Abstract
It appears that the story on vitamin E and its role in human health remains incomplete. It is apparent that vitamin E supplementation involves many variables, some of which include its uptake from the intestine, the preference for α-tocopherol, transport by tocopherol specific proteins and lipid transporters and the differential metabolism of different vitamin E isoforms. The fundamental differences within population genetics can have significant implications for the effect that dietary supplementation might have on human health. When evaluating the efficacy of vitamin E prophylactic or therapeutic use in previous and future studies, it is critical to consider dosage to be administered, form of vitamin E and source (such as whether from synthetic or purified from natural sources). Further studies are needed to determine the effects of all vitamin E isoforms on cell growth, tumorigenicity, to clarify its possible use as an adjuvant to existing chemotherapeutics. The Alpha-Tocopherol, Beta Carotene (ATBC) Cancer Prevention Study Group and Selenium and Vitamin E Cancer Prevention Trial (SELECT) studies along with the numerous studies of vitamin E should help guide the next chapter of vitamin E research.
Keywords: Tocopherol, Tocotrienol, vitamin E transport proteins, antioxidant, cancer prevention, cancer therapy
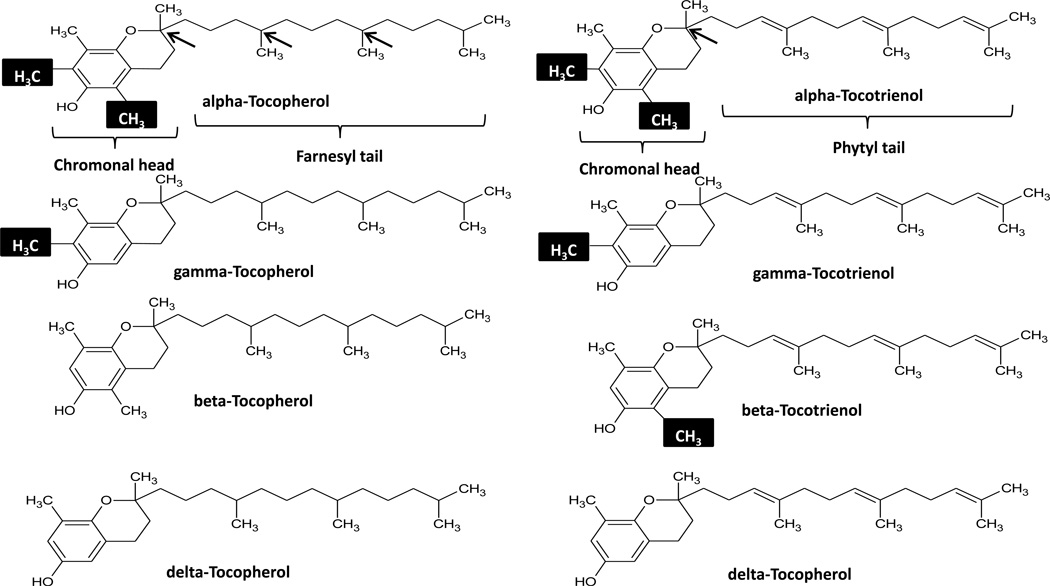
Members of the vitamin E family are hydrophobic fat-soluble compounds found in a variety of food sources such as corn oil, peanuts, vegetable oils, fruits and vegetables consumed through diet (table 1). Vitamin E exists in 8 isoforms, α, β, γ, δ-tocopherol and α, β, γ, δ-tocotrienol (figure 1). The small structural differences between vitamin E isoforms have a significant impact on vitamin E metabolism. Dietary habits thus play a significant role in which vitamin E isoform is primarily consumed; a Mediterranean diet, for example contains a significant amount of leafy greens, which contain high levels of α-tocopherol [1]. The health benefits of consuming vitamin E through diet or supplementation are believed to be for its antioxidant properties as a peroxyl radical scavenger. Vitamin E protects cells from cell damage caused by free radicals that damage cell membranes through lipid oxidation (lipid peroxidation) leading to DNA damage and cancer development [2– 4]. Vitamin E acts as an efficient antioxidant, by reducing the peroxyl radical and eliminating the chain reaction of fatty acid radical propagation [2]. It is generally believed that the tocotrienols exhibit stronger antioxidant activity when compared to the tocopherols [5]. Although past epidemiological studies, as well as in vivo and in vitro studies indicate vitamin E intake has protective properties against carcinogenesis [7–11]; recent studies on vitamin E show the contrary [12–14]. These findings were further substantiated by the results from the largest vitamin E randomized trial to date, Selenium and Vitamin E Cancer Prevention Trial (SELECT), which showed that α-tocopherol supplementation increased prostate cancer incidence [15]. The seemingly contradictory results of the SELECT trial should not discourage the use of vitamin E as a protective agent, but rather should be a pointer to the complexities of vitamin E uptake, and metabolism. The multiple iso- and stereoisomers (synthetic) existing within the vitamin E family should prompt a closer look at the specific properties within this family. It should not be expected that all isoforms are created equal as some studies suggest. Depending on the system studied there exist opposing effects amongst the various vitamin E isoforms [16–17]. Studies indicate that the natural vitamin E isoform RRR-α-tocopherol is preferentially incorporated in lipoproteins and maintained in plasma [18–20], while all other stereo and iso-forms are quickly eliminated through excretion and metabolite formation [21,22]. Besides vitamin E isoforms, transport mechanisms including vitamin E transport proteins and digestive defects can all have significant impacts on the benefits of vitamin E supplementation. Prompted by groundbreaking studies such as the ATBC and SELECT trials, future investigations trying to fill in the gaps widened by contradicting data will surely help clear a field at a crossroad, and try to answer questions such as, is vitamin E beneficial, should synthetics be disregarded, and should the lesser studied tocotrienols be reconsidered as potentially beneficial agents in cancer prevention. This commentary provides a broad overview of vitamin E, from structure to metabolism, while highlighting key studies regarding the use of vitamin E as a cancer preventative before ending on the relatively unexplored tocotrienols and vitamin E transport proteins.
Table 1.
Vitamin E content of some common foods.
| Source | alpha-T | beta-T | gamma-T | delta-T | alpha-T3 | gamma-T3 |
|---|---|---|---|---|---|---|
| Almondb | 25.87±1.35 | .89±.08 | ||||
| Black walnutsb | 1.8 | 28.48 | ||||
| Avacado (Haas)a | 1.93 | 0.06 | 0.69 | 0.03 | 0.04 | |
| Plumsa | .28±.11 | .05±.08 | 0.02±.04 | .22±.19 | ||
| Spinach (raw)a | 1.96 ±0.43 | 0.21±0.06 | ||||
| Tomato (raw unpeeled)a | 0.53± | |||||
| Broccoli (raw)a | 1.44±0.22 | Tr | 0.31±0.12 | Tr | ||
| Bananaa | 0.13±0.1 | Tr | 0.02±0.03 | |||
| mg/100g |
Figure 1.
Vitamin E isoforms: Methyl groups within the chromonal head determine alpha, beta, and delta status (highlighted). Arrows point to existing chiral centers located in the farnesyl tail (3) and phytl tail (1) of tocopherols and tocotrienols respectively. An unsaturated tail distinguishes the tocotrienols form the saturated tail of the tocopherol vitamin E isoforms.
1. Structure and function
1.1
As shown in figure 1, the α, β, γ, δ vitamin E isoforms vary in the methylation status of the chroman ring, which is composed of a phenolic ring joined to a heterocyclic ring. The structural difference conferred by methylation status within the phenolic ring is believed to determine the preferential uptake and secretion of the various vitamin E isoforms [23]. An unsaturated tail within the tocotrienol structure results in the only structural difference from its tocopherol family member. While there are 3 chiral centers within the unsaturated phytol tail of tocopherols at positions 2, 4 and 8; tocotrienols with an unsaturated tail possess only one chiral center at position 2. Thus tocopherol isoforms can exist as 8 possible stereoisomers depending on the orientation of these 3 chiral centers and there are only two possible stereoisomers for tocotrienol isoforms. Naturally occurring vitamin E exist in the RRR stereo-isoform, while the synthetic alpha-tocopherol or all-rac-alpha tocopherol used in many studies exists as 8 stereoisomers in equal amounts The absolute configuration of the enantiomers is sometimes used to identify the vitamin E isoform in the literature i.e. RRR-α-tocopherol to denote the natural form of natural vitamin E (α –tocopherol), other nomenclature found in the literature include, d- α-tocopherol, ddd-α-tocopherol. Conversely synthetics can sometimes be written as dl-α-tocopherol or all-rac-α-tocopheryl acetate. The impact of the vitamin sources in future studies must not be disregarded and caution must be taken when comparing studies, particularly if results are different from what has been previously established in multiple studies by various groups.
1.2. Vitamin E as an antioxidant
In lipid peroxidation, Reactive Oxygen Species (ROS) such as hydroxyl and peroxyl radicals created and released primarily by the mitochondria attack polyunsaturated fatty acids (PUFAs) within cell membranes [24–27]. Lipid peroxidation results in the production of lipid radicals and lipid peroxide, which in turn can propagate the formation of lipid radicals eventually altering the cell membrane, leading to modified proteins and DNA bases, mutagenic properties that have been shown to be carcinogenic [27–29].
The lipid peroxidation product, 4-hydroxynonenal is used as a reliable biomarker of increased oxidative stress found in many cancers. While vitamin E inhibits lipid peroxidation, this activity varies amongst the vitamin E family. Although it is generally regarded that the alpha isoform has the highest antioxidant activity [α-tocopherol > β-tocopherol > γ-tocopherol > δ-tocopherol] as shown by Müller et. al, by following the decrease in absorbtion of the reference dye pyrogallolsulfonphthalein by peroxyl radicals, which was slowed dependent on the vitamin E isoform used [5]. The tocotrienols have been considered to be stronger antioxidants in in vivo and in vitro models, although the antioxidant capacity of the different alpha isoforms is debatable [30]. The debate seems to stem from the specific assay and experimental conditions in which the various isoforms are evaluated [5,6,30]. It is important to bear in mind that there exists preference for the α-tocopherol isoform in the human body. Therefore this aspect needs to be taken into account in future studies, to make studies on vitamin E isoforms relevant to human biology.
2. Metabolism
2.1. Uptake
Vitamin E uptake into plasma is similar to fat uptake through the small intestine. Bile acid secretions in the intestinal lumen help in forming micelles containing vitamin E and lipid hydrolysis products which are absorbed through the membrane of intestinal cells and transported as lipoproteins known as chylomicrons in the lymph and blood [20,31]. Chylomicron catabolism must take place for further vitamin E processing. This is achieved by lipoprotein lipase secreted by the pancreas resulting in free fatty acids, vitamin E containing lipoproteins, chylomicron remnants, or LDL and HDL containing vitamin E that can be taken up by tissue or by the liver [20,31]. Selective transport of α-tocopherol occurs within the liver by the α-tocopherol transfer protein (α-TTP) effectively selecting α-tocopherol over the various vitamin E isoforms, which are degraded or excreted in urine or bile [18,19,22–23,31]. Competitive binding experiments performed by Hosomi et al., using radio-labeled tocopherols resulted in higher α-tocopherol uptake between membranes providing a better understanding of the higher levels of α-tocopherol as compared to other vitamin E isoforms found in blood and tissue samples [23]. From these experiments we know that α-tocopherol transfer protein has the greatest binding affinity for α-tocopherol, followed by β, γ and δ-tocopherol. This is one mechanistic explanation for how α-tocopherol is selectively rescued from biliary or urinary excretion and incorporated into VLDL that is recycled into the plasma to maintain α-tocopherol levels [32–34].
2.2. Diet
Since vitamin E is consumed through diet, it should be expected that dietary habits significantly impact the amount and type of vitamins we consume. An epidemiological study by Seaverson et al., showed that iron intake in Hispanic men was 43% lower compared to non-Hispanic white men. This study showed that cultural variation in dietary patterns does influence the composition of nutrient (vitamin C) and mineral (iron and calcium) uptake [35]. In fact global, cultural and economic status play a role in the amount and vitamin E isoform consumed [36]. For example in comparing the westernized diet with a Mediterranean diet we see a majority of food consumed within the Mediterranean diet consisting of leafy greens, nuts and olive oils which contain high quantities of α-tocopherol [37] while the westernized diet is high in soybean and soybean oil which contain a larger proportion of γ-tocopherol [38]. Epidemiological data suggest that there exists a cultural variation in vitamin E serum levels, and a link between vitamin E serum levels and cancer prevalence/incidence [7,11,35,36,39,40]. Vitamin E deficiency is rarely due to poor diet but rather due to genetic defects leading to poor vitamin E absorption/processing, and lipoprotein synthesis [39– 41], α-tocopherol transfer protein (α-TTP) expression, [42] deficiency in bile secretions [43] and defects in lipoprotein synthesis due to microsomal transfer protein. There is extensive data regarding the negative impact of increased free radicals on healthy cells with a strong correlation between increased ROS and prostate, colon, breast, ovarian as well as other cancers [11,44–47]. Therefore it should be expected that differences in global cancer incidences could in part be due to environment and diet. With respect to vitamin E, this would include the different forms of vitamin E consumed.
3. Vitamin E and cancer prevention studies: A short summary of some key studies on vitamin E and cancer management is shown in Table 2
Table 2.
Summary of cancer related vitamin E trials.
| Study type | Findings | Isoform | Result | Ref |
|---|---|---|---|---|
| in-vitro mouse melanoma model | Growth inhibition | Semi-synthetic form of α-tocopherol | (+) | ref. 49 |
| in-vivo colon cancer mouse model | Inhibited tumorigenesis | Unspecified | (+) | ref. 51 |
| Breast cancer for at risk women | Remission of mammary dysplasia | Synthetic α-tocopherol | (+) | ref. 50 |
| Retrospective epidemiological study of breast cancer | Negative association between breast cancer and vitamin E plasma | Vit. E in serum (α-tocopherol) | (+) | ref. 11 |
| Micronutrient intervention study of esophageal/gastric cancer | Lower mortality in β-carotene, vitamin E and selenium group | Unspecified | (+) | ref. 52 |
| Randomized primary-prevention of lung cancer | Significant reduction in prostate and increase in stomach cancer | Synthetic α-tocopherol | (+) prostate, (−) stomach | ref 56 |
| Meta-analysis of various cancers | Only prostate cancer is lower | (−) prostate | ref. 57 | |
| Epidemiological study of breast cancer | No association with breast cancer | Unspecified | no connection | ref. 58 |
| Epidemiological study of breast cancer | No association with breast cancer | Unspecified | no connection | ref. 59 |
| Prospective cohort study of breast cancer | No association with cancer incidence | Unspecified | no connection | ref. 60 |
| Breast and gynecological cancer study | Higher vitamin E levels in cervical and endometrial cancers | Unspecified α-tocopherol | (−) | ref. 61 |
| Randomized control study of total and prostate cancer | No effect of total or prostate cancer incidence | Synthetic α-tocopherol | no connection | ref. 62 |
| Prostate cancer prevention trial | No effect on prostate cancer incidence | Unspecified | no connection | ref. 63 |
| Prostate cancer prevention trial | Supplementation increased prostate cancer incidence | Synthetic α-tocopherol | (−) | ref. 15 |
3.1. Cancer preventing effects
Of the vitamin E isoforms, α-tocopherol has been extensively studied and there is much data indicating that it has a role in cancer prevention and as a possible therapeutic agent [48]. Early studies treating mouse melanoma with vitamin E acid succinate resulted in growth inhibition, morphological changes and a differentiated phenotype [49]. A subsequent study using vitamin E in women at a high risk of developing breast cancer resulted in a remission in 15 out of 17 women [50]. High-performance liquid chromatography analysis of samples from 5000 women for retinol, β-carotene and vitamin E levels showed that women who developed breast cancer had on average lower levels of vitamin E compared to controls (4.7 mg l−1 vs. 6.0 mg l−1). Women who developed breast cancer were in the quintile with the lowest vitamin E plasma levels and these data were statistically significant.
A large randomized trial of 29,600 Chinese participants was conducted to determine if micronutrients could impact the incidence of esophageal, stomach cancer and overall cancers. The trial design did not allow for data to be deemed statistically significant for any one micronutrient. However, when grouped with β-carotene and selenium, vitamin E had a significant benefit on total mortality and this was largely due to lower cancer-related mortality when compared to placebo group [52]. Prior to this study the beneficial role of vitamin E in gastric cancers showed conflicting results in preclinical models and case control studies [53–55].
A randomized, double-blind, placebo-controlled primary prevention trial called Alpha-Tocopherol, Beta Carotene, (ATBC) investigated the effects of antioxidant supplements on the incidence of lung cancer. Twenty-nine thousand Finnish male smokers between the ages of 50–69 years old were randomly assigned to take dl-α-tocopheryl acetate (50 mg/day), β-carotene (20mg/day), both or placebo. A statistically significant negative correlation was found between α-tocopherol levels and prostate cancer incidence [10,56]. In 2007 a meta-analysis of 12 randomized control trials in which vitamin E was given as a supplement concluded that the only significant values were for lowered prostate cancer incidence [57].
3.2 Lack of association
A prospective analysis of vitamin intake and risk of breast cancer in 34,000 post-menopausal women in The Iowa Women’s Health Study (IWHS) by a food frequency questionnaire found no association between vitamin E intake and breast cancer incidence [58]. The lack of strong supplementation data, duration of supplementation and direct plasma measurements weakend the overall impact of the IWHS findings [58]. Several other investigations failed to show a correlation between vitamin E intake and breast cancer (Table 2) [58–60]. A study on antioxidant levels in tissue samples of female patients with gynecological cancers showed heterogeneity in regards to origin and type of cancers and vitamin E levels and a significant positive correlation with cervical and endometrial cancers [61]. Although the ATBC trial found that α-tocopherol supplementation did not lower incidence of lung cancer at end point, base line serum α-tocopherol levels did have a negative correlation with lung cancer incidence. The contradictory results could be a result of the form of α-tocopherol used as the supplement; α-tocopherol baseline levels have a high proportion of the natural vitamin E isoform as this isoform is shown to persist substantially longer in the serum compared to all other isoforms, secondly, there is little data on the effects of stereoisomers found in synthetic α-tocopherol and cancer incidence [18–19,22,31].
The promise of significant reduction in prostate cancer incidence seen in the ATBC prompted a number of randomized trials to look at vitamin E and prostate cancer [15,62,63]. The Physicians Health Study II, a randomized double-blind placebo controlled 24 factorial designed trial (n=14,641) determined if long term vitamin E, and/or β-carotene supplementation had a significant impact on prostate and total cancer incidence. There was no significant effect on the incidence of prostate cancer or total cancer mortality when compared to placebo control [62]. A few caveats when comparing the results of the Physicians Health Study trial must be taken into consideration: 1) the sample was of a small subgroup of participants, US physicians who are more likely to live a healthier life, 2) serum samples were not used to determine compliance or in analyzing the results of supplementation during the trial, 3) compliance was evaluated by self-reports, 4) unlike other studies supplementation was every other day as opposed to daily intake and 5) previous history of cancer did not result in exclusion [62]. Based on a food frequency questionnaire, The Prostate Cancer Prevention Trial (PCPT; a randomized placebo-controlled trial of finasteride vs. placebo) found no association between dietary nutrient or supplemental vitamin E intake and total prostate cancer [63]. The result on vitamin E was confounded by the lack of serum sample measurements to determine vitamin E levels. There was however, an association between risk of high-grade cancer and high intake of polyunsaturated fats. Since high intake of dietary unsaturated fats can lead to a decrease in α-tocopherol levels, it implies that vitamin E could be lower in these participants who subsequently had a higher incidence of high-risk prostate cancer [64].
3.3. Cancer promoting effects
SELECT has been the most comprehensive study to determine the long-term effects of vitamin E and selenium on prostate cancer risk in healthy men [15]. Based on the observed trend of vitamin E increasing prostate cancer incidence (p=0.06) with no significant benefit to justify continuation supplementation was discontinued after 5.5 years. Continued follow-up analysis of 7–12 years found vitamin E increasing prostate cancer risk by 17% (p=0.008). The results of the SELECT trial have been highly publicized, and vitamin E’s beneficial role as a supplement must now be carefully weighed against potential risks specifically for those men taking higher dosage of 400 IU vitamin E supplements. Most multivitamin dosages tend to be closer to the suggested daily allowance of 33.3 IU for synthetic forms of Vitamin E by the Food and Nutrition Board [64]. Results of the SELECT trial and the conflicting findings with the ATBC study has left the prostate cancer scientific community conflicted as well. To fully understand these results, we must first analyze some key difference, some of which were touched on by the SELECT group [15]. The most apparent difference between these trials includes the cohort, participant genetic makeup, difference in primary end point and the chosen dosage. The benefits of vitamin E on prostate cancer incidence seen with the lower dosage 50 mg/d (ATBC) may yet prove to be crucial as we see with many things in nature, even a good thing can be harmful if used in excess. The synthetic racemic mixture of α tocopherol contains all eight stereoisomers in equal amounts of which only the R,R,R-α-tocopherol is preferentially maintained within tissues and serum. It is important not to disregard other members of the vitamin E family as well since there are studies indicating γ-tocopherol, the second most common vitamin E isoform could be a beneficial micronutrient. Further, vitamin E transport proteins could prove to be key factors in determining the efficacious property of vitamin E [66–67].
4. Tocopherol & Tocotrienol
4.1. Cell culture studies
A promising study by Torricelli et al., showed that tocopherols affect cell function beyond a mere antioxidant mechanism [66]. Prostate Cancer-3 (PC-3) cells are cells derived from a patient with grade IV androgen independent metastatic prostate cancer and are frequently used in cell culture models. Using an in vitro prostate cancer androgen-independent model, α- and γ-tocopherol treated PC-3 cell growth was inhibited 3–5 fold. γ-tocopherol exhibited greater potency including increased expression and activity of transglutaminase 2, a cell differentiation marker that induces differentiation and eventual apoptosis. Given that γ-tocopherol is more readily metabolized into γ-carboxyethyl hydroxychroman and previous studies with tocopherol metabolites have shown similar results, it is possible that γ-tocopherol metabolites may be the effector agent resulting in growth inhibition of PC-3 cells, although other studies suggest metabolite formation is largely a detoxification mechanism with little or no effect on proliferation [66–69]. Tocotrienols were consistently more potent at inhibiting cell growth and inducing apoptosis in mouse mammary epithelial cells, CL-S1 when compared to tocopherols [70]. Perhaps the most intriguing of these findings was that malignant cells were more sensitive to the growth inhibitory effects of tocotrienols than that of normal mammary epithelial cells. The promising potential of tocotrienols as inhibitors of tumor growth is hindered by its poor delivery to tumor cells without affecting normal tissue [71–72]. By encapsulating a tocotrienol rich fraction (TRF) in tranferrin bearing vesicles, Fu et al., effectively increased cellular uptake of TRF resulting in a 2–5 fold increase in cytotoxicity (0.17±0.14 vs. 0.97±0.48 IC50 µg/mL]). Trienols have also been tested for therapeutic use in non-small cell lung cancer (NSCLC) cells. NSCLC cell lines A549 and H1299 treated with 30 µM 5-tocotrienol resulted in inhibiton of growth, migration and invasion that was mediated by Notch-1 at the transcriptional and translational level [73]. This led to further studies to test the potential use of 5-tocotrienol in combination with cisplatin. Given that drug resistance is commonly seen in cisplatin therapy and that increased Notch-1 signaling is observed in 30% of NSCLC cells the combinaiton of δ-tocotrienol and cisplatin was tested as a combinaiton therapy and found to have an additive effect on apoptosis, cell migration and cell invasion and inhibition of Notch-1 signaling [74]. Perhaps the most promising finding was the combination treatment resulted in a net inhibition of NF-κB binding activity; a significant finding in that NF-κB is associated with drug resistance, and cisplatin treatment is known to increase NF-κB activity. These and other studies are exemplary on the potential use of vitamin E isoforms not traditionally studied as possible chemotherapeutic therapies or used in combination to sensitize drug resistant cancers. Additional studies of vitamin E isoforms have shown opposing regulatory effects on leucocyte recruitment and migration during inflammation. For instance McCary et al., showed that phosphatidylserine-dependent PKCα activation was enhanced by γ-tocopherol treatment or inhibited with α-tocopherol treatment, thus an intricate interplay between levels of γ- and α-tocopherol help determine PKCα activation within the cell [16]. In the androgen independent prostate cancer cell line PC-3, γ- and α-tocopherol treatment resulted in decreased progression through the S-phase of the cell cycle, although γ-tocopherol treatment produces a significantly greater growth inhibitory effect when compared to α-tocopherol [66].
4.2. In vivo studies
In an in vivo mouse model a tocotrienol-rich fraction (TRF) encapsulated in transferrin bearing vesicles was able to reduce tumor volume within 24h after intravenous tail vein injections, and after 10 days of vitamin E treatments tumor regression was substantial.. Systemic administration of transferrin-TRF in animals with subcutaneously implanted tumors resulted in either stable or partial responsiveness (tumor regression) compared with non-treated mice which were 100% progressive [71]. Although there have been fewer studies on the tocotrienols, data indicate its potential use as a therapeutic [71]. While there is evidence that vitamin E isoforms may work better in combination rather than singly, yet other studies suggest vitamin E isoforms may interact antagonistically in regulating cell signaling pathways. In 1989 blood specimens were collected from 10,456 men over the age of 45 in Washington County MD for analysis in a nested case-control study. Termed as the “CLUE II” study, (referencing to the campaign slogan ‘Give Us A Clue about Heart Disease and Cancer’), it was conducted to determine the association of α-tocopherol, γ-tocopherol, selenium and prostate cancer incidence [38]. CLUE II was a 6 year study that identified low levels of α-tocopherol and γ-tocopherol levels in prostate cancer subjects, although only γ-tocopherol was statistically significant (p=0.0002), and for subjects in the highest 5th quintile γ-tocopherol resulted in a 5-fold reduction in prostate cancer. Finally, when comparing all three micronutrients, when α-tocopherol was high, and γ-tocopherol and selenium were low the odds ratio for the development of prostate cancer was 0.77(95% CI=0.33–1.80; [38]). This study highlights the need for careful consideration of other vitamin E isoforms and their potential antagonistic interactions. In a study measuring α-and γ-tocopherol serum levels, increasing dl-α-tocopheryl acetate supplementation resulted in an increase of α-tocopherol levels and decreasing levels of γ-tocopherol [75]. Although vitamin E isoform, dosage and measurements of baseline levels are important factors in determining if the effects of vitamin E are harmful or beneficial in supplement use, cellular transport or uptake of vitamin E also has a significant impact on treatment efficacy.
5. Vitamin E transporters
To date, only one vitamin E-specific transporter has been found, the α-tocopherol transfer protein (α-TTP) found in the cytosol and highly expressed in hepatocytes. Being a hydrophobic compound and associating with lipoproteins similar to cholesterol it should be of no surprise that vitamin E absorption also involves the same transport proteins as cholesterol. In fact, mice deficient in the ATP-binding cassette transporter A1 (ABCA1) have no detectable plasma α-tocopherol. Since α-tocopherol is incorporated into HDL and ABCA1 is needed for HDL biogenesis the afore mentioned observation maybe a secondary effect [76–77]. The high-affinity HDL-receptor scavenger receptor class B type I (SR-BI), a membrane receptor with wide tissue distribution including, ovaries, testis, lung and brain is believed to have many physiological functions. SR-BI deficient mice have low levels of α-tocopherol in brain, ovaries, lung and testis, but elevated levels in plasma suggesting a cellular uptake function. In the SR-BI deficient mice, liver α-tocopherol levels were unchanged while a decrease in biliary secretion was observed. The increased bioavailability of γ-tocopherol in these mice suggest non-specificity to α-tocopherol and substantiates the evidence implying that α-tocopherol transfer protein is largely responsible for the α-tocopherol isoform bioavailability preference and that absorption and uptake is non-specific to all vitamin E isoforms and similar to cholesterol absorption and uptake. To underscore the similarities between vitamin E and cholesterol absorption, Takada et al., overexpressed a protein essential for intestinal absorption of cholesterol namely Niemann-Pick C1-like 1 (NPC1L1) in human colorectal adenocarcinoma (Caco-2) cells, which resulted in increased uptake of all vitamin E isoforms. There was significant inhibition of absorption by the NCP1L1 specific inhibitor Ezetimibe [76]. A recent study of tocopherol-associated protein (TAP) revealed a negative correlation between TAP and prostate cancer cell lines [78]. TAP overexpression in the human prostate cancer cell line, LNCaP resulted in increased vitamin E uptake, which included vitamin E isoforms γ- and δ-tocopherol. This increased uptake correlated with LNCaP growth inhibition. To further substantiate these findings; knockdown of TAP in the non-malignant human prostate epithelial cells (HPr-1) resulted in increased cell growth. Interestingly, the growth inhibitory effects conferred by TAP overexpression was independent of vitamin-E, as vitamin E treatment of TAP overexpressing cells did not further decrease cell growth significantly. Furthermore, Ni et al., showed that the PI3K subunits, p110 and p85 interaction was significantly decreased resulting in lower pAkt; functionally this resulted in growth inhibition, as constitutively active Akt rescued TAP inhibition. To impart relevant significance, mouse xenografts of LNCaP control or TAP overexpressing cells showed inverse correlation between TAP overexpression and tumor incidence and size [78]. In situ hybridization of human tissue arrays for TAP expression revealed cytoplasmic and nuclear staining, while neoplastic samples showed primarily nuclear staining and an overall significantly lower TAP expression pattern. Studies show that the tocopherol transfer protein (TTP) has a function in sensitizing prostate cancer cells to the apoptotic effects of α-tocopherol [79]. The over-expression of TTP in prostate cancer cells increased apoptosis, inhibited tumorigenicity and significantly inhibited growth when treated with vitamin E. Endogenous TTP expression had a linear correlation with basal apoptotic levels. The knockdown of TTP resulted in resistance to the apoptotic effects of vitamin E. A finding with possible implications for the conflicting data observed between the ATBC and SELECT trials was the observation that polymorphic variants of TTPA and SEC14L2 correlated with increased prostate cancer incidence (polymorphisms differences can exist between diverse ethnic populations), some variants correlated with increased incidence, lowered baseline α-tocopherol levels, while other alleles had contradictory correlations depending on whether it was haplotype or homozygous [80]. Additional proteins involved in vitamin E transport include phospholipid transfer protein, LDL receptor and lipoprotein lipase (chylomicron hydrolysis) [76,81].
6. Conclusion
In order to establish vitamin E as a potential ally in health, particularly in the fight against cancer dosage appears to be a variable that must be empirically resolved. While one can understand the rationale of high dosage in past studies; a reasonable dosage recommended by the Food and Nutrition Board of 15 milligrams (22.4 IU) daily intake of natural vitamin E should be the baseline for future studies (1 mg = 1.49 IU for natural α-tocopherol, 0.67 mg = 2.22 IU for synthetic α-tocopherol). Of particular concern is the use of synthetic vitamin E which includes equal mixture of all eight possible stereoisomers. Vitamin E is shown to modulate many molecular pathways [16–17,66,71,73,74,76–78] and this complexity could in part be explained by the various stereoisomers modulating the cellular response. These effects could be non-existent in an organism that selectively removes all non-α tocopherol isoforms although studies by Muller et al, suggests gene expression is similar in comparison between natural and synthetic forms [22,31,80–83]. Treating the hepatocellular carcinoma cell line HepG2 with either natural or synthetic forms of vitamin E did not result in a significant difference in gene expression [82]. TTP isoforms should also be evaluated to determine if polymorphisms significantly impact vitamin E metabolism and if these polymorphisms are common within populations [80]. Lastly how can we, and should we adjust vitamin E levels in plasma/tissues, and if so, should there be better methods to deliver vitamin E to the desired tissue [72,84]. Recent studies on vitamin E analogs could play major roles in this regard [84,85]. Cheng et al, synthesized an acetylated vitamin E analog conjugated to a modified lipophilic cation (Mito-ChM) that selectively targeted mitochondria from cancer cells by taking advantage of their higher membrane potential [84]. In vitro treatments of human breast cancer cell lines MCF-7, MDA-MB-231 and the non-cancerous cell line MCF-10A resulted in a significantly higher growth inhibition in breast cancer cell lines [84]. This is a very exciting time in vitamin E research, yet it is evident that we are far away from making the final decision on the benefit vs. risk for the potential use of vitamin E in human health. Apparently we (both laboratory scientists and clinicians) have an obligation to make a concerted effort to establish the usefulness or lack thereof of vitamin E before branding it as being harmful for human health, especially if this relatively inexpensive compound can provide prophylactic benefit from multiple human diseases.
Acknowledgements
We acknowledge the following funding sources: 5R01 CA149516 (RG) and 1T32CA148724 (EC).
Abbreviations
- ABCA1
ATP-binding cassette A1
- ATBC
alpha-tocopherol, beta-carotene
- HDL
high density lipoproteins
- LDL
low density lipoproteins
- NSCLC
non-small cell lung cancer
- NPC1L1
Niemann-Pick C1-like 1
- PKC
protein kinase C
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- SR-BI
scavenger receptor class B type 1
- SELECT
selenium and vitamin E cancer prevention trial
- TAP
Tocopherol associated protein;
- TTP
tocopherol transfer protein
- TRF
tocotrienol rich fraction
- VLDL
very low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang Q, Christen S, Shigenaga MK, Ames BN. gammα-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001 Dec;74(6):714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C. An update on products and mechanisms of lipid peroxidation. Mol Nutr Food Res. 2009 Mar;53(3):315–321. doi: 10.1002/mnfr.200800131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai F, Dupertuis YM, Pichard C. Role of polyunsaturated fatty acids and lipid peroxidation on colorectal cancer risk and treatments. Curr Opin Clin Nutr Metab Care. 2012 Mar;15(2):99–106. doi: 10.1097/MCO.0b013e32834feab4. [DOI] [PubMed] [Google Scholar]
- 4.Green RM, Hodges NJ, Chipman JK, O'Donovan MR, Graham M. Reactive oxygen species from the uncoupling of human cytochrome P450 1B1 may contribute to the carcinogenicity of dioxin-like polychlorinated biphenyls. Mutagenesis. 2008 Nov;23(6):457–463. doi: 10.1093/mutage/gen035. [DOI] [PubMed] [Google Scholar]
- 5.Müller L, Theile K, Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010 May;54(5):731–742. doi: 10.1002/mnfr.200900399. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, Niki E, Noguchi N. Comparative study on the action of tocopherols and tocotrienolls as antioxidant: chemical and physical effects. Chem Phys Lipids. 2003 Mar;123(1):63–75. doi: 10.1016/s0009-3084(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 7.Longnecker MP, Martin-Moreno JM, Knekt P, Nomura AM, Schober SE, Stähelin HB, Wald NJ, Gey KF, Willett WC. Serum alpha-tocopherol concentration in relation to subsequent colorectal cancer: pooled data from five cohorts. J Natl Cancer Inst. 1992 Mar 18;84(6):430–435. doi: 10.1093/jnci/84.6.430. [DOI] [PubMed] [Google Scholar]
- 8.Gunawardena K, Murray DK, Meikle AW. Vitamin E and other antioxidants inhibit human prostate cancer cells through apoptosis. Prostate. 2000 Sep 1;44(4):287–295. doi: 10.1002/1097-0045(20000901)44:4<287::aid-pros5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Fleshner N, Fair WR, Huryk R, Heston WD. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J Urol. 1999 May;161(5):1651–1654. [PubMed] [Google Scholar]
- 10.Venkateswaran V, Fleshner NE, Klotz LH. Modulation of cell proliferation and cell cycle regulators by vitamin E in human prostate carcinoma cell lines. J Urol. 2002 Oct;168(4 Pt 1):1578–1582. doi: 10.1016/S0022-5347(05)64524-7. [DOI] [PubMed] [Google Scholar]
- 11.Wald NJ, Boreham J, Hayward JL, Bulbrook RD. Plasma retinol, beta-carotene and vitamin E levels in relation to the future risk of breast cancer. Br J Cancer. 1984 Mar;49(3):321–324. doi: 10.1038/bjc.1984.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller ER3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 13.Chen CS, Wells PG. Enhanced tumorigenesis in p53 knockout mice exposed in utero to high-dose vitamin E. Carcinogenesis. 2006 Jul;27(7):1358–1368. doi: 10.1093/carcin/bgi325. [DOI] [PubMed] [Google Scholar]
- 14.Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, Estaquio C, Briançon S, Favier A, Latreille J, Malvy D. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007 Sep;137(9):2098–2105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- 15.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011 Oct 12;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin E isoforms directly bind PKCα and differentially regulate activation of PKCα. Biochem J. 2012 Jan 1;441(1):189–198. doi: 10.1042/BJ20111318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torricelli P, Caraglia M, Abbruzzese A, Beninati S. γ-Tocopherol inhibits human prostate cancer cell proliferation by up-regulation of transglutaminase 2 and down-regulation of cyclins. Amino Acids. 2012 Mar 30; doi: 10.1007/s00726-012-1278-y. [DOI] [PubMed] [Google Scholar]
- 18.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr. 1989 Mar;49(3):517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 19.Traber MG, Kayden HJ. Alpha-tocopherol as compared with gamma-tocopherol is preferentially secreted in human lipoproteins. Ann N Y Acad Sci. 1989;570:95–108. doi: 10.1111/j.1749-6632.1989.tb14911.x. [DOI] [PubMed] [Google Scholar]
- 20.Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res. 1993 Mar;34(3):343–358. [PubMed] [Google Scholar]
- 21.Mustacich DJ, Shields J, Horton RA, Brown MK, Reed DJ. Biliary secretion of alpha-tocopherol and the role of the mdr2 P-glycoprotein in rats and mice. Arch Biochem Biophys. 1998 Feb 15;350(2):183–192. doi: 10.1006/abbi.1997.0529. [DOI] [PubMed] [Google Scholar]
- 22.Traber MG, Kayden HJ. Alpha-tocopherol as compared with gamma-tocopherol is preferentially secreted in human lipoproteins. Ann N Y Acad Sci. 1989;570:95–108. doi: 10.1111/j.1749-6632.1989.tb14911.x. [DOI] [PubMed] [Google Scholar]
- 23.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997 Jun 2;409(1):105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 24.Thomas C, Mackey MM, Diaz AA, Cox DP. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 2009;14(3):102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- 25.Freinbichler W, Colivicchi MA, Stefanini C, Bianchi L, Ballini C, Misini B, Weinberger P, Linert W, Varešlija D, Tipton KF, Della Corte L. Highly reactive oxygen species: detection, formation, and possible functions. Cell Mol Life Sci. 2011 Jun;68(12):2067–2079. doi: 10.1007/s00018-011-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colquhoun A. Lipids, mitochondria and cell death: implications in neuro-oncology. Mol Neurobiol. 2010 Aug;42(1):76–88. doi: 10.1007/s12035-010-8134-4. [DOI] [PubMed] [Google Scholar]
- 27.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009 Sep 1;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Poli G, Biasi F, Leonarduzzi G. 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol Aspects Med. 2008 Feb-Apr;29(1–2):67–71. doi: 10.1016/j.mam.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda T, Tao H, Goto M, Yamada H, Suzuki M, Wu Y, Xiao N, He Q, Guo W, Cai Z, Kurabe N, Ishino K, Matsushima Y, Shinmura K, Konno H, Maekawa M, Wang Y, Sugimura H. Lipid Peroxidation-Induced DNA Adducts in Human Gastric Mucosa. Carcinogenesis. 2012 Oct 11; doi: 10.1093/carcin/bgs327. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, Packer L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993 Oct 12;32(40):10692–10699. doi: 10.1021/bi00091a020. [DOI] [PubMed] [Google Scholar]
- 31.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 32.Uchida T, Abe C, Nomura S, Ichikawa T, Ikeda S. Tissue distribution of a- and γ-tocotrienol and γ-tocopherol in rats and interference with their accumulation by α-tocopherol. Lipids. 2012 Feb;47(2):129–139. doi: 10.1007/s11745-011-3620-7. [DOI] [PubMed] [Google Scholar]
- 33.Baxter LL, Marugan JJ, Xiao J, Incao A, McKew JC, Zheng W, Pavan WJ. Plasma and Tissue Concentrations of α-tocopherol and 5-Tocopherol Following High Dose Dietary Supplementation in Mice. Nutrients. 2012 Jun;4(6):467–490. doi: 10.3390/nu4060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005 Apr 1;38(7):857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Seaverson EL, Buell JS, Fleming DJ, Bermudez OI, Potischman N, Wood RJ, Chasan-Taber L, Tucker KL. Poor iron status is more prevalent in Hispanic than in non-Hispanic white older adults in Massachusetts. J Nutr. 2007 Feb;137(2):414–420. doi: 10.1093/jn/137.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi T, Hildesheim A, Sinha R. Opportunities for cancer epidemiology in developing countries. Nat Rev Cancer. 2004 Nov;4(11):909–917. doi: 10.1038/nrc1475. [DOI] [PubMed] [Google Scholar]
- 37.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003 Jun 26;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 38.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, Morris JS, Comstock GW. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000 Dec 20;92(24):2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 39.Seaverson EL, Buell JS, Fleming DJ, Bermudez OI, Potischman N, Wood RJ, Chasan-Taber L, Tucker KL. Poor iron status is more prevalent in Hispanic than in non-Hispanic white older adults in Massachusetts. J Nutr. 2007 Feb;137(2):414–420. doi: 10.1093/jn/137.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comstock GW, Bush TL, Helzlsouer K. Serum retinol, beta-carotene, vitamin E, and selenium as related to subsequent cancer of specific sites. Am J Epidemiol. 1992 Jan 15;135(2):115–121. doi: 10.1093/oxfordjournals.aje.a116264. [DOI] [PubMed] [Google Scholar]
- 41.Ben Hamida C, Doerflinger N, Belal S, Linder C, Reutenauer L, Dib C, Gyapay G, Vignal A, Le Paslier D, Cohen D, et al. Localization of Friedreich ataxia phenotype with selective vitamin E deficiency to chromosome 8q by homozygosity mapping. Nat Genet. 1993 Oct;5(2):195–200. doi: 10.1038/ng1093-195. [DOI] [PubMed] [Google Scholar]
- 42.Sokol RJ, Heubi JE, Iannaccone S, Bove KE, Balistreri WF. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 1983 Nov;85(5):1172–1182. [PubMed] [Google Scholar]
- 43.Rader DJ, Brewer HB, Jr Abetalipoproteinemia. New insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease. JAMA. 1993 Aug 18;270(7):865–869. doi: 10.1001/jama.270.7.865. [DOI] [PubMed] [Google Scholar]
- 44.Veeramani S, Yuan TC, Lin FF, Lin MF. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene. 2008 Aug 28;27(37):5057–5068. doi: 10.1038/onc.2008.143. Epub 2008 May 26. PubMed PMID: 18504439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Wang X, Cheng S, Sun L, Son YO, Yao H, Li W, Budhraja A, Li L, Shelton BJ, Tucker T, Arnold SM, Shi X. Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/β-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol Appl Pharmacol. 2011 Oct 15;256(2):114–121. doi: 10.1016/j.taap.2011.07.016. Epub 2011 Aug 11. [DOI] [PubMed] [Google Scholar]
- 46.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. doi: 10.1038/362709a0. Review. [DOI] [PubMed] [Google Scholar]
- 47.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005 Dec;4(12):1367–1373. doi: 10.4161/cbt.4.12.2233. Epub 2005 Dec 12. [DOI] [PubMed] [Google Scholar]
- 48.Abrams A. Use of vitamin E in chronic cystic mastitis. N. Engl. J. Med. 1965;272:2388. [Google Scholar]
- 49.Prasad KN, Edwards-Prasad J. Effects of tocopherol (vitamin E) acid succinate on morphological alterations and growth inhibition in melanoma cells in culture. Cancer Res. 1982 Feb;42(2):550–555. [PubMed] [Google Scholar]
- 50.Sundaram GS, London R, Margolis S, Wenk R, Lustgarten J, Nair PP, Goldstein P. Serum hormones and lipoproteins in benign breast disease. Cancer Res. 1981 Sep;41(9 Pt 2):3814–3816. [PubMed] [Google Scholar]
- 51.Cook MG, McNamara P. Effect of dietary vitamin E on dimethylhydrazine-induced colonic tumors in mice. Cancer Res. 1980 Apr;40(4):1329–1331. [PubMed] [Google Scholar]
- 52.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993 Sep 15;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 53.Buiatti E, Palli D, Bianchi S, Decarli A, Amadori D, Avellini C, Cipriani F, Cocco P, Giacosa A, Lorenzini L, et al. A case-control study of gastric cancer and diet in Italy. III. Risk patterns by histologic type. Int J Cancer. 1991 May 30;48(3):369–374. doi: 10.1002/ijc.2910480310. [DOI] [PubMed] [Google Scholar]
- 54.Risch HA, Jain M, Choi NW, Fodor JG, Pfeiffer CJ, Howe GR, Harrison LW, Craib KJ, Miller AB. Dietary factors and the incidence of cancer of the stomach. Am J Epidemiol. 1985 Dec;122(6):947–959. doi: 10.1093/oxfordjournals.aje.a114199. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M, Furukawa F, Toyoda K, Sato H, Hasegawa R, Hayashi Y. Effects of four antioxidants on N-methyl-N'-nitro-N-nitrosoguanidine initiated gastric tumor development in rats. Cancer Lett. 1986 Feb;30(2):161–168. doi: 10.1016/0304-3835(86)90084-4. [DOI] [PubMed] [Google Scholar]
- 56.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994 Apr 14;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 57.Alkhenizan A, Hafez K. The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trials. Ann Saudi Med. 2007 Nov-Dec;27(6):409–414. doi: 10.5144/0256-4947.2007.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kushi LH, Fee RM, Sellers TA, Zheng W, Folsom AR. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. Am J Epidemiol. 1996 Jul 15;144(2):165–174. doi: 10.1093/oxfordjournals.aje.a008904. [DOI] [PubMed] [Google Scholar]
- 59.Rohan TE, Howe GR, Friedenreich CM, Jain M, Miller AB. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993 Jan;4(1):29–37. doi: 10.1007/BF00051711. [DOI] [PubMed] [Google Scholar]
- 60.Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE, Willett WC. A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. N Engl J Med. 1993 Jul 22;329(4):234–240. doi: 10.1056/NEJM199307223290403. [DOI] [PubMed] [Google Scholar]
- 61.Palan PR, Goldberg GL, Basu J, Runowicz CD, Romney SL. Lipid-soluble antioxidants: beta-carotene and alpha-tocopherol levels in breast and gynecologic cancers. Gynecol Oncol. 1994 Oct;55(1):72–77. doi: 10.1006/gyno.1994.1250. [DOI] [PubMed] [Google Scholar]
- 62.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009 Jan 7;301(1):52–62. doi: 10.1001/jama.2008.862. Epub 2008 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, Albanes D, Thompson IM. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010 Sep 1;172(5):566–577. doi: 10.1093/aje/kwq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villaverde C, Baucells MD, Manzanilla EG, Barroeta AC. High levels of dietary unsaturated fat decrease alpha-tocopherol content of whole body, liver, and plasma of chickens without variations in intestinal apparent absorption. Poult Sci. 2008 Mar;87(3):497–505. doi: 10.3382/ps.2007-00292. [DOI] [PubMed] [Google Scholar]
- 65.Institute of Medicine. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. Food and Nutrition Board. [Google Scholar]
- 66.Torricelli P, Caraglia M, Abbruzzese A, Beninati S. γ-Tocopherol inhibits human prostate cancer cell proliferation by up-regulation of transglutaminase 2 and down-regulation of cyclins. Amino Acids. 2012 Mar 30; doi: 10.1007/s00726-012-1278-y. [DOI] [PubMed] [Google Scholar]
- 67.Conte C, Floridi A, Aisa C, Piroddi M, Floridi A, Galli F. Gamma-tocotrienol metabolism and antiproliferative effect in prostate cancer cells. Ann N Y Acad Sci. 2004 Dec;1031:391–394. doi: 10.1196/annals.1331.054. [DOI] [PubMed] [Google Scholar]
- 68.Galli F, Stabile AM, Betti M, Conte C, Pistilli A, Rende M, Floridi A, Azzi A. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys. 2004 Mar 1;423(1):97–102. doi: 10.1016/j.abb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 69.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohé R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002 Oct;132(10):3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 70.McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000 Sep;224(4):292–301. doi: 10.1046/j.1525-1373.2000.22434.x. [DOI] [PubMed] [Google Scholar]
- 71.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008 Aug 15;123(4):739–752. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 72.Fu JY, Blatchford DR, Tetley L, Dufès C. Tumor regression after systemic administration of tocotrienol entrapped in tumor-targeted vesicles. J Control Release. 2009 Dec 3;140(2):95–99. doi: 10.1016/j.jconrel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 73.Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem. 2011 Oct;112(10):2773–2783. doi: 10.1002/jcb.23184. [DOI] [PubMed] [Google Scholar]
- 74.Ji X, Wang Z, Sarkar FH, Gupta SV. Delta-tocotrienol augments cisplatin-induced suppression of non-small cell lung cancer cells via inhibition of the Notch-1 pathway. Anticancer Res. 2012 Jul;32(7):2647–2655. [PubMed] [Google Scholar]
- 75.Lehmann J, Rao DD, Canary JJ, Judd JT. Vitamin E and relationships among tocopherols in human plasma, platelets, lymphocytes, and red blood cells. Am J Clin Nutr. 1988 Mar;47(3):470–474. doi: 10.1093/ajcn/47.3.470. [DOI] [PubMed] [Google Scholar]
- 76.Takada T, Suzuki H. Molecular mechanisms of membrane transport of vitamin E. Mol Nutr Food Res. 2010 May;54(5):616–622. doi: 10.1002/mnfr.200900481. [DOI] [PubMed] [Google Scholar]
- 77.Orsó E, Broccardo C, Kaminski WE, Böttcher A, Liebisch G, Drobnik W, Götz A, Chambenoit O, Diederich W, Langmann T, Spruss T, Luciani MF, Rothe G, Lackner KJ, Chimini G, Schmitz G. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet. 2000 Feb;24(2):192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 78.Ni J, Wen X, Yao J, Chang HC, Yin Y, Zhang M, Xie S, Chen M, Simons B, Chang P, di Sant'Agnese A, Messing EM, Yeh S. Tocopherol-associated protein suppresses prostate cancer cell growth by inhibition of the phosphoinositide 3-kinase pathway. Cancer Res. 2005 Nov 1;65(21):9807–9816. doi: 10.1158/0008-5472.CAN-05-1334. [DOI] [PubMed] [Google Scholar]
- 79.Morley S, Thakur V, Danielpour D, Parker R, Arai H, Atkinson J, Barnholtz-Sloan J, Klein E, Manor D. Tocopherol transfer protein sensitizes prostate cancer cells to vitamin E. J Biol Chem. 2010 Nov 12;285(46):35578–35589. doi: 10.1074/jbc.M110.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wright ME, Peters U, Gunter MJ, Moore SC, Lawson KA, Yeager M, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Association of variants in two vitamin e transport genes with circulating vitamin e concentrations and prostate cancer risk. Cancer Res. 2009 Feb 15;69(4):1429–1438. doi: 10.1158/0008-5472.CAN-08-2343. Epub 2009 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lemaire-Ewing S, Desrumaux C, Néel D, Lagrost L. Vitamin E transport, membrane incorporation and cell metabolism: Is alpha-tocopherol in lipid rafts an oar in the lifeboat? Mol Nutr Food Res. 2010 May;54(5):631–640. doi: 10.1002/mnfr.200900445. [DOI] [PubMed] [Google Scholar]
- 82.Muller PY, Netscher T, Frank J, Stoecklin E, Rimbach G, Barella L. Comparative quantification of pharmacodynamic parameters of chiral compounds (RRR- vs. all-rac-alpha tocopherol) by global gene expression profiling. J Plant Physiol. 2005 Jul;162(7):811–817. doi: 10.1016/j.jplph.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 83.Weiser H, Vecchi M. Stereoisomers of alpha-tocopheryl acetate. II. Biopotencies of all eight stereoisomers, individually or in mixtures, as determined by rat resorption-gestation tests. Int J Vitam Nutr Res. 1982;52(3):351–370. [PubMed] [Google Scholar]
- 84.Cheng G, Zielonka J, McAllister DM, Mackinnon AC, Jr, Joseph J, Dwinell MB, Kalyanaraman B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013 Jun 13;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Husain K, Centeno BA, Chen DT, Fulp WJ, Perez M, Zhang Lee G, Luetteke N, Hingorani SR, Sebti SM, Malafa MP. Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E δ-tocotrienol. Carcinogenesis. 2013 Apr;34(4):858–863. doi: 10.1093/carcin/bgt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Husain K, Centeno BA, Chen DT, Fulp WJ, Perez M, Zhang Lee G, Luetteke N, Hingorani SR, Sebti SM, Malafa MP. Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E δ-tocotrienol. Carcinogenesis. 2013 Apr;34(4):858–863. doi: 10.1093/carcin/bgt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chun J, Lee J, Ye L, Exler J, Eitenmiller RR. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J Food Compost Anal. 2006;(19):196–204. [Google Scholar]
- 88.Thomas RG, Gebhardt Beltsville MD, editor. Nuts and seeds as sources of alpha and gamma tocopherols. USDA-ARS Nutrient Data Laboratory. (USDA-abstract) http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Articles/AICR06 NutSeed.pdf.