Abstract
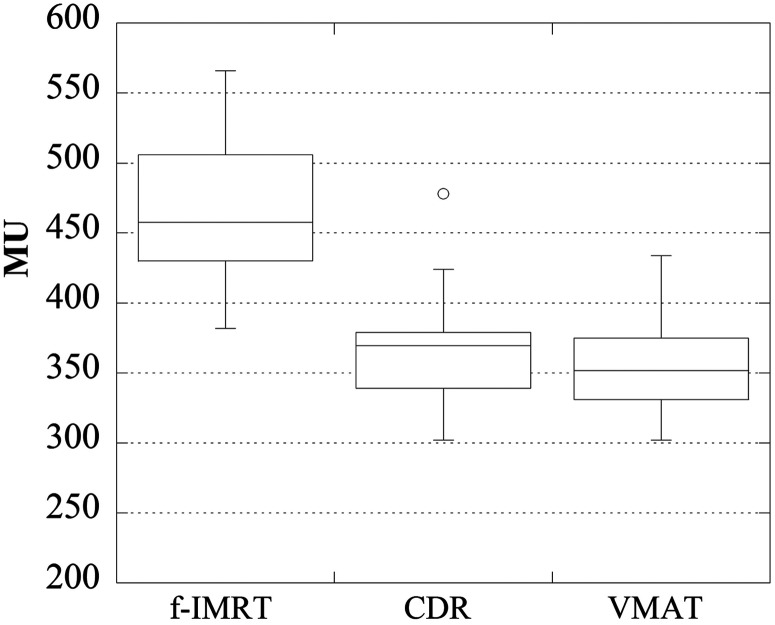
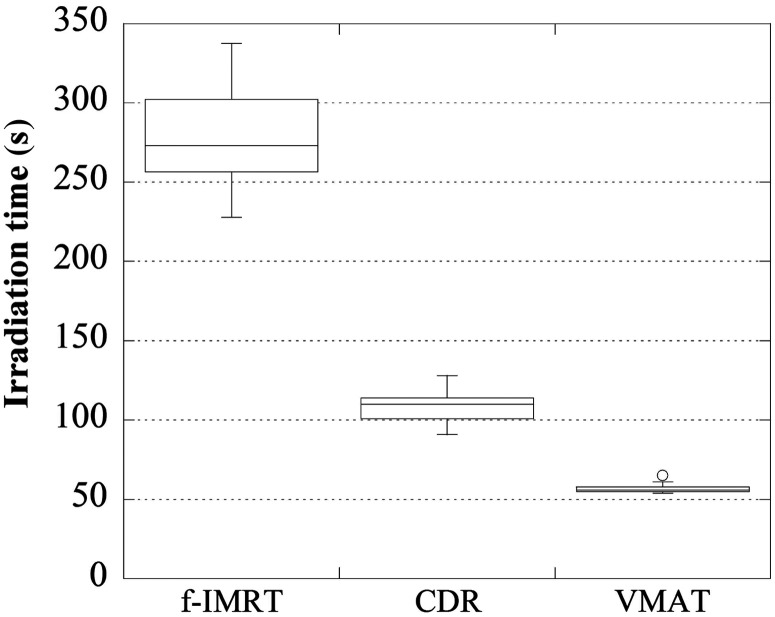
Volumetric-modulated arc therapy (VMAT) is a widespread intensity-modulated radiation therapy (IMRT) method, however, VMAT requires adaptation of the radiation treatment planning system (RTPS) and linear accelerator (linac); these upgrades are quite expensive. The Smart Arc of Pinnacle3 (Philips), which is the software used in VMAT calculations, can select constant dose rate (CDR) mode. This approach has a low initial cost because the linac upgrade is not required. The objective of this study was to clarify the utility of CDR mode for prostate IMRT. Pinnacle3 and Clinac 21EX linac (Varian, 10 MV X-rays) were used for planning. The plans were created for 28 patients using a fixed multi-field IMRT (f-IMRT), VMAT and CDR techniques. The dose distribution results were classified into three groups: optimal, suboptimal and reject. For the f-IMRT, VMAT and CDR results, 25, 26 and 21 patients were classified as ‘optimal’, respectively. Our results show a significant reduction in the achievement rate of ‘optimal’ for a CDR when the bladder volume is <100 cm3. The total numbers of monitoring units (MUs) (average ± 1σ) were 469 ± 53, 357 ± 35 and 365 ± 33; the average optimization times were ∼50 min, 2 h and 2 h 40 min, and the irradiation times were ∼280 s, 60 s and 110 s, respectively. CDR can reduce the total MUs and irradiation time compared with f-IMRT, and CDR has a lower initial cost compared with VMAT. Thus, for institutions that do not currently perform VMAT, CDR is a useful option. Additionally, in the context of patient identification, bladder volume may be useful.
Keywords: constant dose rate, intensity-modulated radiation therapy, prostate cancer, Smart Arc CDR, volumetric-modulated arc therapy
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is a technology that delivers radiation more precisely to the tumor than earlier techniques, sparing the surrounding normal tissue from unnecessary exposure [1]. However, the required irradiation time is longer and the total number of monitoring units (MUs) is greater for fixed multi-field IMRT (f-IMRT) compared with conventional methods [1]. The increased irradiation time may increase the intrafractional error and the patient restriction time; additionally, an increase in the total number of MUs may cause problems with radiation shielding [2].
More recently, volumetric-modulated arc therapy (VMAT), a widespread rotational IMRT method using a dynamic gantry speed and multileaf collimator (MLC) field shape, has gained considerable attention [3]. The dose–volume histograms (DVHs) of VMAT and that of f-IMRT are equivalent; however, the total number of MUs and required irradiation time are less for VMAT than for f-IMRT [4, 5]. To use the VMAT approach, the hardware and software systems of the radiation treatment planning system (RTPS), as well as the linear accelerator (linac), must be upgraded; these upgrades are costly. Thus, many institutions do not have the means to perform VMAT and must continue with the problems specified above.
The Smart Arc system of Pinnacle3 (RTPS by Philips) software used for rotational IMRT calculations, can select VMAT mode or constant-dose-rate (CDR) mode. Details of the SmartArc planning algorithm have been described by Bzdusek et al. [6]. CDR mode is a rotational IMRT technique that uses a fixed gantry rotational speed and a fixed dose rate. Compared with f-IMRT, the potential advantages of CDR include a large reduction in irradiation time and in the total number of MUs [5]. Moreover, the system can be used by institutions that cannot perform VMAT (because the linac upgrade is not required for CDR mode). Therefore, such institutions can reduce both the required irradiation time and the total number of MUs to make the initial cost low. For institutions that do not currently perform VMAT, CDR may be a useful option. The objective of this study was to clarify the utility of Smart Arc CDR for prostate IMRT. We expected that achieving the defined dose limit with CDR may be more difficult than with VMAT. Of the two approaches, VMAT has more parameters that can be adjusted for optimization. The second objective of this study was to identify the subgroup of patients in whom CDR cannot achieve the optimal dose distribution.
MATERIALS AND METHODS
Pinnacle3 version 9.2 was used as the RTPS in this study. The linac model (Clinac 21EX, Varian; X-ray energy: 10 MV) was used for planning. The plans were created using f-IMRT with the direct machine parameter optimization [7], VMAT and CDR techniques for 28 patients (average age: 75 years; range: 59–88 years) based on computed tomography (CT) images of their prostate cancer. Optimization was carried out using an inverse planning technique. Details of these methods are provided below.
Planning CT
A LightSpeed RT16 (GE) was used for the planning CT. Body-fix (Medical Intelligence) and supine set-ups were used to keep the patients stationary. CT images were acquired in 2.5-mm slice thicknesses. The procedure to acquire the CT images is described below.
Patients ingested 350-mg magnesium oxide tablets 2 d before CT-image acquisition at a dose rate of three tablets per day.
CT images were acquired after 10:00 AM.
Patients were instructed to drink a fixed volume of water 60–90 min before CT image acquisition; urination was prohibited to enable acquisition of the CT image.
The CT image was examined for gas in the rectum by the radiation oncologist after image acquisition. If needed, additional CT images were acquired based on the recommendation that the diameter of the rectum, transversely at the base, was > 4 cm [8].
Contours
The patients were classified into the following four groups according to the National Comprehensive Cancer Network (NCCN) [9] criteria: ‘low risk’ (T1–T2a, Gleason score 2–6 and PSA < 10 ng/ml), ‘intermediate risk’ (T2b–T2c or Gleason score 7 or PSA 10–20 ng/ml), ‘high risk’ (T3a or Gleason score 8–10 or PSA > 20 ng/ml), and ‘very high risk’ (T3b–T4). The target volume was determined based on the risk group. There were 3 low risk patients, 16 intermediate risk patients, 9 high risk patients, and no very high risk patients in this study.
Clinical target volume
The clinical target volume (CTV) for the ‘low risk group’ was the prostate only, for the ‘intermediate risk group’ was the prostate + seminal vesicle surrounding 1 cm of the prostate, for the ‘high risk group’ was the prostate + seminal vesicle surrounding 2 cm of the prostate, with prostate+5-mm margin (T3a), and for the ‘very high risk group’ the prostate + seminal vesicle (T3b), prostate + 5-mm margin + seminal vesicle surrounding 2 cm of the prostate (T4).
Planning target volume
The planning target volume (PTV) was defined by the CTV + 10-mm margin (rectum side: 6 mm).
Organ at risk
The rectum (from the ischial tuberosities to the rectosigmoid flexure), the whole bladder, the small intestine surrounding 1.5 cm of the PTV, the sigmoid colon surrounding 1.5 cm of the PTV, and the femoral heads and body were defined as the organs at risk (OARs).
Optimization and plan evaluation
Table 1 shows the characteristics and planning conditions of f-IMRT, CDR and VMAT. f-IMRT was planned using seven beams; VMAT and CDR were planned using a single arc. The prescribed dose to 95% (D95) of the PTV was 74 Gy in 37 fractions. The dose calculation grid size was set to 2 mm for all examples in this study. The isocenter was set to the PTV center. Adaptive Convolve [10] with heterogeneous correction was used for the dose calculation algorithm.
Table 1.
Characteristics and planning conditions of f-IMRT, CDR and VMAT
| f-IMRT | VMAT | CDR | |
|---|---|---|---|
| IMRT technique | Static MLC | Dynamic MLC (Rotational) | Dynamic MLC (Rotational) |
| Gantry rotation speed | Variable | Fixed | |
| Dose rate | Fixed | Variable | Fixed |
| Gantry angle (degrees) | 0, 50, 100, 155, 205, 260, 310 | 230–130 (CW) | 230–130 (CW) |
| Calculation pitch (degrees) | 4 | 4 | |
| Devices that must be upgraded (on the assumption that f-IMRT can be carried out) | RTP and Linac | RTP |
Optimized plans were evaluated with respect to dose distribution, total number of MUs, optimization time and irradiation time. The dose distributions were evaluated with respect to the DVH and the dose limit defined for this study. The dose distribution results were classified into three groups: optimal, suboptimal and reject, based on the achievement level of the dose limit. Table 2 shows the dose limit used. The optimization time corresponds to the time taken to optimize the plan with the RTPS. As a consequence of the difficulties presented by some patients in achieving the appropriate dose limit using CDR, the chi-square test and t-test were used for verification of the correlation between the level of achievement of the dose limit and the organ volume obtained with planning CT. Positioning of the small intestine and sigmoid colon close to the PTV might make achievement of the dose limit problematic. Additionally, a bladder volume of 100–150 cm3 is more suitable for planning CT acquisition and irradiation [11]. Therefore, a bladder volume of 100 cm3 was used as an index of verification of the level of achievement of the dose limit in this study. The chi-square test was carried out to verify the correlations between the achievement rate of the dose limit and the bladder volume and positions of the small intestine and sigmoid colon. SPSS version 17 (IBM) was used for statistical analysis.
Table 2.
Dose limits used in this study
| Optimal | Suboptimal | ||
|---|---|---|---|
| PTV | D95 (D94–D96) | 74 Gy | |
| Mean | <77.7 Gy | <79.2 Gy | |
| Maximum | <81.4 Gy | <85.1 Gy | |
| D98 | >70.3 Gy | ||
| V75 | <5% | < 10% | |
| V70 | <20% | <25% | |
| Rectum | V60 | <40% | <45% |
| V50 | <50% | <50% | |
| V40 | <65% | ||
| Maximum | <78 Gy | <82 Gy | |
| Bladder | V70 | <30% | <35% |
| V50 | <50% | <60% | |
| Femoral heads | Maximum | <50 Gy | D5 < 50 Gy |
| Small intestine | Maximum | <60 Gy | |
| Sigmoid colon | Maximum | <65 Gy | |
| Body | Maximum | <81.4 Gy | <85.1 Gy |
RESULTS
DVH and the achievement rate of the dose limit
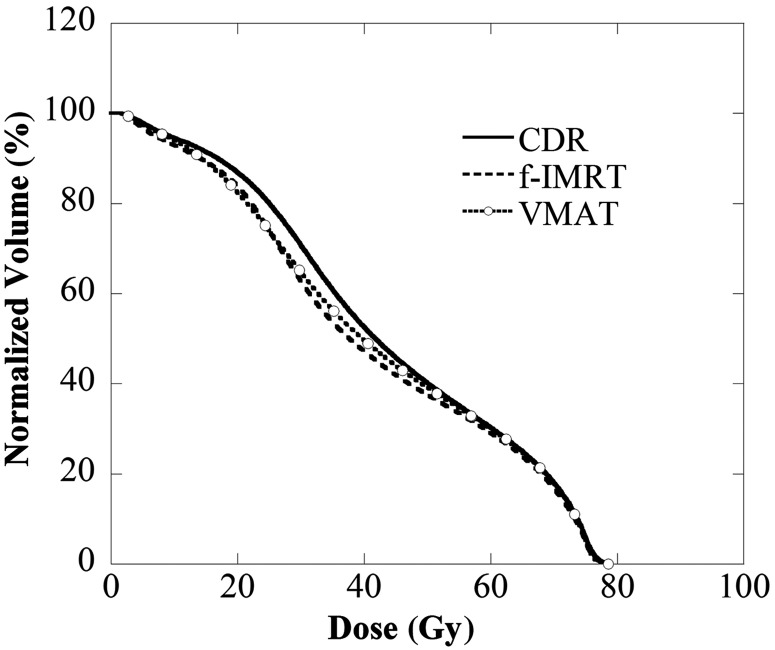
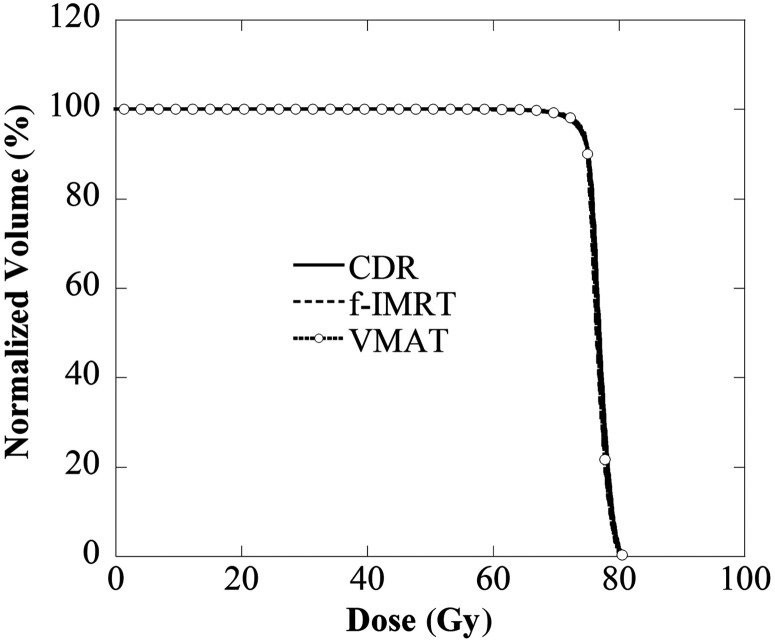
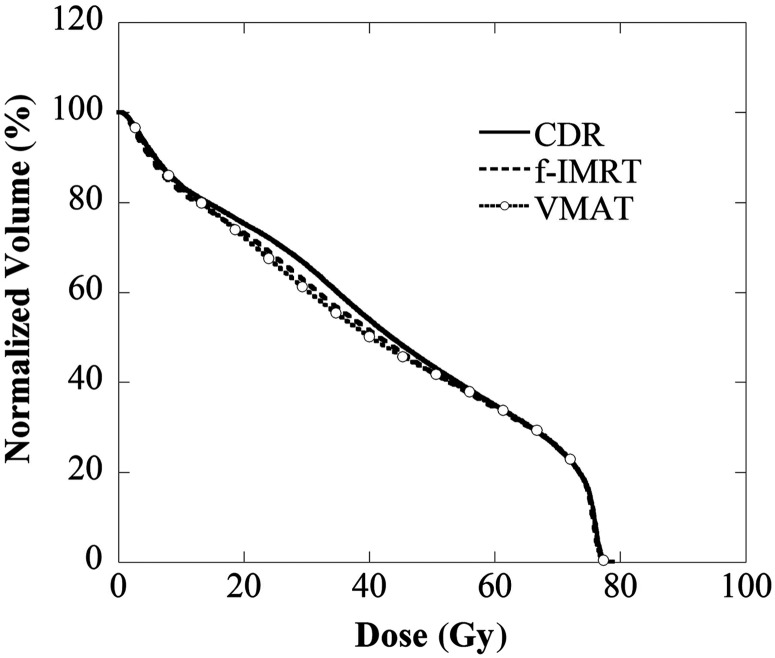
Figures 1–3 display the average DVH for 28 patients of the PTV, rectum and bladder. Table 3 lists the dose indexes of the PTV, rectum and bladder, which are the average of all cases. The DVHs of the PTV for all of the methods were in good agreement, and D2, D50 and D95 were within 0.5 Gy. D2 of the rectum and bladder for all methods were within 0.5 Gy, and the volume receiving 50–70 Gy (V50–V70) were within 3%. The V20–V40 using the CDR approach was ∼5% higher than with the f-IMRT and VMAT methods. The achievement level of the dose limit is evident for the f-IMRT, VMAT and CDR techniques. There were no ‘rejects’; 25 (89%), 26 (93%) and 21 (75%) patients were classified as ‘optimal’ and the remaining patients were classified as ‘suboptimal’ (f-IMRT, VMAT and CDR, respectively). The optimal CDR achievement rate was slightly lower than those of the other two methods. Tables 4 and 5 reveal the correlation between the achievement level of the dose limit and the risk group or organ volume. Our results indicate a significant reduction in the dose limit achievement rate for the CDR method when the bladder volume was <100 cm3.
Fig. 2.
Rectum DVH using the CDR, f-IMRT and VMAT techniques. The V20–V40 of the rectum determined by CDR was higher than that obtained with the other two methods.
Fig. 1.
Comparison of the PTV DVH of the CDR, f-IMRT and VMAT techniques as a function of dose. The results were in good agreement.
Fig. 3.
Bladder DVH using the CDR, f-IMRT and VMAT techniques. The V20–V40 of the bladder determined using CDR was higher than that obtained with the other two methods.
Table 3.
Dose indexes of the PTV, rectum and bladder (average ± 1σ of all cases)
| Dose index | CDR | f-IMRT | VMAT | |
|---|---|---|---|---|
| PTV | D95 (Gy) | 74.10 ± 0.29 | 74.30 ± 0.19 | 74.20 ± 0.17 |
| D50 (Gy) | 76.85 ± 0.45 | 76.45 ± 0.45 | 76.55 ± 0.60 | |
| D2 (Gy) | 79.95 ± 0.51 | 79.70 ± 0.48 | 79.80 ± 0.98 | |
| Rectum | D2 (Gy) | 76.40 ± 0.49 | 76.05 ± 0.67 | 75.95 ± 0.44 |
| V70 (%) | 18.0 ± 2.3 | 16.8 ± 2.7 | 17.9 ± 1.9 | |
| V60 (%) | 30.3 ± 4.4 | 29.1 ± 4.1 | 30.0 ± 4.5 | |
| V50 (%) | 40.2 ± 5.8 | 37.6 ± 5.6 | 39.2 ± 6.2 | |
| Bladder | D2 (Gy) | 76.85 ± 0.47 | 76.60 ± 0.63 | 76.65 ± 0.41 |
| V70 (%) | 25.4 ± 4.8 | 25.5 ± 4.9 | 25.6 ± 4.8 | |
| V60 (%) | 35.0 ± 5.9 | 34.3 ± 6.7 | 34.8 ± 6.0 | |
| V50 (%) | 43.6 ± 7.3 | 42.2 ± 8.1 | 42.2 ± 7.3 |
Table 4.
Results of the t-test for verification of the correlation between the level of achievement of the dose limit and organ volume obtained with planning CT
| Variable | Average (Range) | P |
|---|---|---|
| Prostate volume | 30.6 (6.3–71.9) cm3 | 0.776 |
| PTV volume | 142.9 (71.6–212.1) cm3 | 0.643 |
| Rectum volume | 51.7 (33.4–107.9) cm3 | 0.719 |
| Bladder volume | 159.6 (69.6–386.4) cm3 | 0.148 |
Table 5.
Results of the chi-square test for verification of the correlations between the achievement rate of the dose limit and risk group, the bladder volume and the positions of the small intestine and sigmoid colon
| Variable | Optimal | P | |
|---|---|---|---|
| Risk group | Low (3) Intermediate (16) High (9) |
1/3 14/16 6/9 |
0.108 |
| Bladder volume | <=100 cm3 (9) >100 cm3 (19) |
4/9 17/19 |
0.020 |
| Sigmoid colon surrounding 1.5 cm of PTV? | Yes (19) No (9) |
13/19 8/9 |
0.371 |
| Small intestine surrounding 1.5 cm of PTV? | Yes (9) No (19) |
7/9 14/19 |
1.000 |
Total MU, optimization time and irradiation time
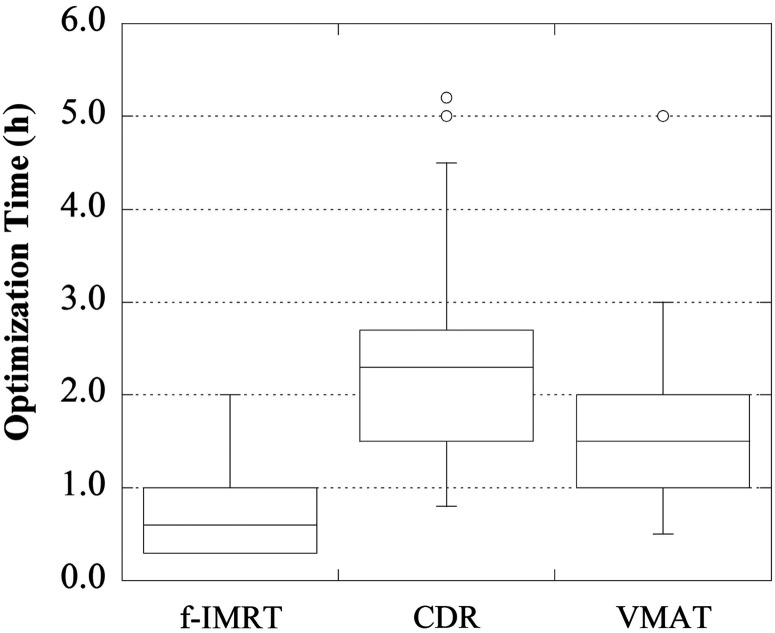
Figures 4–6 display the total number of MUs, the optimization time, and the irradiation time, respectively. For the f-IMRT, VMAT and CDR methods, the total numbers of MUs (average ± 1σ) were 469 ± 53, 357 ± 35 and 365 ± 33, respectively; the optimization times were ∼50 min, 2 h and 2 h 40 min, respectively; and the irradiation times were ∼280 s, 60 s and 110 s, respectively. For f-IMRT, the beam loading time for each field and the time between each segment could not be calculated accurately because this method used a step-and-shoot approach. Therefore, the irradiation times for f-IMRT were acquired using a stopwatch.
Fig. 5.
Optimization times of the f-IMRT, CDR and VMAT techniques. The optimization time for CDR was the longest.
Fig. 4.
Total numbers of MUs determined using the f-IMRT, CDR and VMAT techniques. The f-IMRT technique exhibited the largest total number of MUs.
Fig. 6.
Irradiation times for the f-IMRT, CDR and VMAT techniques. f-IMRT required the longest irradiation time.
DISCUSSION
As our results indicate, the optimal CDR achievement rate was slightly lower than that of the other two methods. It has been reported that the DVHs of VMAT and f-IMRT are equivalent [4, 5]. However, the CDR technique offers fewer parameters that can be optimized compared with VMAT. Therefore, it is more difficult to achieve the defined dose limit with CDR than with VMAT. Moreover, it becomes increasingly difficult to achieve the dose limit when the bladder volume during planning CT is <100 cm3. This suggests that if the bladder volume is >100 cm3, it may be possible to improve the achievement rate of the dose limit for CDR. Additionally, it has been reported that a bladder volume of 100–150 cm3 is adequate for planning [11]; thus, levels closer to 100 cm3 appear to be reasonable in practice. Figures 2–3 reveal that V20–V40 of the rectum and bladder for CDR was higher than that for f-IMRT and VMAT. V20–V40 of the rectum and bladder may not be related to an increased incidence of adverse events [12] . Therefore, our results indicated that the dose distribution and DVH of the CDR, f-IMRT and VMAT methods were equivalent clinically. The total number of MUs for the CDR and VMAT methods were almost equal and <20–25% of that of f-IMRT. Additionally, the required irradiation time for CDR was <60% of that of f-IMRT. CDR also offered improved throughput and a reduced intrafractional error compared with f-IMRT. However, the required irradiation time of CDR was longer by ∼50 s compared with that of VMAT; this was attributed to the fixed gantry rotational speed and dose rate of the CDR technique (i.e. these two parameters could not be optimized).
The optimization time of CDR was ∼3 times that of f-IMRT because CDR was calculated by each 4° increment of gantry angle (large number of fields). VMAT was also calculated using a number of fields, however, the optimization time of CDR was longer than that of VMAT. This result was attributed to the numerous iterations required with IMRT planning using CDR to achieve the dose limit. Compared with f-IMRT, the potential advantages of CDR include a large reduction in irradiation time and in the total number of MUs, however, the optimization time of CDR is longer than that of f-IMRT. Therefore, for institutions using CDR for patients who can reach the dose limit easily, it may be reasonable to use f-IMRT for those who cannot. Additionally, the bladder volume may be useful for identifying into which category a patient falls in order to determine the appropriate treatment.
CONCLUSION
The utility of Smart Arc CDR for prostate IMRT was clarified in this study. CDR can reduce the total number of MUs and the patient irradiation time compared with f-IMRT, resulting in a lower initial cost compared with that of VMAT. For institutions that do not have the means to perform VMAT, CDR can be used instead. Identifying which patients will have difficulty in achieving the dose limit in advance is important. Bladder volume may be a useful index for determining this patient subset, and for these patients a combination of CDR and f-IMRT should provide a better outcome.
REFERENCES
- 1.Pizkall A, Carol M, Lohr F, et al. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys. 2000;48:1371–80. doi: 10.1016/s0360-3016(00)00772-0. [DOI] [PubMed] [Google Scholar]
- 2.Ruben JD, Lancaster CM, Jones P, et al. A comparison of out-of-field dose and its constituent components for intensity-modulated radiation therapy versus conformal radiation therapy: implications for carcinogenesis. Int J Radiat Oncol Biol Phys. 2011;81:1458–64. doi: 10.1016/j.ijrobp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–7. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P, Happersett L, Hunt M, et al. Volumetric modulated arc therapy: planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys. 2010;76:1456–62. doi: 10.1016/j.ijrobp.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Palma D, Vollans E, James K, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Bzdusek K, Friberger H, Eriksson K, et al. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys. 2009;36:2328–39. doi: 10.1118/1.3132234. [DOI] [PubMed] [Google Scholar]
- 7.Hårdemark B, Liander A, Rehbinder H, et al. RaySearch White Paper. Stockholm, Sweden: RaySearch Laboratories AB; 2003. Direct machine parameter optimization with Ray Machine in Pinnacle3. [Google Scholar]
- 8.de Crevoisier R, Tucker SL, Dong L, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–73. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. http://www.nccn.org/index.asp. (18 June 2013, date last accessed) [Google Scholar]
- 10.Ahnesjo A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Med Phys. 1989;16:577–92. doi: 10.1118/1.596360. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura N, Shikama N, Takahashi O, et al. The relationship between the bladder volume and optimal treatment planning in definitive radiotherapy for localized prostate cancer. Acta Oncol. 2012;51:730–4. doi: 10.3109/0284186X.2011.639388. [DOI] [PubMed] [Google Scholar]
- 12.Michalski JM, Gay H, Jackson A, et al. Radiation dose–volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl) doi: 10.1016/j.ijrobp.2009.03.078. S123–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]