Abstract
In this study, WPT-A, a type of water-soluble homogeneous lichen polysaccharide, was isolated and purified from Parmelia tinctorum. We investigated whether WPT-A has radioprotective effects when administered before total-body irradiation (TBI). Mice were treated with WPT-A via intraperitoneal injection (i.p.) once per day for three consecutive days prior to 7, 7.5, 8.5, 10 or 10.5-Gy TBI. Our results indicated that the survival rate was enhanced at a range of levels of TBI. The calculated dose reduction factor (DRF) was 1.2. White blood cell (WBC) counts, spleen colony forming units (CFU-S) and bone marrow nucleated cell (BMNC) counts were used to investigate the radioprotective effects of WPT-A on the hematopoietic system. The treatment groups received WPT-A at 20, 50 and 80 mg/kg b.w. doses before 6.5-Gy TBI and showed significantly higher BMNC and WBC counts compared with the radiation-only group. The groups administered 50 and 80 mg/kg b.w. WPT-A showed a significant increase in CFU-S compared with the radiation-only group. We also carried out a single cell gel electrophoresis assay to explore the radioprotective effects of WPT-A on DNA damage. The results from single-cell gel electrophoresis of peripheral blood leukocytes showed that WPT-A attenuated radiation-induced DNA damage. These results indicate a potential use for WPT-A as a radioprotector.
Keywords: radioprotection, Parmelia tinctorum, hematopoietic system, single cell gel electrophoresis, comet assay
INTRODUCTION
The development of safe and more effective radioprotectors is very important in view of their potential application during unintended radiation exposure and radiotherapy. Over the past 50 years, the possible radioprotective effects of many synthetic and natural agents have been investigated. Currently, there is no safe and effective non-toxic radioprotector available for human use [1]. Amifostine, also known as Ethyol or WR2721 is the only clinically accepted radioprotector, but it has inherent dose-limiting toxicities [2]. Several drugs are in different stages of evaluation, but so far none possess all the requisite qualities of an optimum radioprotector [3, 4].
Parmelia tinctorum belongs to the Parmeliae family of lichens. Lichens have been used for medicinal purposes throughout the ages, and beneficial claims have to some extent been correlated with their polysaccharide content. Of 13 500 lichen species growing worldwide, less than 100 species have been investigated for polysaccharide content. A number of investigations have been carried out on the biological effects of lichen polysaccharides [5], most notably antitumor [6], immunomodulating [7], antiviral [8, 9] and memory-enhancing effects [10, 11].
In our study, a homogeneous polysaccharide, WPT-A, was isolated and identified from Parmelia tinctorum. The chemical and physical characteristics of WPT-A were determined. In addition, its protective effects in mice exposed to radiation were explored in terms of whole-body survival, protection of the hematopoietic system and attenuation of DNA damage.
MATERIALS AND METHODS
Preparation of Parmelia tinctorum polysaccharide
Parmelia tinctorum (1.5 kg) was crushed and first extracted with ethanol. The residue was extracted with boiling water. The resultant aqueous extract was concentrated in a rotary evaporator under reduced pressure, precipitated by ethanol at 4°C for 24 h, and then centrifuged (5000 rpm, 10 min). The precipitate was dissolved in water and then deproteinated with chloroform and n-butanol eight times. The resulting aqueous fraction was extensively dialyzed against running water for 3 d and then against distilled water for 1 d. The retentate was concentrated under reduced pressure to a small volume, and 4 volumes of ethanol were added stepwise with stirring at 4°C. The mixture was stored overnight at 4°C. The resulting precipitate obtained by centrifugation consisted of crude polysaccharide.
To purify the crude polysaccharide, sequential column filtration was used. First, the extract was applied to a DEAE-32-Cellulose column and eluted with 0.1 M NaCl. No carbohydrates were detected via a phenol-sulfuric acid color reaction in the fractions prior to linear gradient elution at 2 M and 0.1 M NaCl. The corresponding fractions, WPT-A and WPT-B, were pooled, dialyzed, and lyophilized. WPT-A, which had the highest activity, was further fractionated on a Sephadex G-150 column and eluted with 0.1 M NaCl, resulting in one fraction. This fraction was purified by rechromatography on the same exclusion column three times. The resulting homogeneous polysaccharide obtained was designated WPT-A (yield: 3.2 g).
The sugar composition of WPT-A was analyzed by nuclear magnetic resonance spectroscopy (NMR) and gas chromatography (GC), and the linkage position of sugar was analyzed by GC–MS (mass spectroscopy). The carbohydrate and protein contents were measured using the phenol-sulfuric acid method [12] and the Lowry method [13], respectively. The glucuronic acid content was measured using the sulfuric acid-carbazole method [14]. Homogeneity and molecular weight measurements were completed with high power liquid chromatography (HPLC).
Animals
ICR mice (6–8 weeks old) with an average body weight of 20 ± 2 g were obtained from the Animal Center of the Chinese Academy of Medical Sciences. They were maintained under controlled laboratory conditions at a temperature of 25 ± 2°C with a controlled light cycle (14 h of light and 10 h of darkness). The mice were fed standard animal food pellets and tap water ad libitum. All animal experiments were conducted according to the guidelines of the institutional Ethics Committee.
Irradiation of animals
Total body gamma irradiation (TBI) was accomplished at room temperature using a 137Cs Gamma Tissue Irradiator at a dose rate of 0.78 Gy/min (Theratron 780E, Canada) during the experimental period. Each mouse was kept in a perforated plastic container. When irradiated, the mice were placed on a rotating platform to ensure even dose delivery to all tissues.
Administration of WPT-A
WPT-A was dissolved in normal saline for administration at the desired concentrations, and the dose was expressed in mg/kg b.w. Different doses of WPT-A were administered to mice via the intraperitoneal (i.p.) route in a maximum volume of 0.2 ml. Control animals received 0.2 ml of normal saline.
Maximum tolerated dose
The acute toxicity of WPT-A was studied in terms of percent survival, change in behavior, alteration in neuromuscular coordination, and respiratory disorders for 14 d after the administration of a single dose of WPT-A at 125, 250, 500, 1000, 2000 and 2500 mg/kg b.w. The maximum dose of WPT-A that yielded no toxic manifestations was considered the maximum tolerated dose (MTD).
Survival and dose reduction factor analysis
Mice (n = 10 mice/group) were administered WPT-A at a dose of 80 mg/kg once a day for three consecutive days, and then on the third day they were irradiated with gamma rays at a dose of 7, 7.5, 8.5, 10 or 10.5-Gy 30 min after the administration of WPT-A. The mice were monitored daily for 30 d, to determine survival rates. On Day 31, all the surviving mice were euthanized by cervical dislocation. The data were reported as the percentage of animals surviving. The protective capacity of WPT-A was expressed as the dose reduction factor (DRF). The DRF was calculated by dividing the LD50/30 of the radiation plus WPT-A group by the LD50/30 of the radiation-only group [15].
Studies on radioprotection of the hematopoietic system
The animals were randomly divided into five groups of eight mice. The mice received WPT-A at a dose of 20, 50 and 80 mg/kg or normal saline administered i.p. once a day for three consecutive days, and then on the third day, they were irradiated with gamma rays at a dose of 6.5-Gy 30 min after administration of WPT-A. The mice were sacrificed by cervical dislocation on the ninth post-irradiation day in all groups. Peripheral blood was collected from the tail vein, and their spleens and femoral bones were collected. The white blood cells (WBCs), endogenous spleen clone-forming unit, and bone marrow nucleated cells (BMNCs) were investigated to estimate the radioprotective effects of WPT-A on the hematopoietic system. All experiments were repeated twice.
BMNC counts
Mouse femoral bones were collected, and bone marrow was flushed out with 3% acetic acid. The number of BMNCs was counted using a microscope.
WBC counts
Peripheral blood was collected from the tail vein on the ninth post-irradiation day. WBCs were determined using a Coulter LH755 Hematology Analyzer.
Endogenous spleen colony-forming units
Spleens were removed from mice and fixed in Bouin's solution (trinitrophenol and methanal) for 24 h. Macroscopic spleen colony-forming units (CFU-S), visible to the naked eye, were scored in each spleen [16].
Studies on radioprotection of DNA damage
DNA damage was detected using the comet assay. The mouse groups and administration of WPT-A in this assay were as for the studies on radioprotection of the hematopoietic system (see above).
Blood from the tail vein of each animal was collected in heparinized vials 30 min after irradiation, and a lymphocyte suspension was prepared. The comet assay, also called single cell gel electrophoresis (SCGE), was performed under alkaline conditions as described by Banath et al. [17]; two layers of agarose were used. First, the comet slides were coated with 100 μl of normal-melting-point agarose, then, once the first agarose layer was coagulated, a mixture of 75 μl of low-melting-point agarose and 25 μl of lymphocyte suspension was applied as the second layer. The comet slides were immersed in cold alkaline lysis solution for 2 h at 4°C. After lysis, double-distilled water was used to rinse away excess salt. All the comet slides were then placed in buffer for 20 min in a horizontal electrophoresis tank pre-filled with cold alkaline electrophoresis buffer to loosen the tight double-helical structure of DNA for electrophoresis. Electrophoresis was then performed at 200 mA for 20 min in electrophoresis buffer at room temperature. The slides were then rinsed twice with distilled water and stained with ethidium bromide (2 μg/ml). All of the above procedures were carried out in the dark to avoid additional DNA damage. The comets were viewed using a Nikon 90i fluorescence microscope, and images of 100 comets were collected for each subject using a digital imaging system. Cells that overlapped were not counted. All the comet images were analyzed using Comet Assay Software Project (CASP, Wroclaw University, Poland) [18], and the DNA percentage in the comet tail (TDNA %), tail moment (TM), and Olive tail moment (OTM) were recorded to describe DNA damage to the lymphocytes. All the experiments were repeated twice.
Statistical analysis
Statistical analysis was performed using SPSS 12.0 for Windows. Data obtained were expressed as the mean±SD. The data were analyzed by one-way ANOVA to confirm the variability of data and validity of results. A Student's t-test was used for statistical comparisons between the groups. The significance levels were set at P < 0.05, P < 0.01 and P < 0.001. The LD50/30 values and the DRF were determined by regression analysis.
RESULTS
Structural features of WPT-A
The alditol acetate composition of WPT-A was determined by GC, which indicated that WPT-A consisted of galactose, mannose and glucose in a 9.7:7.2:1 molar ratio. 1,3-galactose, 1,6-galactose and 1,3,4-mannose were found at the backbone, and 1,4-glucose was found at the branch.
The polysaccharide was eluted as a single symmetric peak on UltrahydrogelTM 2000, UltrahydrogelTM 500 and UltrahydrogelTM Linear serial columns, corresponding to a molecular weight of 45 000 daltons, which suggested WPT-A was homogeneous.
MTD
The acute toxicity of WPT-A was studied in terms of percent survival, change in behavior, alteration in neuromuscular coordination, and respiratory disorders for 14 d post-administration of a single dose of different concentrations of WPT-A. Single doses of WPT-A up to 2500 mg/kg b.w. were well tolerated by the mice, and no deaths were noted.
Survival, LD50/30 and DRF analysis
The experimental data showed that mice treated with irradiation alone displayed survival rates of 60%, 50%, 20%, 0% and 0% when irradiated with 7.0, 7.5, 8.5, 10 and 10.5-Gy, respectively. Administration of WPT-A after various doses of TBI enhanced the survival rates, yielding rates of 90%, 80%, 60%, 20% and 10% at 7.0, 7.5, 8.5, 10 and 10.5-Gy, respectively. Regression analysis of the radiation survival data produced an LD50/30 value of 8.8 for the radiation plus WPT-A group and 7.4 for the radiation-only group (Fig. 1). The calculated DRF was 1.2. These results indicated that WPT-A administered before TBI enhanced the survival of mice.
Fig 1.
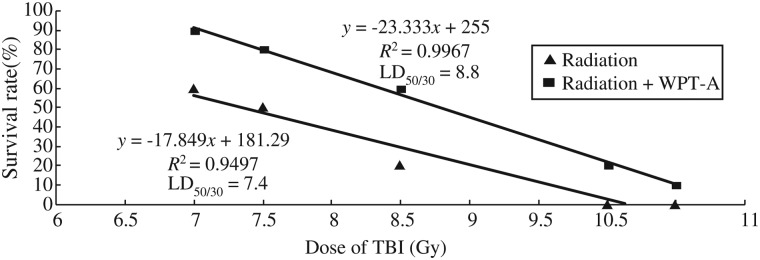
The survival curves for the radiation-only group and the radiation plus WPT-A group. Mice (n = 10 mice/group) were administered WPT-A at a dose of 80 mg/kg once a day for three consecutive days, and then on the third day they were irradiated with gamma rays at a dose of 7, 7.5, 8.5, 10 or 10.5-Gy 30 min after the administration of WPT-A. The mice were monitored daily for 30 d to determine survival rates.
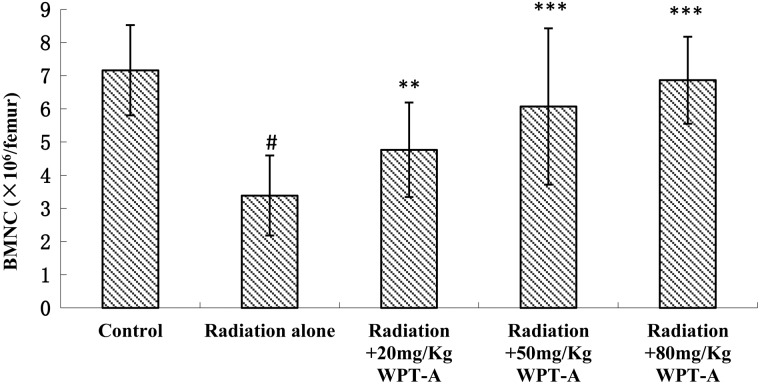
BMNC counts
As shown in Fig. 2, the number of nucleated cells in bone marrow in the radiation-only group decreased markedly compared with the control group. The treatment groups, which received 20, 50 and 80 mg/kg b.w. doses of WPT-A prior to radiation, had significantly higher BMNC counts compared with the radiation-only group.
Fig 2.
Effects of pre-irradiation WPT-A on BMNC counts in mice. Femoral bones were collected, and the bone marrow was flushed out with 3% acetic acid. The number of BMNCs was counted using a microscope. Results are presented as means ± SD (n = 24). **P < 0.01, ***P < 0.001 compared with the radiation-only group. #P < 0.001 compared with the control group. The Student's t-test was used for statistical comparisons between the groups. The P values of the different treatment groups were as follows: Radiation and 20 mg/kg WPT-A, 0.0014; Radiation and 50 mg/kg WPT-A, 8.744 × 10−5; Radiation and 80 mg/kg WPT-A, 1.765 × 10−9.
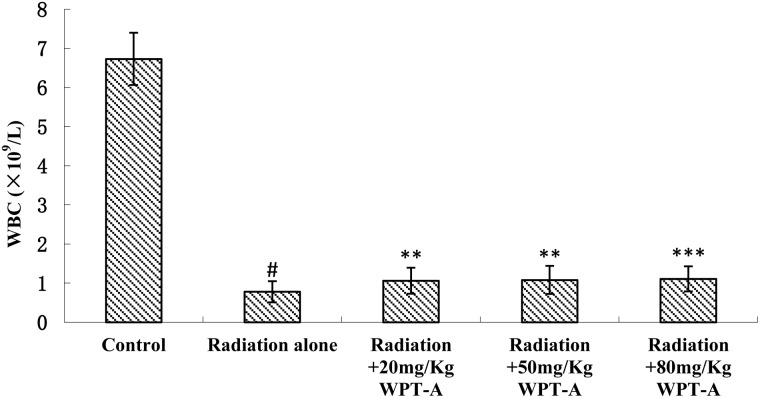
WBC counts
The WBC counts decreased sharply in the radiation-only group compared with the control group. The treatment groups, which received WPT-A at 20, 50 and 80 mg/kg b.w. prior to radiation, showed a marked increase in WBC counts in comparison with the radiation-only group (Fig. 3). The difference was statistically significant.
Fig 3.
Effects of pre-irradiation WPT-A on WBC counts in mice. Blood was collected from the caudal vein into heparinized tubes on the ninth day post-irradiation, and the number of WBC was detected using a Hematology Analyzer. The results are presented as means±SD (n = 24). **P < 0.01, ***P < 0.001 compared with the radiation-only group. #P < 0.001 compared with the control group. The Student's t-test was used for statistical comparisons between the groups. The P values of the different treatment groups were as follows: Radiation and 20 mg/kg WPT-A, 0.0038; Radiation and 50 mg/kg WPT-A, 0.0034; Radiation and 80 mg/kg WPT-A, 0.0008.
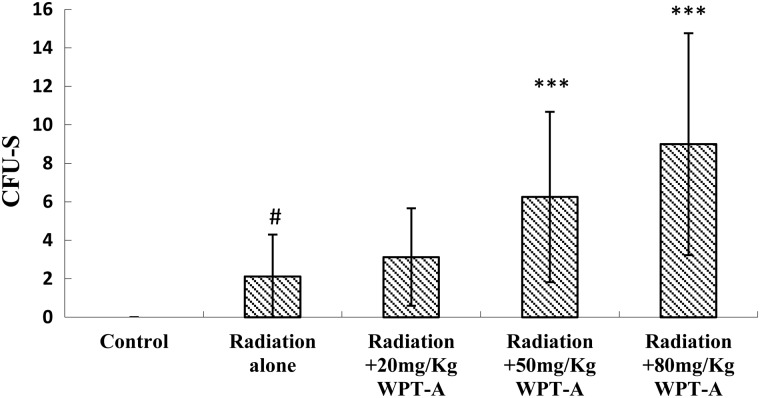
Endogenous CFU-S
CFU-Ss in the radiation-only group were observed. The treatment groups that received 20, 50 and 80 mg/kg b.w. of WPT-A prior to irradiation had more CFU-Ss compared with the radiation-only group (Fig. 4). The treatment groups administered WPT-A at 50 and 80 mg/kg b.w. demonstrated a significant change in CFU-Ss compared with the radiation-only group. The difference was statistically significant.
Fig 4.
Effects of pre-irradiation WPT-A on CFU-Ss in mice. In addition, the number of CFU-Ss per spleen was counted. Results are presented as means±SD (n = 24). ***P < 0.001 compared with the radiation-only group. #P < 0.001 compared with the control group. The Student's t-test was used for statistical comparisons between the groups. The P values of different treatment groups were as follows: Radiation and 20 mg/kg WPT-A, 0.1725; Radiation and 50 mg/kg WPT-A, 0.0004; Radiation and 80 mg/kg WPT-A, 1.469 × 10−5.
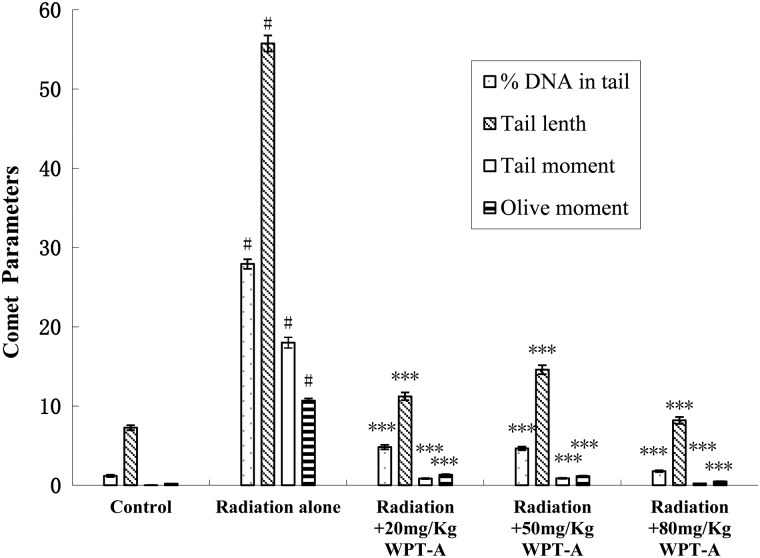
Radioprotective effects on DNA damage
The effects of WPT-A against radiation-induced DNA damage in peripheral blood leukocytes were investigated using SCGE. Nucleoids of the cells in the control group appeared circular, whereas those in the radiation group looked like comets, with fluorescence intensity diminishing from the head to the tail, indicating DNA damage. Figure 5 shows the frequency distribution histograms of TL, TM, OTM and percentage of DNA in the tail of leukocytes isolated from the animals. Pre-irradiation WPT-A treatment in mice significantly decreased TL, TM, OTM and percentage of DNA in the tail compared with those observed in the radiation groups.
Fig 5.
Effects of pre-irradiation WPT-A on γ-radiation-induced DNA damage in mice. The alkali comet assay was adopted to determine the effects of WPT-A on γ-radiation-induced DNA damage in mouse peripheral blood leukocytes. The bar graph represents means ± SD obtained by analyzing 100 cells/mouse (n = 24). ***P < 0.001 compared with the radiation-only group. #P < 0.001 compared with the control group.
DISCUSSION
In the present study, we demonstrated for the first time that lichen polysaccharides can provide protection against radiation toxicity in a murine model. The study revealed that pre-irradiation administration of WPT-A at a dose of 80 mg/kg b.w. enhanced survival rates. Regression analysis of the radiation survival data resulted in an LD50/30 value of 8.8 for the radiation plus WPT-A group, and 7.4 for the radiation-only group. The calculated DRF was 1.2. In our preliminary experiment, the same results were obtained with eight mice in each group.
Most synthetic and herbal radioprotective agents show their maximum radioprotective effect at a dose approaching the MTD [19–22]. A single dose of WPT-A up to 2500 mg/kg b.w. was tolerated well by mice and provided significant radioprotection at a dose of 80 mg/kg b.w. Therefore, WPT-A may have potential as an agent for human application due to its low toxicity.
It is well known that the hematopoietic system is a critically important system for radiation survival, but is susceptible to radiation-induced damage. Radiation survival is the result of several factors including the prevention of damage through the inhibition of free-radical generation, efficient scavenging of free radicals [23], repair of DNA [24, 25], repair of membrane and other damaged target molecules [26], and the replenishment of severely damaged or dead cells. The recruitment of cells to substitute for damaged cells could add to survival. The present study demonstrated that WPT-A pretreatment elevated the number of radiation-induced endogenous CFU-Ss and increased BMNCs when compared with the radiation-only group. The CFU-Ss exhibited many characteristics of primitive hematopoietic stem cells, such as extensive proliferative capacity, the capacity for self-renewal, and the capability of generating multiple hematopoietic lineages that prevented radiation damage in mice. The enhancement of CFU-Ss indicated a role of WPT-A in protecting the primitive stem cells, such as stimulating the generation of primitive stem cells and reconstitution of hematopoiesis after TBI. As hematopoiesis is stimulated in the bone marrow, BMNCs and WBCs decreased because irradiation increased. These effects of WPT-A on enhancement of myelopoiesis and alleviation of DNA damage will be extremely beneficial for radiation survival of the hematopoietic system.
Although our data revealed that WPT-A treatment ameliorated ionizing irradiation-induced damage in mice, the mechanisms by which WPT-A facilitated this effect are unknown. Early experiments reported that polysaccharides isolated from the lichen Thamnolia vermicularis var. subuliformis significantly stimulated TNF-α secretion in rat peritoneal macrophages and exhibited immunomodulatory activity [27]. The structural characteristics of WPT-A are similar to those of the polysaccharides isolated from Thamnolia vermicularis var. subuliformis. TNF-α has been shown to have a role in radioprotection. Anti-TNF-α antibody reduced the survival of irradiated mice, indicating that the natural level of TNF-α in the body contributes to radioresistance. TNF-α can protect mice from doses of radiation that would be fatal to untreated animals, if given before irradiation [28–31]. Furthermore, it was suggested that TNF-α might exert its radioprotective effects on a pool of primitive multipotential hematopoietic cells [30]. The administration of a high dose of TNF-α 1–3 h after irradiation significantly protected mice [32]. It was also shown to induce mRNA of MnSOD by TNF-α [33].
The precise mechanisms of the radioprotective action of WPT-A remain uncertain. It is suggested that the principal mechanism of radioprotection by WPT-A is enhanced restoration of the innate immune system. Therefore, it is speculated that radioprotection by WPT-A when administrated before irradiation is mediated by the simultaneous secretion of TNF-α. It is known that a large amount of exogenous TNF-α has radioprotective effects and is toxic, whereas endogenous TNF-α by simultaneous immune system secretion exerted its effects modulated by the complicated immune system. The relationship between the amount of endogenous TNF-α and its radioprotective effect and toxicity is unclear. WTF-A stimulated TNF-α secretion remains to be confirmed.
Ionizing radiation can directly induce DNA damage or indirectly induce DNA damage by producing reactive oxygen species such as superoxide anions and hydrogen peroxide. Antioxidants ameliorate DNA damage and exert radioprotection effects [23]. Behera reported that polysaccharide from the tissue culture of some lichens showed antioxidant effects [34]. Thus, we suggest that decreased radiation-induced DNA damage in peripheral leukocytes by WPT-A may be attributed to its antioxidant effects. DNA damage and repair involve many factors. Oxidative stress is one cause of DNA damage. The mechanism of the radioprotective action of WPT-A on DNA damage demonstrated in the comet parameters may also be related to other mechanisms and requires further study.
Our results demonstrated that the polysaccharide from Parmelia tinctorum ameliorated ionizing irradiation-induced damage in mice and possesses the potential properties of an ideal radioprotector.
FUNDING
This work was supported by National Natural Science Foundation of China (grant number 81273005) and the Tianjin Municipal Science and Technology Commission (grant number 12JCYBJC16300). Funding to pay the Open Access publication charges for this article was provided by National Natural Science Foundation of China (grant number 81273005).
REFERENCES
- 1.Maurya DK, Devasagayam TP, Nair CK. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J Exp Biol. 2006;44:93–114. [PubMed] [Google Scholar]
- 2.Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Semin Radiat Oncol. 2003;13:62–72. doi: 10.1053/srao.2003.50006. [DOI] [PubMed] [Google Scholar]
- 3.Seed T, Kumar S, Whitnall M, et al. New strategies for the prevention of radiation injury. J Radiat Res. 2002;43:S239–44. doi: 10.1269/jrr.43.s239. [DOI] [PubMed] [Google Scholar]
- 4.Seed TM. Radiation protectants: current status and future prospects. Health Phys. 2005;89:531–45. doi: 10.1097/01.hp.0000175153.19745.25. [DOI] [PubMed] [Google Scholar]
- 5.Elin S. Olafsdottir, Kristín Ingólfsdottir. Polysaccharides from lichens: structural characteristics and biological activity. Planta Med. 2001;67:199–208. doi: 10.1055/s-2001-12012. [DOI] [PubMed] [Google Scholar]
- 6.Leao AM, Buchi DF, Iacomini M, et al. Cytotoxic effect against Hela cells of polysaccharides from the lichen Ramalina celastri. J Submicrosc Cytol Pathol. 1997;29:503–9. [PubMed] [Google Scholar]
- 7.Olafsdottir ES, Omarsdottir S, Paulsen BS, et al. Rhamnopyranosylgalactofuranan, a new immunologically active polysaccharide from Thamnolia subuliformis. Phytomedicine. 1999;6:273–9. doi: 10.1016/S0944-7113(99)80020-8. [DOI] [PubMed] [Google Scholar]
- 8.Stübler D, Buchenauer H. Antiviral activity of the glucan lichenan (poly-β-(1-3, 1-4)-D-anhydroglucose). I. Biological activity in tobacco plants. J Phytopathol. 1996;144:37–43. [Google Scholar]
- 9.Stübler D, Buchenauer H. Antiviral activity of the glucan lichenan (poly-β-(1-3, 1-4)-D-anhydroglucose). II. Studies on the mode of action. J Phytopathol. 1996;144:45–52. [Google Scholar]
- 10.Smriga M, Chen J, Zhang JT, et al. Isolichenan, an α-glucan isolated from the lichen Cetrariella islandica, repairs impaired learning behaviour and facilitates hippocampal synaptic plasticity. Proc Jpn Acad B Phys Biol Sci. 1999;75:219–23. [Google Scholar]
- 11.Smriga M, Saito H. Effect of selected thallophytic glucans on learning behaviour and short-term potentiation. Phytother Res. 2000;14:153–5. doi: 10.1002/(sici)1099-1573(200005)14:3<153::aid-ptr666>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. [Google Scholar]
- 13.Waterborg JH, Matthews HR. The Lowry Method for protein quantitation. Methods Mol Biol. 1994;32:1–4. doi: 10.1385/0-89603-268-X:1. [DOI] [PubMed] [Google Scholar]
- 14.Filisetti-Cozzi TM, Carpita NC. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991;197:157–62. doi: 10.1016/0003-2697(91)90372-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Lin J, Cui J-G, et al. Radioprotection of bone marrow hematopoiesis by CpG-oligodeoxynucleotides administered to mice after total-body irradiation. J Radiat Res. 2012;52:828–33. doi: 10.1269/jrr.10098. [DOI] [PubMed] [Google Scholar]
- 16.Till JE, McCulloch EA. A direct measurement of radiation sensitivity of normal bone marrow. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 17.Banath JP, Fushiki M, Olive PL. Rejoining of DNA single and double-strand breaks in human white blood cells exposed to ionizing radiation. Int J Radiat Biol. 1998;73:649–60. doi: 10.1080/095530098141906. [DOI] [PubMed] [Google Scholar]
- 18.Konca K, Lankoff A, Banasik A, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534:15–20. doi: 10.1016/s1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 19.Uma Devi P, Ganasoundari A, Rao BS, et al. In vivo radioprotection by ocimum flavonoids: survival of mice. Radiat Res. 1999;151:74–8. [PubMed] [Google Scholar]
- 20.Zois CE, Giatromanolaki A, Sivridis E, et al. Narrow amifostine dose windows define radioprotection outcome, following fractionated whole-body irradiation of mice. In Vivo. 2011;25:191–6. [PubMed] [Google Scholar]
- 21.Goel HC, Ganguly SK, Prasad J, et al. Radioprotective effects of diltiazem on cytogenetic damage and survival in gamma ray exposed mice. Indian J Exp Biol. 1996;34:1194–200. [PubMed] [Google Scholar]
- 22.Riklis E, Kol R, Marko R. Trends and developments in radioprotection: the effect of nicotinamide on DNA repair. Int J Radiat Biol. 1990;57:699–708. doi: 10.1080/09553009014550871. [DOI] [PubMed] [Google Scholar]
- 23.Weiss JF, Landauer MR. Radioprotection by anti-oxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 24.Dittmann K, Mayer C, Wanner G, et al. The radioprotector O-phospho-tyrosine stimulates DNA-repair via epidermal growth factor receptor and DNA-dependent kinase phosphorylation. Radiother Oncol. 2007;84:328–34. doi: 10.1016/j.radonc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekharan DK, Kagiya TV, Nair CKK. Radiation protection by 6-palmitoyl ascorbic acid-2-glucoside: studies on DNA damage in vitro, ex vivo, in vivo and oxidative stress in vivo. J Radiat Res. 2009;50:203–12. doi: 10.1269/jrr.08090. [DOI] [PubMed] [Google Scholar]
- 26.Belli M, Sapora O, Tabocchini MA. Molecular targets in cellular response to ionizing radiation and implications in space radiation protection. J Radiat Res. 2002;43:S13–9. doi: 10.1269/jrr.43.s13. [DOI] [PubMed] [Google Scholar]
- 27.Omarsdottir S, Freysdottir J, Olafsdottir ES. Immunomodulating polysaccharides from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine. 2007;14:179–84. doi: 10.1016/j.phymed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Dalmau SR, Freitas CS, Tabak DG. Interleukin-1 and tumor necrosis factor-α as radio- and chemoprotectors of bone marrow. Bone Marrow Transplant. 1993;12:551–63. [PubMed] [Google Scholar]
- 29.Neta R. Cytokines in radioprotection and therapy of radiation injury. Biotherapy. 1988;1:41–5. doi: 10.1007/BF02170134. [DOI] [PubMed] [Google Scholar]
- 30.Slordal L, Muench MO, Warren DJ, et al. Radioprotection by murine and human tumor-necrosis factor: dose dependent effects on hematopoiesis in the mouse. Eur J Haematol. 1989;43:428–34. doi: 10.1111/j.1600-0609.1989.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 31.Urbaschek R, Mannel DN, Urbaschek B. Tumor necrosis factor induced stimulation of granulopoiesis and radioprotection. Lymphokine Res. 1987;6:179–86. [PubMed] [Google Scholar]
- 32.Neta R, Oppenheim JJ, Douches SD. Interdependence of the radioprotective effects of human recombinant interleukin-1α, tumor necrosis factor-α, granulocyte colony-stimulating factor, and murine recombinant granulocyte–macrophage colony-stimulating factor. J Immunol. 1988;140:108–11. [PubMed] [Google Scholar]
- 33.Wong GH, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–4. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 34.Behera BC, Verma N, Sonone A, et al. Tissue culture of some lichens and screening of their antioxidant, antityrosinase and antibacterial properties. Phytother Res. 2007;21:1159–70. doi: 10.1002/ptr.2228. [DOI] [PubMed] [Google Scholar]