs et al., 1997; Shifman et al., 2008). As in mammals, the regenerative process after SCI in lamprey is influenced by age, conditioning lesion, and cAMP, indicating that at least some pathways relevant to regeneration are shared across vertebrates with different spontaneous regeneration capacities (Cohen et al., 1989; Cohen et al., 1999; Jin et al., 2009; Zhang et al., 2004). For an illustration of lamprey axons regenerating across the lesion site, see Figure 2.
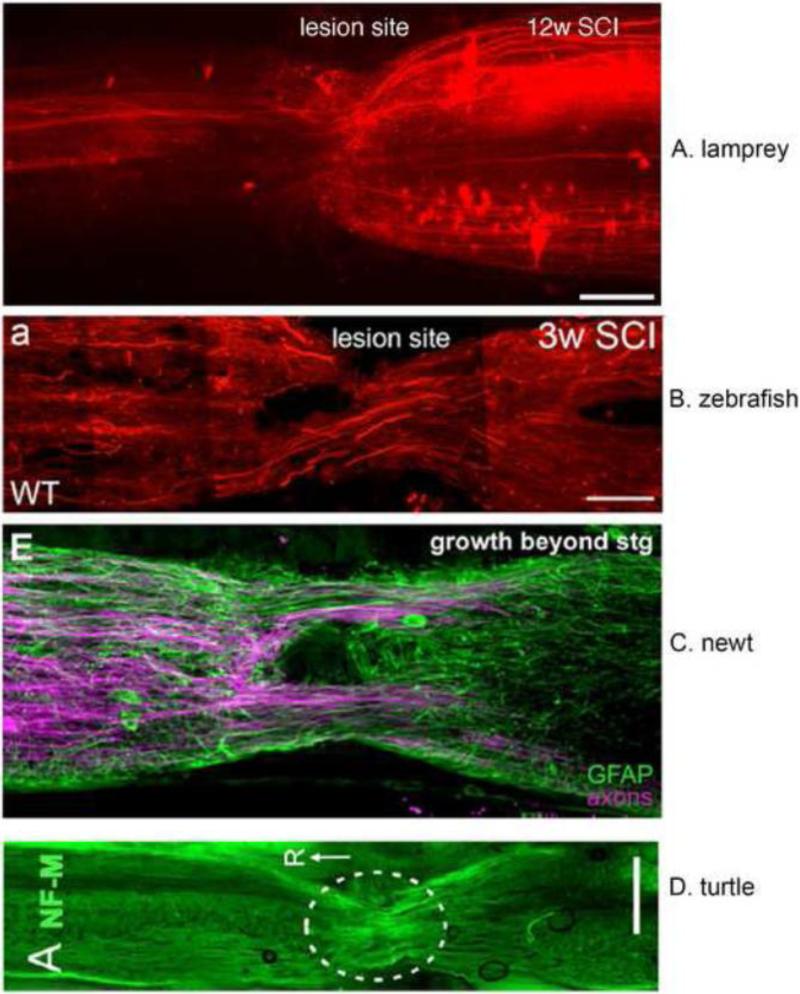
Figure 2.
Robust axonal regeneration after spinal cord injury occurs in multiple non-mammalian model organisms.
(A) Retrograde tracing with DTMR (red) was performed on lampreys twelve weeks after complete spinal cord transection, when behavioral recovery is maximal. DTMR was applied 1cm caudal to the original transection site. Arrowheads indicate three regenerated axons. Since this is a complete transection injury model, any axons crossing the original lesion site have regenerated. Scale bar: 200μM. Image provided by Angelos Papatheodorou and Ona Bloom.
(B) Retrograde axonal tracing was performed on adult zebrafish three weeks after complete spinal cord transection, when swimming ability is almost normal. Experimental details and original image from: Figure 6A, Goldshmit, Sztal, Jusuf, Hall, Nguyen-Chi, Currie PD. Journal of Neuroscience, May 30, 2012, 32(22):7477-7492.
(C) Axonal tracing and anti-GFAP antibody labeling was performed on adult newt after complete spinal cord transection. Experimental details and original image from: Figure 6E, Zukor K, Kent D, Odelberg S. Neural Development, January 4 2011, 6:1-22.
(D) Regenerating axons visualized by neurofilament M antibody labeling in a turtle after complete spinal cord transection. Experimental details and original image from: Figure 10A, Rehermann M, Marichal N, Russo R, Trujillo-Cenoz O. The Journal of Comparative Neurology March 20, 2009, 515: 197-214.
A series of recent, elegant studies from the laboratory of Max Cooper (Emory University) and others, demonstrated that lampreys have both innate and adaptive immune systems (Guo et al., 2009; Khalturin et al., 2004; Sato et al., 2003; Uinuk-Ool et al., 2002). Similar to mammals, cellular elements of the lamprey immune system include neutrophils, microglia, B cells and at least two distinct T cell populations (Alder et al., 2008; Alder et al., 2005; Cannon et al., 2005; Das et al., 2013; Guo et al., 2009; Hirano et al., 2013; Kasamatsu et al., 2010; Khalturin et al., 2004; Mayer et al., 2002; Pancer et al., 2004; Sato et al., 2003; Slack et al., 2008; Uinuk-Ool et al., 2002; Uinuk-ool et al., 2003). Despite differences in the molecular mechanisms of antigen presentation and the structure of antigen receptors, general strategies of adaptive immunity appear to be similar between lamprey and jawed vertebrates (Boehm, 2011). Lamprey hematopoetic tissues include the typhlosole, a tissue that is analogous to bone marrow, as well as the gills, which are thymus-like (Bajoghli et al 2011). The lamprey CNS contains macrophages/microglia, as defined by their morphology and binding to isolectin-B4 (Shifman and Selzer, 2007; Shifman et al., 2009). While the source of lamprey microglia in the injured spinal cord is not well understood, there is evidence consistent with a peripheral origin (Laramore et al., 2011). In mammals, resident microglia appear to derive from cells that migrate to the brain early in development, which are distinct from monocyte-derived macrophages that infiltrate the spinal cord from the periphery after injury (Ginhoux et al., 2013; Shechter and Schwartz, 2013). Evidence suggests that ratio and function of these distinct cell types may influence outcomes in mouse model of SCI, including regeneration of damaged neurons (Donnelly et al., 2011).
Immediately after lamprey spinal cord transection, the central canal enlarges at the lesion, a blood clot forms, blood cells accumulate, ependymal cells from the central canal proliferate across the lesion, and a glial-ependymal bridge forms (Lurie et al., 1994; Lurie and Selzer, 1991a, b; Rovainen, 1976; Selzer, 1978). Starting at 2-4 weeks post-injury, regenerating axons grow across the lesion site, even when allowed access to uninjured tissue, as in a hemisection injury (Lurie and Selzer, 1991b; Wood and Cohen, 1981). It appears that glial cells, and not just their secreted products, are necessary for axonal regeneration in lamprey, since a reduction in glial cell number by freezing reduced axon regeneration (Lurie and Selzer, 1991a). Unlike mammalian astrocytes, after SCI in lampreys, glial processes reorient and extend across the lesion site, parallel to the normal axonal trajectory and form a bridge (Boehm, 2011; Lau et al., 2013; Lurie et al., 1994). A glial bridge is also observed in most of the other species discussed here, although it is unclear what aspects of glial responses to SCI are conserved across species. For example, it is unclear if lampreys have reactive astrocytes, as they do not appear to accumulate at the scar and a common marker of astrocytes, GFAP, has not yet been identified in the lamprey genome (Lurie et al., 1994; Smith et al., 2013). Recently, extensive gliogenesis, as well as limited neurogenesis, both mostly associated with the ependymal region, was reported (Zhang et al., 2013a). Interestingly, as mentioned above, in mammals, GFAP- astrocytes, derived from ependymal cells, play a positive role in limiting spinal cord damage after SCI (Sabelstrom et al., 2013). Glial organization in lamprey can be modulated, as cAMP promotes a greater and more rapid organization of glial fibers after SCI, which are arranged longitudinally through the lesion site (Lau et al., 2013). Molecular constituents of the scar that are shared with or distinct from mammals are largely unknown in lamprey, although preliminary evidence suggests that CSPG does accumulate at the lesion, where they are detrimental to axon regeneration (Hu et al., 2013). As in mammals, lamprey microglia/macrophages are increased in number after SCI and subsets of microglia have been shown to express both axon guidance and repulsion molecules, weeks to months after injury (Laramore et al., 2011; Lau et al.; Shifman and Selzer, 2007; Shifman et al., 2009). Application of cAMP to the spinal cord at the time of transection increased the numbers of microglia one-month post injury (Lau et al., 2013).
In contrast to the detailed knowledge of molecular pathways induced after SCI in mammals, genomic resources in lamprey have been lacking and only a few cytokines have been identified conclusively thus far (Guo et al., 2009; Najakshin et al., 1999; Sato et al., 2003; Tsutsui et al., 2007). Similarly to what is known about the conservation of inflammatory responses across species, lamprey lymphocytes stimulated with classic, non-traumatic inflammatory stimuli upregulate expression of macrophage migration inhibitory factor (MIF), interleukin (IL)-8 and IL-17 (Alder et al., 2008; Tsutsui et al., 2007). Whether these and other pro-inflammatory mediators are induced by SCI in lamprey is currently unknown. Recent publication of the lamprey genome has increased the potential to pursue such studies (Smith et al., 2013) and gene expression profiling of immune-related transcripts upregulated by SCI in lamprey are currently being pursued (Bloom O, Brown CT, Buxbaum J, Li W, Morgan JR, Smith J, unpublished studies). Therefore, while the effects of immune responses on recovery from SCI in lamprey are largely unexplored, molecular tools are increasingly available to address this question. The first question to address is whether the immune response to SCI in lamprey supports or impedes regeneration.
Zebrafish
Due to the general experimental advantages of zebrafish and their capacity to regenerate spontaneously after CNS injury, they are rapidly emerging as an attractive model organism in which to study immune responses to SCI (Becker and Becker, 2008; Lieschke and Currie, 2007). In adult zebrafish, it is possible to perform assays of anatomical recovery, cellular activity, and behavioral responses, in intact animals in real time. Imaging in vivo immune responses to SCI should be a major advantage in the zebrafish, as an increasing number of studies have demonstrated cellular immune responses in other pathological settings (Renshaw and Trede, 2012), and transgenic lines selectively labeling a variety of central and peripheral immune cell types relevant to SCI including T and B cells, macrophage/microglia, and neutrophils are available (Ellett et al., 2010; Page et al., 2013; Renshaw et al., 2006). Providing an opportunity to couple in vivo with in vitro studies, a method to culture primary zebrafish spinal cord neurons was recently described (Chen et al., 2013). Astrocyte biology and its relation to radial glial cells and neurogenesis is a rapidly growing area of research in zebrafish, although it is mostly studied in the context of brain and not spinal cord injury (Kyritsis et al., 2013).
Adult zebrafish can regenerate axons after SCI and recover behaviors such as swimming and escape response (Becker et al., 2004; Becker et al., 1997). The time course to regain substantial behavioral recovery in adult zebrafish is approximately 1 month for either a crush or transection injury (Becker et al., 2004; Becker et al., 1997; Hui et al., 2010). After spinal cord transection and treatment with cAMP in larval zebrafish, recovery of escape responses can be observed 3-5 days after injury (Becker et al., 2004; Becker et al., 1997; Bhatt et al., 2004; Bhatt et al., 2007). For an illustration of zebrafish axons regenerating across the lesion site, see Figure 2B. As in lamprey, not all zebrafish reticulospinal neurons have the capacity to regenerate equally, and poor regenerators, such as the Mauthner neurons, which also regenerate poorly in the lamprey, can be induced to regenerate by intracellular injection of cAMP (Becker et al., 1998; Becker et al., 1997; Bhatt et al., 2004). In the spinal cord, cervical transection is followed by neurogenesis that results in new motor neurons that are functionally integrated (Reimer et al., 2008). Behavioral recovery is achievable by a varying number of regenerating neurons (Reimer et al., 2009), suggesting the importance of network plasticity, as well as neuronal regeneration (Reimer et al., 2009). Regeneration of spinal motor neurons was recently shown to depend on brain-derived dopamine from descending dopaminergic axons, demonstrating the importance of interneuronal signaling in regeneration (Reimer et al., 2013).
Zebrafish possess all major immune cell types present in mammals, including neutrophils, macrophage/microglia, dendritic cells, B and T lymphocytes (Becker and Becker, 2007; Sunyer, 2013). Zebrafish microglia are present in the brain by day 3 of development, being derived from myeloid precursors, they resemble the morphologies of their mammalian counterparts and have the ability to phagocytose both pathogens and apoptotic neurons (Herbomel et al., 2001; Peri and Nusslein-Volhard, 2008; Svahn et al., 2012). At steady state, microglial activity in the brain has been linked to regulation of neuronal activity (Li et al., 2012). After targeted laser-induced brain injury, microglia accumulate at the lesion site, where their migration is influenced by calcium waves (Sieger et al., 2012). While understudied in SCI, a series of recent elegant experiments have tied inflammation to neurogenesis after traumatic brain injury (TBI). After stab lesion in the adult zebrafish brain, microglia proliferate, accumulate at the lesion site and neurogenesis is induced (Baumgart et al., 2011; Kyritsis et al., 2012; Marz et al., 2011). Acute inflammation drives neurogenesis and neuronal regeneration (Kizil et al., 2012a; Kizil et al., 2012b; Kyritsis et al., 2013; Kyritsis et al., 2012). The mechanism of reactive neurogenesis depends on the transcription factor gata-3 (Kizil et al., 2012a; Kyritsis et al., 2012). Immunosuppression, either by the steroid dexamethasone or by an antagonist of the leukotriene system (Pranlukast), reduced neurogenesis after stab injury or sterile inflammation (Kyritsis et al., 2012). This effect was also observed in 2 separate studies that showed regeneration of the fin after mechanical injury and lateral line regeneration, suggesting that acute inflammation may promote repair in multiple zebrafish tissues (Barros-Becker et al., 2012; Kyritsis et al., 2012).
It should be noted that in mammals, CNS regional differences in regenerative capacity exist, with robust neurogenesis in the lateral ventricles and dentate gyrus of the hippocampus and little appreciable neurogenesis in the spinal cord (Ihrie and Alvarez-Buylla, 2011; Thuret et al., 2006). So whether inflammation would promote neurogenesis in the zebrafish spinal cord, as it does in the brain, is an open question. After complete spinal cord transection of adult zebrafish, the number of microglia/macrophages increased significantly at 14 days post injury, caudal to the lesion site, where they were observed clearing myelin debris (Becker and Becker, 2001). After a crush induced injury to the adult zebrafish spinal cord, macrophages and other immune cells also increased in number at the injury site acutely, where they were observed clearing myelin debris and dying neurons (Becker and Becker, 2002, 2007; Hui et al., 2010). A recent study demonstrated that acutely (<1 week) post SCI, GFAP+ glial cells and macrophages increased near the lesion site and at three weeks post SCI, astrocytes differentiated into presumptive radial glial (GFAP+nestin+) cells that extended processes across the lesion site (Goldshmit et al., 2012b). A bridge resulting from bipolar glial cells provided a permissive substrate for axons from newborn and established neurons to cross the lesion site, supporting functional recovery (Goldshmit et al., 2012b). By five weeks post SCI, the glial bridge disassembled, coinciding with reassembled spinal cord tissue (Goldshmit et al., 2012b).
Establishment of a regeneration-promoting glial cell bridge across the lesion site is regulated by fibroblast growth factor (FGF), which triggers glial cell proliferation and migration (Goldshmit et al., 2012b). Significantly, application of FGF to primate astrocytes in culture promotes changes in their morphology that resembled the zebrafish astrocytes, demonstrating the translational relevance of SCI studies in zebrafish. FGF also promotes recovery from SCI in Xenopus laevis tadpoles and adult rats (Lin et al., 2012b; Lu et al., 2012). Pro-inflammatory and anti-regenerative effects of the lysophosphatidic acid (LPA) on SCI have been shown in both zebrafish and mice (Goldshmit et al., 2012a). In contrast, HMGb1, which promotes developing neurite outgrowth and is also a pro-inflammatory cytokine, had pro-regenerative effects in zebrafish after SCI (Fang et al., 2013).
Thus, despite evidence of acute inflammation promoting regeneration in the brain and elsewhere, the effects of the immune response on regeneration in the zebrafish spinal cord are still not well understood. Unlike many other non-mammalian species discussed here, the zebrafish is an NIH-designated model organism and extensive molecular resources facilitating experimentation are available. In particular, several experimental tools exist to study immune responses to SCI, including the ability to manipulate and monitor gene expression via transgenic reporter lines, morpholinos, the Cre recombinase system, and photoconversion (Gemberling et al., 2013). In addition, the zebrafish research community has long history of sharing protocols, fish lines and reagents to support the use of zebrafish in biomedical research, e.g. (http://zfin.org/). A recent study of the zebrafish genome demonstrated that 82% of the genes listed in the Online Mendelian Inheritance in Man database had at least one zebrafish ortholog, further supporting the use of zebrafish as a translational model of SCI (Howe et al., 2013).
Salamanders and Frogs
Immune responses have been observed to correlate with regenerative capacity after SCI in amphibians (Harty et al., 2003; Mescher and Neff, 2005, 2006; Mescher et al., 2007; Tanaka and Ferretti, 2009). Experimental paradigms used to study responses to SCI in salamanders (axolotls and newts) are tail amputation and spinal cord transection. For a review of the responses to SCI during tail regeneration, see (Tanaka and Ferretti, 2009). Spinal cord transection is followed by axon regeneration, neurogenesis and recovery of almost normal swimming ability over 2-3 months, which depends on regeneration of descending neurons (Davis et al., 1990; Hui et al., 2013). Newts have microglia with morphologies similar to those in other species, while the presence of astrocytes is still an open question (Kirkham et al., 2011). Ablation of tyrosine hydroxylase-containing neurons in the brain induced microglial activation and proliferation (Kirkham et al., 2011). Inhibition of inflammation via the introduction of dexamethasone acutely after neuronal ablation decreased the number of microglia and increased neuronal regeneration, consistent with an inhibitory effect of inflammation on recovery (Kirkham et al., 2011). Interestingly, a recent study of limb regeneration in axolotl concluded that an early macrophage response, which included upregulation of both inflammatory and anti-inflammatory cytokines, is necessary for successful regeneration (Godwin et al., 2013).
Immune responses to SCI in salamander is largely unexplored. In the axolotl, a crush model of spinal cord injury induced accumulation of macrophage/microglial cells at the injury site and peripheral to the spinal cord (Zammit et al., 1993). In the newt, complete transection of the spinal cord induced a cellular inflammatory response, including an influx of lymphocytes and phagoyctic macrophage-like cells, as indicated by hematoxylin/eosin staining (Zukor et al., 2011). In the newt, as in lampreys and zebrafish, GFAP+ astrocytes do not appear to form a dense scar at the lesion site. In fact, astrocytes and ependymal-derived glial cells appear to extend processes across the lesion, parallel to the direction of regenerating axons forming a bridge (Zukor et al., 2011). For an illustration of newt axons regenerating across the lesion site and the glial cell bridge, see Figure 2C (Godwin et al., 2013).
At the molecular level, gene expression profiling after tail amputation in the axolotl demonstrated immune and injury response genes upregulated during the first week after injury (Monaghan et al., 2007). Genome and transcriptome resources are available and growing in axolotl (www.ambystoma.org). A reference transcriptome has also just been reported for the newt (Abdullayev et al., 2013). A recent RNA-Seq analysis of the developing blastema formed after forelimb amputation in axolotl demonstrated that immune genes were upregulated in the initial 24h after amputation (Stewart et al., 2013). Proteomics are also becoming available as a resource in axolotl (Rao et al., 2009).
Frogs
Historically, tadpoles have been a classic model used to delineate cellular and molecular mechanisms governing vertebrate development and are being increasingly used to understand questions in regeneration (Slack et al., 2008). The injury model most commonly used to study SCI in tadpoles is tail amputation and signals are required from the spinal cord to accomplish tail tissue regeneration (Lin et al., 2012a). Recovery of swimming after spinal cord hemisection depends on regeneration of descending axons (Gibbs and Szaro, 2006). Until relatively recently, tadpoles were considered to be able to regenerate their spinal cord after injury throughout their development and unable to do so after metamorphosis. However, Slack and colleagues discovered a “refractory period,” at 4-6 days of development, during which time tadpoles (X. laevis) transiently lose their ability to regenerate muscle, spinal cord and notochord (Beck et al., 2003). The Szaro laboratory conducted a study of cellular and molecular processes differentially expressed before and after metamorphosis, corresponding to conditions when tadpoles are able or unable to regenerate (Gibbs et al., 2011). Importantly, results of this investigation demonstrated a link between regenerative capacity and activation of the immune system. Thus, when tadpoles were kept in prolonged larval state by pharmacological blockade of thyroid hormone with methimazole, wound healing was less complete than in untreated animals at 3 weeks post injury; this treatment led to upregulation in MHC class II and T cell receptor (TCR) components, which are critical for function of the adaptive immune system, supporting the important role of immune system activation for successful spinal cord repair (Gibbs et al., 2011). Gene expression profiling after tail amputation of tadpoles in another frog species also showed early inflammatory/wound healing responses (Tazaki et al., 2005). Surprisingly, at the cellular level, few studies have been performed in tadpoles to investigate their immune response to SCI. After optic nerve crush, an extensive macrophage/microglia response was observed, which peaked at 5 days post injury and declined by about a month after injury (Goodbrand and Gaze, 1991; Wilson et al., 1992). Since the microglial response was initiated acutely and its peak coincided with axon regeneration, the authors proposed a beneficial role of microglia on axon regeneration (Goodbrand and Gaze, 1991). Reflecting both potential positive and negative influences of the immune response on regeneration, immune genes induced in a model of tail amputation were either delayed or prolonged during the refractory period, when tadpoles cannot regenerate (Fukazawa et al., 2009). For example, expression of the chemokine CXCR2 was upregulated during the post-refractory regeneration period, which the authors suggest may induce invasion of peripheral leukocytes (Fukazawa et al., 2009). Perhaps such cells represent anti-inflammatory cell populations that promote remodeling of the tissue and removal of debris. On the contrary, MHCII, which is expressed on antigen presenting cells such as monocytes and macrophages/microglia, was upregulated during the refractory period. Perhaps these cells represent inflammatory macrophages/microglia that are deleterious to recovery. Treatment of tadpoles with immunosuppressive drugs or immune cell depletion during the refractory period restored regenerative capacity. Notably, the authors suggested that regulatory T cells in particular, whose development coincides with the post-refractory period, may dampen immune signals that inhibit regeneration (Fukazawa et al., 2009). Cellular and molecular inflammatory responses in X. tropicalis were initiated within 6 hours of amputation and sustained for several days, with gene ontology terms “immune/defense response” enriched among transcripts upregulated at 6h post amputation (Love et al., 2011). The role of reactive oxygen species (ROS), which are produced by inflammatory cells in many injury models across species, is currently unclear in frog after SCI. ROS were shown to increase dramatically during the first three days following tail amputation in X. laevis, where they promoted regeneration (Love et al., 2013). However, while inflammatory cells were also increased in number after tail amputation, they did not appear to be the source of ROS (Love et al., 2013).
As with the other model organisms described above, the experimental tools available to investigate the role of the immune response in SCI in frog are growing. Tadpoles have many of the experimental advantages of zebrafish and many of the features that made it a classic model organism to study nervous system development also make it attractive to study nervous system regeneration, including their small size, affordability, availability of transgenic lines, transparency of CNS tissues and the ability to assay simple regeneration-dependent behaviors, such as swimming, (Cline and Kelly, 2012; Pratt and Khakhalin, 2013). X. tropicalis is an NIH-designated model organism whose genome is publicly available. This species can also regenerate its spinal cord after tail amputation (Love et al., 2011). To support the use of Xenopus in biomedical research, the National Xenopus Resource Center was recently established at the Marine Biological Laboratory (http://www.mbl.edu/xenopus/) and the Xenopus laevis Research Resource for Immunobiology promotes the study of immunological questions by providing a variety of experimental resources (http://www.urmc.rochester.edu/mbi/resources/Xenopus/index.cfm). In Europe, the European Xenopus Resource Centre is located at the University of Portsmouth (http://www.port.ac.uk/research/exrc/) and Xenbase offers a variety of genomic and other resources to the research community (http://www.xenbase.org/common/). Together, these organizations offer reagents to target specific immune cell types, including transgenic stocks, genetic manipulations of gene expression and other resources, which should enable future studies of the immune response to SCI in frogs.
Turtle
A few recent studies have also demonstrated axon regeneration after complete spinal cord transection in the turtle (Garcia et al., 2012; Rehermann et al., 2009; Rehermann et al., 2011). After injury, axon regeneration, primarily of propriospinal and sensory neurons, is followed by partial restoration of locomotion in ~50% of spinalized turtles (Rehermann et al., 2009; Rehermann et al., 2011). In this model, histological assays demonstrated that a blood clot filled with red and white blood cells formed immediately after SCI. As in the other regenerative non-mammalian species discussed above, after SCI, glial cell processes grew parallel to the long axis of the spinal cord. At the same time, phagocytic macrophages were observed at the lesion (Rehermann et al., 2009). Studies are just beginning to explore cellular aspects of neurogenesis and molecular programs influencing regeneration in this organism (Garcia et al., 2012; Rehermann et al., 2011). For an illustration of turtle axons regenerating across the lesion site, see Figure 2D.
Advantages and Caveats
The major and significant advantage to studying immune (or any type of) responses to SCI in the non-mammalian vertebrates described here is that they have a high rate of success in recovery of function (Figure 2). Therefore, in these models, the emphasis is on understanding mechanisms promoting, rather than inhibiting, the desired outcome. There are also a range of experimental advantages, including: small organism size, low cost, availability of transgenic models, ability to do forward and reverse genetics, visibility of relevant tissues and cells in vivo, identifiable cell types with predictable regenerative responses, simple behavioral assays that depend directly on axon regeneration, and the relative simplicity of CNS circuits.
Of course, as with any model system, there are several caveats in interpreting insights gained from studying immune responses to SCI in non-mammalian model organisms. For example, in several of the species described above, only subpopulations of neurons regenerate and yet, functional recovery is still achieved. How much regeneration is needed in order to promote functional recovery from SCI in mammals? Historically, the biggest obstacle to studying regeneration in non-mammalian species was a lack of molecular resources to investigate such questions, but with next generation sequencing technologies, that is changing rapidly. Proteomic resources are still lagging behind those available in mammals, which impacts the availability of antibodies for immunohistochemistry and flow cytometry. This is less of a challenge in the CNS and innate immune system, where proteins are often highly conserved, than in the adaptive immune system, where molecular strategies are more diverse across species. Because inflammation is widely conserved across species and induced by a variety of traumatic and non-traumatic neurodegenerative settings in mammals, it is a reasonable candidate pathway to study in any vertebrate after SCI. The increasing number of available molecular resources should encourage the development of translational studies in these animals.
Challenges and Opportunities
Despite such caveats, the potential opportunity to translate knowledge gained from studying cellular and molecular responses to SCI in non-mammalian model organisms to mammals is growing. The underlying rationale for studying SCI-triggered responses in a variety of organisms is based on the hypothesis that neuronal regeneration, like neuronal function, relies on fundamental pathways conserved across species. It would follow that regenerative pathways activated in non-mammalian vertebrates after SCI could be activated in mammals, leading to better outcomes. While it was once challenging to investigate the mechanisms leading to functional recovery after SCI in non-mammalian vertebrates, next generation sequencing technologies have enabled the analysis of molecular pathways modulated by SCI in these species (Smith et al., 2011). Molecular data from transcriptome and genome studies in these organisms is leading to the generation or identification of reagents that can mark specific cell types and molecules. These resources will then enable more mechanistic studies. At the cellular level, resources accumulating in these species also enable critical questions that are unapproachable in mammalian systems. For example, what intrinsic signals distinguish typically selectively vulnerable or regenerating neurons in zebrafish or lamprey? At the tissue level, comparing molecular data across multiple non-mammalian species should reveal fundamental and basic aspects of pro-regenerative responses that are conserved throughout evolution (Figure 3). By contrasting those responses with data similarly collected in mammalian models of SCI, it will also be possible to reveal anti-regenerative responses that are specific to mammals or shared across vertebrates. For example, identifying beneficial aspects of the immune response to SCI that are shared across non-mammalian vertebrates may identify aspects of the immune response predicted to promote recovery from SCI in mammals. Future studies are necessary to test these predictions and identify new targets for intervention in mammalian models of SCI.
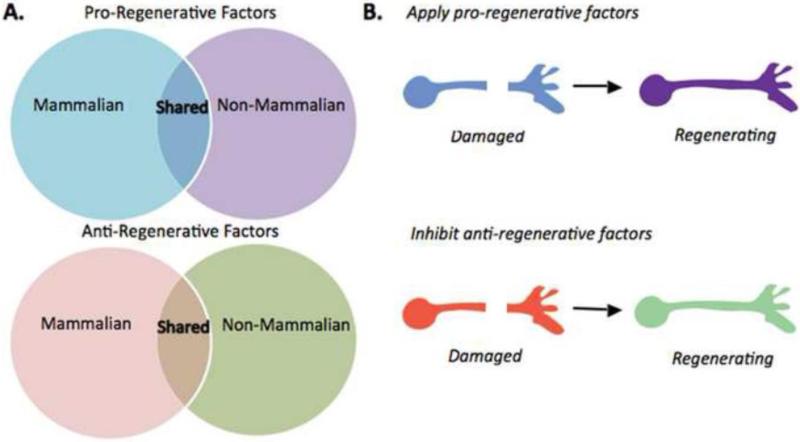
Figure 3.
Comparative approach to discovering biological responses that promote or inhibit regeneration after spinal cord injury.
(A) Gene expression profiling of responses to SCI in non-mammalian model organisms that recover function after SCI will provide a critical step towards understanding the responses able to regulate this process in mammals. By comparing responses shared across regeneration-competent species and contrasting with those in mammals, we can predict pro-regenerative factors that are evolutionarily conserved and those that are unique to non-mammalian vertebrates. It will also allow us to predict anti-regenerative factors regulated by SCI that are evolutionarily conserved and those that are unique to mammals. (B) Ongoing and future studies using tools of genetic and pharmacologic manipulation will functionally test these predictions by applying candidate pro-regenerative factors (upper) or inhibiting candidate anti-regenerative factors (lower). Together, such experiments will identify new targets for intervention in mammalian models of axonal injury and regeneration.
Highlights.
Many non-mammalian vertebrate species exhibit significant recovery after SCI.
The immune response influences regenerative capacity across species.
Understanding immune responses to SCI across vertebrates may promote recovery.
ACKNOWLEDMENTS
I thank all of my colleagues in the area of spinal cord injury research and apologize to those whose work was not discussed due to space limitations. I thank Dr. Jennifer Morgan for comments on the manuscript (The Marine Biological Laboratory), Dr. Elena Nikulina and Ms. Paige Herman of the Bloom Lab for comments on the manuscript and assistance in preparing the figures. Additionally, I gratefully acknowledge support from the National Institutes of Health R03NS078519, The Feinstein Institute for Medical Research and The Marine Biological Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdullayev I, Kirkham M, Bjorklund AK, Simon A, Sandberg R. A reference transcriptome and inferred proteome for the salamander Notophthalmus viridescens. Exp Cell Res. 2013;319:1187–1197. doi: 10.1016/j.yexcr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Adrian EK, Jr., Williams MG, George FC. Fine structure of reactive cells in injured nervous tissue labeled with 3H-thymidine injected before injury. J Comp Neurol. 1978;180:815–839. doi: 10.1002/cne.901800412. [DOI] [PubMed] [Google Scholar]
- Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, Gartland GL, Boydston JA, Turnbough CL, Jr., Cooper MD. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Bao F, Bailey CS, Gurr KR, Bailey SI, Rosas-Arellano MP, Brown A, Dekaban GA, Weaver LC. Human spinal cord injury causes specific increases in surface expression of beta integrins on leukocytes. J Neurotrauma. 2010;28:269–280. doi: 10.1089/neu.2010.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Becker F, Romero J, Pulgar A, Feijoo CG. Persistent oxytetracycline exposure induces an inflammatory process that improves regenerative capacity in zebrafish larvae. PLoS One. 2012;7:e36827. doi: 10.1371/journal.pone.0036827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdi D, Schwab ME. Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–186. doi: 10.1016/0006-8993(94)01410-j. [DOI] [PubMed] [Google Scholar]
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J. Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia. 2011;60:343–357. doi: 10.1002/glia.22269. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Becker CG, Becker T. Repellent guidance of regenerating optic axons by chondroitin sulfate glycosaminoglycans in zebrafish. J Neurosci. 2002;22:842–853. doi: 10.1523/JNEUROSCI.22-03-00842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Becker T. Growth and pathfinding of regenerating axons in the optic projection of adult fish. J Neurosci Res. 2007;85:2793–2799. doi: 10.1002/jnr.21121. [DOI] [PubMed] [Google Scholar]
- Becker CG, Becker T. Adult zebrafish as a model for successful central nervous system regeneration. Restor Neurol Neurosci. 2008;26:71–80. [PubMed] [Google Scholar]
- Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T, Schachner M. L1.1 is involved in spinal cord regeneration in adult zebrafish. J Neurosci. 2004;24:7837–7842. doi: 10.1523/JNEUROSCI.2420-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Becker CG. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J Comp Neurol. 2001;433:131–147. doi: 10.1002/cne.1131. [DOI] [PubMed] [Google Scholar]
- Becker T, Bernhardt RR, Reinhard E, Wullimann MF, Tongiorgi E, Schachner M. Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules. J Neurosci. 1998;18:5789–5803. doi: 10.1523/JNEUROSCI.18-15-05789.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011 doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DH, Otto SJ, Depoister B, Fetcho JR. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. doi: 10.1126/science.1098439. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Patzelova H, McLean DL, Fetcho JR, Zottoli SJ. Functional regeneration in the larval zebrafish spinal cord. In: Becker CG, Becker T, editors. Model Organisms in Spinal Cord Regeneration. WILEY-VCH Verlag GmbH & Co.; KgaA, Weihnheim: 2007. pp. 263–288. [Google Scholar]
- Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011;11:307–317. doi: 10.1038/nri2944. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2010 doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Busch DJ, Morgan JR. Synuclein accumulation is associated with cell-specific neuronal death after spinal cord injury. J Comp Neurol. 2012;520:1751–1771. doi: 10.1002/cne.23011. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, McCurley N, Bockman DE, Schorpp M, Cooper MD, Boehm T. A thymus candidate in lampreys. Nature. 2011;470:90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- Cannon JP, Haire RN, Pancer Z, Mueller MG, Skapura D, Cooper MD, Litman GW. Variable domains and a VpreB-like molecule are present in a jawless vertebrate. Immunogenetics. 2005;56:924–929. doi: 10.1007/s00251-004-0766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER, Schwartz JH, Wilson FD, Nairn AC, Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980;77:7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lee H, Henle SJ, Cheever TR, Ekker SC, Henley JR. Primary neuron culture for nerve growth and axon guidance studies in zebrafish (Danio rerio). PLoS One. 2013;8:e57539. doi: 10.1371/journal.pone.0057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT, Kelly D. Xenopus as an experimental system for developmental neuroscience: introduction to a special issue. Dev Neurobiol. 2012;72:463–464. doi: 10.1002/dneu.22012. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Baker MT, Dobrov TA. Evidence for functional regeneration in the adult lamprey spinal cord following transection. Brain Res. 1989;496:368–372. doi: 10.1016/0006-8993(89)91090-1. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Kiemel T, Pate V, Blinder J, Guan L. Temperature can alter the function outcome of spinal cord regeneration in larval lampreys. Neuroscience. 1999;90:957–965. doi: 10.1016/s0306-4522(98)00502-8. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Mackler SA, Selzer ME. Functional regeneration following spinal transection demonstrated in the isolated spinal cord of the larval sea lamprey. Proc Natl Acad Sci U S A. 1986;83:2763–2766. doi: 10.1073/pnas.83.8.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH, Mackler SA, Selzer ME. Behavioral recovery following spinal transection: functional regeneration in the lamprey CNS. Trends Neurosci. 1988;11:227–231. doi: 10.1016/0166-2236(88)90131-2. [DOI] [PubMed] [Google Scholar]
- Cohen S, Levi-Montalcini R, Hamburger V. A Nerve Growth-Stimulating Factor Isolated from Sarcom as 37 and 180. Proc Natl Acad Sci U S A. 1954;40:1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Das S, Hirano M, Aghaallaei N, Bajoghli B, Boehm T, Cooper MD. Organization of lamprey variable lymphocyte receptor C locus and repertoire development. Proc Natl Acad Sci U S A. 2013;110:6043–6048. doi: 10.1073/pnas.1302500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Davis BM, Ayers JL, Koran L, Carlson J, Anderson MC, Simpson SB., Jr. Time course of salamander spinal cord regeneration and recovery of swimming: HRP retrograde pathway tracing and kinematic analysis. Exp Neurol. 1990;108:198–213. doi: 10.1016/0014-4886(90)90124-b. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature reviews. Drug discovery. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2010;117:e49–56. doi: 10.1182/blood-2010-10-314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer BM, McAllister AK. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 2012;35:660–670. doi: 10.1016/j.tins.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Pan HC, Lin SL, Zhang WQ, Rauvala H, Schachner M, Shen YQ. HMGB1 Contributes to Regeneration After Spinal Cord Injury in Adult Zebrafish. Mol Neurobiol. 2013 doi: 10.1007/s12035-013-8533-4. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951;115:320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. 2009;136:2323–2327. doi: 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- Garcia G, Libisch G, Trujillo-Cenoz O, Robello C, Russo RE. Modulation of gene expression during early stages of reconnection of the turtle spinal cord. J Neurochem. 2012;121:996–1006. doi: 10.1111/j.1471-4159.2012.07750.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013 doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P, Cuzzocrea S. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. The Journal of pharmacology and experimental therapeutics. 2006;316:1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Esposito E, Bramanti P, Cuzzocrea S. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008a;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Esposito E, Di Paola R, Caminiti R, Meli R, Bramanti P, Cuzzocrea S. Effect of thalidomide on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008b;30:231–240. doi: 10.1097/shk.0b013e318162d290. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Donnelly DJ, Popovich PG. Spinal cord injury therapies in humans: an overview of current clinical trials and their potential effects on intrinsic CNS macrophages. Expert Opin Ther Targets. 2011;15:505–518. doi: 10.1517/14728222.2011.553605. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Kigerl KA, Mandrekar-Colucci SS, Gaudet AD, Popovich PG. Achieving CNS axon regeneration by manipulating convergent neuro-immune signaling. Cell Tissue Res. 2012;349:201–213. doi: 10.1007/s00441-012-1425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs KM, Chittur SV, Szaro BG. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. Eur J Neurosci. 2011;33:9–25. doi: 10.1111/j.1460-9568.2010.07477.x. [DOI] [PubMed] [Google Scholar]
- Gibbs KM, Szaro BG. Regeneration of descending projections in Xenopus laevis tadpole spinal cord demonstrated by retrograde double labeling. Brain Res. 2006;1088:68–72. doi: 10.1016/j.brainres.2006.02.126. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Frontiers in cellular neuroscience. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Matteo R, Sztal T, Ellett F, Frisca F, Moreno K, Crombie D, Lieschke GJ, Currie PD, Sabbadini RA, Pebay A. Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes. Am J Pathol. 2012a;181:978–992. doi: 10.1016/j.ajpath.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen-Chi M, Currie PD. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci. 2012b;32:7477–7492. doi: 10.1523/JNEUROSCI.0758-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzenbach RR, Schwab ME. Disinhibition of neurite growth to repair the injured adult CNS: focusing on Nogo. Cell Mol Life Sci. 2008;65:161–176. doi: 10.1007/s00018-007-7170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbrand IA, Gaze RM. Microglia in tadpoles of Xenopus laevis: normal distribution and the response to optic nerve injury. Anat Embryol (Berl) 1991;184:71–82. doi: 10.1007/BF01744263. [DOI] [PubMed] [Google Scholar]
- Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L, Brewer CR, Collins WF, Goldberger ME, Perl ER. Criteria for evaluating spinal cord regeneration experiments. Surg Neurol. 1980;14:392. [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: an immunologic perspective. Dev Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, Popovich PG. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T, Cooper MD. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–438. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Action potentials recorded from inside a nerve fiber. Nature. 1939;144:710–711. [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Eliott D, Threadgold G, Harden G, Ware D, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang KG, Selzer ME. Effects of chondroitnase on regeneration and survival of axotomized spinal-projecting neurons in the lamprey. Neuroscience Meeting Planner online. 2013 [Google Scholar]
- Hui SP, Dutta A, Ghosh S. Cellular response after crush injury in adult zebrafish spinal cord. Dev Dyn. 2010;239:2962–2979. doi: 10.1002/dvdy.22438. [DOI] [PubMed] [Google Scholar]
- Hui SP, Monaghan JR, Voss SR, Ghosh S. Expression pattern of Nogo-A, MAG, and NgR in regenerating urodele spinal cord. Dev Dyn. 2013;242:847–860. doi: 10.1002/dvdy.23976. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J Neurosci. 1997;17:5206–5220. doi: 10.1523/JNEUROSCI.17-13-05206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Zhang G, Jamison C, Jr., Takano H, Haydon PG, Selzer ME. Axon regeneration in the absence of growth cones: acceleration by cyclic AMP. J Comp Neurol. 2009;515:295–312. doi: 10.1002/cne.22057. [DOI] [PubMed] [Google Scholar]
- Kanyilmaz S, Hepguler S, Atamaz FC, Gokmen NM, Ardeniz O, Sin A. Phagocytic and oxidative burst activity of neutrophils in patients with spinal cord injury. Arch Phys Med Rehabil. 2012;94:369–374. doi: 10.1016/j.apmr.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci U S A. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalturin K, Panzer Z, Cooper MD, Bosch TC. Recognition strategies in the innate immune system of ancestral chordates. Mol Immunol. 2004;41:1077–1087. doi: 10.1016/j.molimm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Popovich PG. Toll-like receptors in spinal cord injury. Curr Top Microbiol Immunol. 2009;336:121–136. doi: 10.1007/978-3-642-00549-7_7. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Madhavan M, Call MK, Santiago W, Tsonis PA, Lambris JD, Del Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- Kirkham M, Berg DA, Simon A. Microglia activation during neuroregeneration in the adult vertebrate brain. Neurosci Lett. 2011;497:11–16. doi: 10.1016/j.neulet.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Kizil C, Dudczig S, Kyritsis N, Machate A, Blaesche J, Kroehne V, Brand M. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 2012a;7:27. doi: 10.1186/1749-8104-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C, Kyritsis N, Dudczig S, Kroehne V, Freudenreich D, Kaslin J, Brand M. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev Cell. 2012b;23:1230–1237. doi: 10.1016/j.devcel.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997;762:173–184. doi: 10.1016/s0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nature reviews. Drug discovery. 2010;9:703–718. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Kandel ER. Neuronal controls of a behavioral response mediated by the abdominal ganglion of Aplysia. Science. 1969;164:847–850. doi: 10.1126/science.164.3881.847. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Casha S, Hurlbert RJ, Yong VW. Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med. 2010;49:425–433. doi: 10.1515/CCLM.2011.068. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Brand M. Neuroinflammation and central nervous system regeneration in vertebrates. Trends Cell Biol. 2013 doi: 10.1016/j.tcb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Laramore C, Maymind E, Shifman MI. Expression of neurotrophin and its tropomyosin-related kinase receptors (Trks) during axonal regeneration following spinal cord injury in larval lamprey. Neuroscience. 2011;183:265–277. doi: 10.1016/j.neuroscience.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BY, Fogerson SM, Walsh RB, Morgan JR. Cyclic AMP promotes axon regeneration, lesion repair and neuronal survival in lampreys after spinal cord injury. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lin G, Chen Y, Slack JM. Imparting regenerative capacity to limbs by progenitor cell transplantation. Dev Cell. 2012a;24:41–51. doi: 10.1016/j.devcel.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Chen Y, Slack JM. Transgenic analysis of signaling pathways required for Xenopus tadpole spinal cord and muscle regeneration. Anat Rec (Hoboken) 2012b;295:1532–1540. doi: 10.1002/ar.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love NR, Chen Y, Bonev B, Gilchrist MJ, Fairclough L, Lea R, Mohun TJ, Paredes R, Zeef LA, Amaya E. Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Dev Biol. 2011;11:70. doi: 10.1186/1471-213X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, Gallop JL, Dorey K, Amaya E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol. 2013;15:222–228. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie DI, Pijak DS, Selzer ME. Structure of reticulospinal axon growth cones and their cellular environment during regeneration in the lamprey spinal cord. J Comp Neurol. 1994;344:559–580. doi: 10.1002/cne.903440406. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. The need for cellular elements during axonal regeneration in the sea lamprey spinal cord. Exp Neurol. 1991a;112:64–71. doi: 10.1016/0014-4886(91)90114-r. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. Preferential regeneration of spinal axons through the scar in hemisected lamprey spinal cord. J Comp Neurol. 1991b;313:669–679. doi: 10.1002/cne.903130410. [DOI] [PubMed] [Google Scholar]
- Marder E. Non-mammalian models for studying neural development and function. Nature. 2002;417:318–321. doi: 10.1038/417318a. [DOI] [PubMed] [Google Scholar]
- Marz M, Schmidt R, Rastegar S, Strahle U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev Dyn. 2011;240:2221–2231. doi: 10.1002/dvdy.22710. [DOI] [PubMed] [Google Scholar]
- Mayer WE, Uinuk-Ool T, Tichy H, Gartland LA, Klein J, Cooper MD. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc Natl Acad Sci U S A. 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine C.f.S.C. Early Acute Managment in Adults with Spinal Cord Injury: A Clinical Practice Guideline for Health-Care Providers, Clinical Practice Guidelines. Paralyzed Veterans of America; Washington, DC: 2013. [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Limb regeneration in amphibians: immunological considerations. ScientificWorldJournal 6 Suppl. 2006;1:1–11. doi: 10.1100/tsw.2006.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Wolf WL, Moseman EA, Hartman B, Harrison C, Nguyen E, Neff AW. Cells of cutaneous immunity in Xenopus: studies during larval development and limb regeneration. Dev Comp Immunol. 2007;31:383–393. doi: 10.1016/j.dci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Monaghan JR, Walker JA, Page RB, Putta S, Beachy CK, Voss SR. Early gene expression during natural spinal cord regeneration in the salamander Ambystoma mexicanum. J Neurochem. 2007;101:27–40. doi: 10.1111/j.1471-4159.2006.04344.x. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M. Janeway's Immunobiology. 7th ed. Garland, New York: 2008. [Google Scholar]
- Najakshin AM, Mechetina LV, Alabyev BY, Taranin AV. Identification of an IL-8 homolog in lamprey (Lampetra fluviatilis): early evolutionary divergence of chemokines. Eur J Immunol. 1999;29:375–382. doi: 10.1002/(SICI)1521-4141(199902)29:02<375::AID-IMMU375>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature medicine. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DM, Wittamer V, Bertrand JY, Lewis KL, Pratt DN, Delgado N, Schale SE, McGue C, Jacobsen BH, Doty A, Pao Y, Yang H, Chi NC, Magor BG, Traver D. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood. 2013;122:e1–e11. doi: 10.1182/blood-2012-12-471029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Pernet V, Schwab ME. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012;349:97–104. doi: 10.1007/s00441-012-1432-6. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Hickey WF. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J Neuropathol Exp Neurol. 2001;60:676–685. doi: 10.1093/jnen/60.7.676. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Streit WJ, Stokes BT. Differential expression of MHC class II antigen in the contused rat spinal cord. J Neurotrauma. 1993;10:37–46. doi: 10.1089/neu.1993.10.37. [DOI] [PubMed] [Google Scholar]
- Popovich PG, van Rooijen N, Hickey WF, Preidis G, McGaughy V. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Exp Neurol. 2003;182:275–287. doi: 10.1016/s0014-4886(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Khakhalin AS. Modeling human neurodevelopmental disorders in the Xenopus tadpole: from mechanisms to therapeutic targets. Dis Model Mech. 2013;6:1057–1065. doi: 10.1242/dmm.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N, Jhamb D, Milner DJ, Li B, Song F, Wang M, Voss SR, Palakal M, King MW, Saranjami B, Nye HL, Cameron JA, Stocum DL. Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biol. 2009;7:83. doi: 10.1186/1741-7007-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann MI, Marichal N, Russo RE, Trujillo-Cenoz O. Neural reconnection in the transected spinal cord of the freshwater turtle Trachemys dorbignyi. J Comp Neurol. 2009;515:197–214. doi: 10.1002/cne.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann MI, Santinaque FF, Lopez-Carro B, Russo RE, Trujillo-Cenoz O. Cell proliferation and cytoarchitectural remodeling during spinal cord reconnection in the fresh-water turtle Trachemys dorbignyi. Cell Tissue Res. 2011;344:415–433. doi: 10.1007/s00441-011-1173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Kuscha V, Wyatt C, Sorensen I, Frank RE, Knuwer M, Becker T, Becker CG. Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J Neurosci. 2009;29:15073–15082. doi: 10.1523/JNEUROSCI.4748-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Norris A, Ohnmacht J, Patani R, Zhong Z, Dias TB, Kuscha V, Scott AL, Chen YC, Rozov S, Frazer SL, Wyatt C, Higashijima S, Patton EE, Panula P, Chandran S, Becker T, Becker CG. Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Dev Cell. 2013;25:478–491. doi: 10.1016/j.devcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Reimer MM, Sorensen I, Kuscha V, Frank RE, Liu C, Becker CG, Becker T. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28:8510–8516. doi: 10.1523/JNEUROSCI.1189-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Renshaw SA, Trede NS. A model 450 million years in the making: zebrafish and vertebrate immunity. Dis Model Mech. 2012;5:38–47. doi: 10.1242/dmm.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). II. Dorsal cells and giant interneurons. J Neurophysiol. 1967;30:1024–1042. doi: 10.1152/jn.1967.30.5.1024. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Regeneration of Muller and Mauthner axons after spinal transection in larval lampreys. J Comp Neurol. 1976;168:545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JS, Smith GM, Silver J. An in vitro model of wound healing in the CNS: analysis of cell reaction and interaction at different ages. Exp Neurol. 1989;103:1–16. doi: 10.1016/0014-4886(89)90180-5. [DOI] [PubMed] [Google Scholar]
- Sabelstrom H, Stenudd M, Reu P, Dias DO, Elfineh M, Zdunek S, Damberg P, Goritz C, Frisen J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- Sato A, Uinuk-ool TS, Kuroda N, Mayer WE, Takezaki N, Dongak R, Figueroa F, Cooper MD, Klein J. Macrophage migration inhibitory factor (MIF) of jawed and jawless fishes: implications for its evolutionary origin. Dev Comp Immunol. 2003;27:401–412. doi: 10.1016/s0145-305x(02)00136-2. [DOI] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Berman MA, Perry VH, Schwab ME. Lymphocyte recruitment following spinal cord injury in mice is altered by prior viral exposure. Eur J Neurosci. 1997;9:1000–1007. doi: 10.1111/j.1460-9568.1997.tb01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ME. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol. 1978;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. The Journal of pathology. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Differential expression of class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration. J Comp Neurol. 2007;501:631–646. doi: 10.1002/cne.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Yumul RE, Laramore C, Selzer ME. Expression of the repulsive guidance molecule RGM and its receptor neogenin after spinal cord injury in sea lamprey. Exp Neurol. 2009;217:242–251. doi: 10.1016/j.expneurol.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Zhang G, Selzer ME. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J Comp Neurol. 2008;510:269–282. doi: 10.1002/cne.21789. [DOI] [PubMed] [Google Scholar]
- Sieger D, Moritz C, Ziegenhals T, Prykhozhij S, Peri F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev Cell. 2012;22:1138–1148. doi: 10.1016/j.devcel.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Slack JM, Lin G, Chen Y. The Xenopus tadpole: a new model for regeneration research. Cell Mol Life Sci. 2008;65:54–63. doi: 10.1007/s00018-007-7431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Miller RH, Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J Comp Neurol. 1986;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Smith J, Morgan JR, Zottoli SJ, Smith PJ, Buxbaum JD, Bloom O. Regeneration in the Era of Functional Genomics and Gene Network Analysis. The Biological Bulletin. 2011;221:18–34. doi: 10.1086/BBLv221n1p18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, Morgan JR, Buxbaum JD, Sachidanandam R, Sims C, Garruss AS, Cook M, Krumlauf R, Wiedemann LM, Sower SA, Decatur WA, Hall JA, Amemiya CT, Saha NR, Buckley KM, Rast JP, Das S, Hirano M, McCurley N, Guo P, Rohner N, Tabin CJ, Piccinelli P, Elgar G, Ruffier M, Aken BL, Searle SM, Muffato M, Pignatelli M, Herrero J, Jones M, Brown CT, Chung-Davidson YW, Nanlohy KG, Libants SV, Yeh CY, McCauley DW, Langeland JA, Pancer Z, Fritzsch B, de Jong PJ, Zhu B, Fulton LL, Theising B, Flicek P, Bronner ME, Warren WC, Clifton SW, Wilson RK, Li W. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet. 2013;45:415–421. 421e411–412. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Spitzbarth I, Bock P, Haist V, Stein VM, Tipold A, Wewetzer K, Baumgartner W, Beineke A. Prominent microglial activation in the early proinflammatory immune response in naturally occurring canine spinal cord injury. J Neuropathol Exp Neurol. 2011;70:703–714. doi: 10.1097/NEN.0b013e3182270f8e. [DOI] [PubMed] [Google Scholar]
- Stein A, Panjwani A, Sison C, Rosen L, Chugh R, Metz C, Bank M, Bloom O. Pilot study: elevated circulating levels of the proinflammatory cytokine macrophage migration inhibitory factor in patients with chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94:1498–1507. doi: 10.1016/j.apmr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stewart R, Rascon CA, Tian S, Nie J, Barry C, Chu LF, Ardalani H, Wagner RJ, Probasco MD, Bolin JM, Leng N, Sengupta S, Volkmer M, Habermann B, Tanaka EM, Thomson JA, Dewey CN. Comparative RNA-seq analysis in the unsequenced axolotl: the oncogene burst highlights early gene expression in the blastema. PLoS Comput Biol. 2013;9:e1002936. doi: 10.1371/journal.pcbi.1002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Sunyer JO. Fishing for mammalian paradigms in the teleost immune system. Nat Immunol. 2013;14:320–326. doi: 10.1038/ni.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svahn AJ, Graeber MB, Ellett F, Lieschke GJ, Rinkwitz S, Bennett MR, Becker TS. Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev Neurobiol. 2012;73:60–71. doi: 10.1002/dneu.22039. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nat Rev Neurosci. 2009;10:713–723. doi: 10.1038/nrn2707. [DOI] [PubMed] [Google Scholar]
- Tazaki A, Kitayama A, Terasaka C, Watanabe K, Ueno N, Mochii M. Macroarray-based analysis of tail regeneration in Xenopus laevis larvae. Dev Dyn. 2005;233:1394–1404. doi: 10.1002/dvdy.20472. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Nakamura O, Watanabe T. Lamprey (Lethenteron japonicum) IL-17 upregulated by LPS-stimulation in the skin cells. Immunogenetics. 2007;59:873–882. doi: 10.1007/s00251-007-0254-2. [DOI] [PubMed] [Google Scholar]
- Uinuk-Ool T, Mayer WE, Sato A, Dongak R, Cooper MD, Klein J. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc Natl Acad Sci U S A. 2002;99:14356–14361. doi: 10.1073/pnas.212527699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uinuk-ool TS, Mayer WE, Sato A, Takezaki N, Benyon L, Cooper MD, Klein J. Identification and characterization of a TAP-family gene in the lamprey. Immunogenetics. 2003;55:38–48. doi: 10.1007/s00251-003-0548-y. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Gaze RM, Goodbrand IA, Taylor JS. Regeneration in the Xenopus tadpole optic nerve is preceded by a massive macrophage/microglial response. Anat Embryol (Berl) 1992;186:75–89. doi: 10.1007/BF00710404. [DOI] [PubMed] [Google Scholar]
- Wood MR, Cohen MJ. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science. 1979;206:344–347. doi: 10.1126/science.482943. [DOI] [PubMed] [Google Scholar]
- Wood MR, Cohen MJ. Synaptic regeneration and glial reactions in the transected spinal cord of the lamprey. J Neurocytol. 1981;10:57–79. doi: 10.1007/BF01181745. [DOI] [PubMed] [Google Scholar]
- Yin HS, Selzer ME. Electrophysiologic evidence of regeneration of lamprey spinal neurons. Exp Neurol. 1984;83:618–628. doi: 10.1016/0014-4886(84)90128-6. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Clarke JD, Golding JP, Goodbrand IA, Tonge DA. Macrophage response during axonal regeneration in the axolotl central and peripheral nervous system. Neuroscience. 1993;54:781–789. doi: 10.1016/0306-4522(93)90247-d. [DOI] [PubMed] [Google Scholar]
- Zhang G, Pizarro IV, Swain GP, Kang SH, Selzer ME. Neurogenesis in the lamprey CNS following spinal cord transection. J Comp Neurol. 2013a doi: 10.1002/cne.23485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Palmer R, McClellan AD. Conditioning lesions enhance axonal regeneration of descending brain neurons in spinal-cord-transected larval lamprey. J Comp Neurol. 2004;478:395–404. doi: 10.1002/cne.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guan Z, Reader B, Shawler T, Mandrekar-Colucci S, Huang K, Weil Z, Bratasz A, Wells J, Powell ND, Sheridan JF, Whitacre CC, Rabchevsky AG, Nash MS, Popovich PG. Autonomic Dysreflexia Causes Chronic Immune Suppression after Spinal Cord Injury. J Neurosci. 2013b;33:12970–12981. doi: 10.1523/JNEUROSCI.1974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukor KA, Kent DT, Odelberg SJ. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 2011;6:1. doi: 10.1186/1749-8104-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]