SUMMARY
Acute myeloid leukemia (AML) therapy involves compounds that are cytotoxic to both normal and cancer cells and relapsed AML is resistant to subsequent chemotherapy. Thus agents are needed that selectively kill AML cells with minimal toxicity. Here we report that AML is dependent on DDX5 and that inhibiting DDX5 expression slows AML cell proliferation in vitro and AML progression in vivo, but is not toxic to cells from normal bone marrow. Inhibition of DDX5 expression in AML cells induces apoptosis via induction of reactive oxygen species (ROS). This apoptotic response can be blocked either by BCL2 overexpression or treatment with the ROS scavenger N-Acetyl-L-cysteine (NAC). Combining DDX5 knockdown with a BCL2 family inhibitor cooperate to induce cell death in AML cells. By inhibiting DDX5 expression in vivo we show that DDX5 is dispensable for normal hematopoiesis and tissue homeostasis. These results validate DDX5 as a potential target for blocking AML.
Keywords: Acute Myeloid Leukemia, DEAD-Box helicase, BCL2, shRNA, cancer therapy validation
INTRODUCTION
Acute myeloid leukemia (AML) is a disease in which the differentiation of hematopoietic progenitor cells is blocked resulting in unregulated expansion of leukemic blasts that is rapidly fatal if untreated. The average age of onset of AML is 66 years and the two-year survival rate for patients older than 56 years is 20-to-25% (Estey and Dohner, 2006). AML chemotherapy is not selective for since it kills normal hematopoietic cells and presents a challenge for treatment, particularly in elderly AML patients due to toxicity. Moreover, chemotherapy resistant AML with unfavorable cytogenetics is markedly prevalent in elderly patients (Yanada and Naoe, 2012). Hence there is a strong demand for new drug targets that when inhibited will selectively block AML without toxicity.
The DEAD-box RNA helicase, DDX5, is a multifunctional protein with an important role in transcription regulation with multiple, sequence-specific transcription factors (Fuller-Pace, 2013), but has been implicated in other processes such as microRNA biology, RNA splicing and ribosome biogenesis (Dardenne et al., 2012; Davis et al., 2008; Fukuda et al., 2007; Saporita et al., 2011). DDX5 is frequently overexpressed in colon, breast, and prostate cancer as well as T-cell acute lymphoblastic leukemia (Causevic et al., 2001; Clark et al., 2008; Lin et al., 2012; Shin et al., 2007; Wortham et al., 2009). Previously we reported that DDX5 functions as a transcriptional co-activator for E2F1 to promote the expression of genes required for cell proliferation and that Ddx5 is frequently amplified in addition to being overexpressed in breast cancer (Mazurek et al., 2012). DDX5 knockdown in breast cancer cells with Ddx5 gene amplification blocked their proliferation and resulted in down-regulated expression of DNA replication factors. In contrast, DDX5 knockdown in breast cancer cells lacking Ddx5 gene amplification did not affect the expression of DNA replication factors and these cells continued to proliferate. Thus epithelial breast cancers that overexpress DDX5 exhibit a greater dependence on DDX5 to proliferate than cancers that do not overexpress DDX5. Recently a requirement for DDX5 in proliferation of T-cell acute lymphoblastic leukemia (T-ALL) cells was described (Lin et al., 2012). In these cells DDX5 interacts with MAML1 to promote the expression of NOTCH-regulated genes, however this study showed that DDX5 is required for initiation of T-ALL in vivo but it remains unclear whether DDX5 inhibition slows progression of established T-ALL or any other cancer.
Here we report results that demonstrate a dependence on DDX5 for proliferation of human acute myeloid leukemia cells containing various genetic lesions. Using a mouse model for chemotherapy resistant AML we demonstrate that inhibition of DDX5 expression slows progression of established AML in vivo. Moreover we developed transgenic mouse lines with doxycycline-inducible, systemic expression of a potent DDX5 shRNA and found that DDX5 depletion did not adversely affect either bone marrow function or adult mouse physiology. These results are consistent with an acquired dependence of AML cells on DDX5 and suggest that DDX5 inhibitors should be effective against AML and well tolerated by normal tissues.
RESULTS
Human AML cell lines are dependent on DDX5 to proliferate
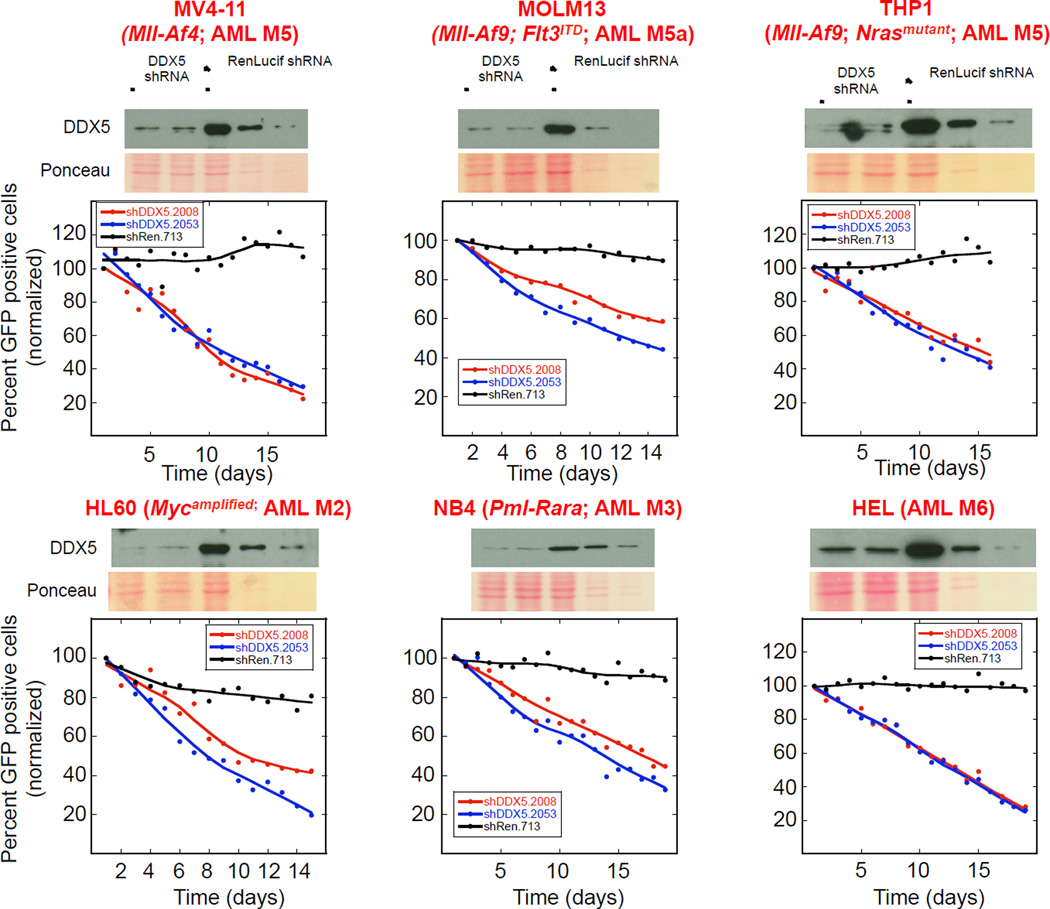
We investigated whether the ability of AML cell lines to proliferate was dependent on DDX5 by measuring the effect of DDX5 depletion on cell proliferation over time after retroviral-mediated shRNA transduction into the cells. Retroviruses encoding either of two potent DDX5 shRNAs (shDDX5.2008 or shDDX5.2053) or a control shRen.713 shRNA (targeting Renilla Luciferase), each linked to GFP, were transduced into AML cell populations that also included GFP negative cells to enable direct comparison in the same culture of the proliferative fitness of DDX5 expressing and depleted cells. DDX5 knockdown impaired proliferation of 7 of 8 human acute myeloid leukemia cell lines having different oncogenic driver mutations (Figure 1 and Figure S1A). Only one cell line, Eol-1, was resistant to DDX5 knockdown (Figure S1B). Immuno-blot analysis of DDX5 in these 8 AML cell lines did not reveal a correlation between DDX5 expression and sensitivity to DDX5 depletion (Figure S1C). These results suggest a broad dependency of genetically diverse human AML cell lines on DDX5 to proliferate in a manner independent of DDX5 protein levels.
Figure 1. AML cell lines are dependent on DDX5 to proliferate.
The indicated AML cell lines were infected with retrovirus encoding GFP expression as well as either of two different DDX5 shRNAs (shDDX5.2008 or shDDX5.2053; first and second lanes on each immuno-blot, respectively) or a control shRNA targeting Renilla Luciferase (shRen.713; third lane on each immuno-blot). Immuno-blots show DDX5 knockdown by the two DDX5 shRNAs. On each immuno-blot whole cell extracts (WCE) prepared from cells infected with the negative control shRen.713 were loaded at either equal total protein as the DDX5 knockdown WCEs (lane 3 on each immuno-blot) or were diluted either 1-to-4 (lane 4 on each immuno-blot) or 1-to-10 (lane 5 on each immuno-blot) so that DDX5 knockdown by either shDDX5.2008 or shDDX5.2053 (lanes 1 and 2 on each immuno-blot) could be determined. Ponceau S stained membranes are shown below each immuno-blot to show protein loading. The effect of DDX5 knockdown on proliferation of each cell line was determined by monitoring the depletion of GFP positive cells expressing the indicated shRNA in each unselected cell culture following infection as described in Experimental Procedures. A reduction in GFP positive cells over time indicates depletion of those cells expressing the indicated shRNA from the cultures. The red and blue lines in each plot shows the fate of cells expressing either the shDDX5.2008 or shDDX5.2053 experimental shRNAs respectively whereas the black line in each plot shows the fate of cells expressing the shRen.713 control shRNA.
DDX5 is required for AML progression in vivo
The dependence of DDX5 for AML progression in vivo was tested using a mouse model of AML (Zuber et al., 2011a; Zuber et al., 2011b). AML is driven by the expression of an MLL-AF9 fusion protein together with constitutively active NRASG12D (Zuber et al., 2011a). The AML cells used in this model express the rtTA tetracycline transactivator allowing doxycycline induced gene knockdown following transduction of the AML cells with a vector encoding an shRNA downstream of a tetracycline-responsive promoter. AML harboring Mll translocations exhibit partial differentiation along the monocytic lineage and AML patients with these mutations have poor prognosis (Schoch et al., 2003). Similar to human Mll-translocated AML, leukemia that develops in this mouse AML model also expresses surface protein markers consistent with partial monocytic differentiation and is refractory to Ara-C chemotherapy (Zuber et al., 2009). Importantly, following AML cell transplantation into recipient mice, leukemia develops with normal expression of the shRNA target gene. Once leukemia is established, as determined by bioluminescence imaging, the mice are given doxycycline in their food and water to induce expression of the shRNA and knockdown of the shRNA target. The effect of target inhibition on progression of established AML is then measured by bioluminescence imaging and monitoring of animal survival (Zuber et al., 2011a; Zuber et al., 2011b).
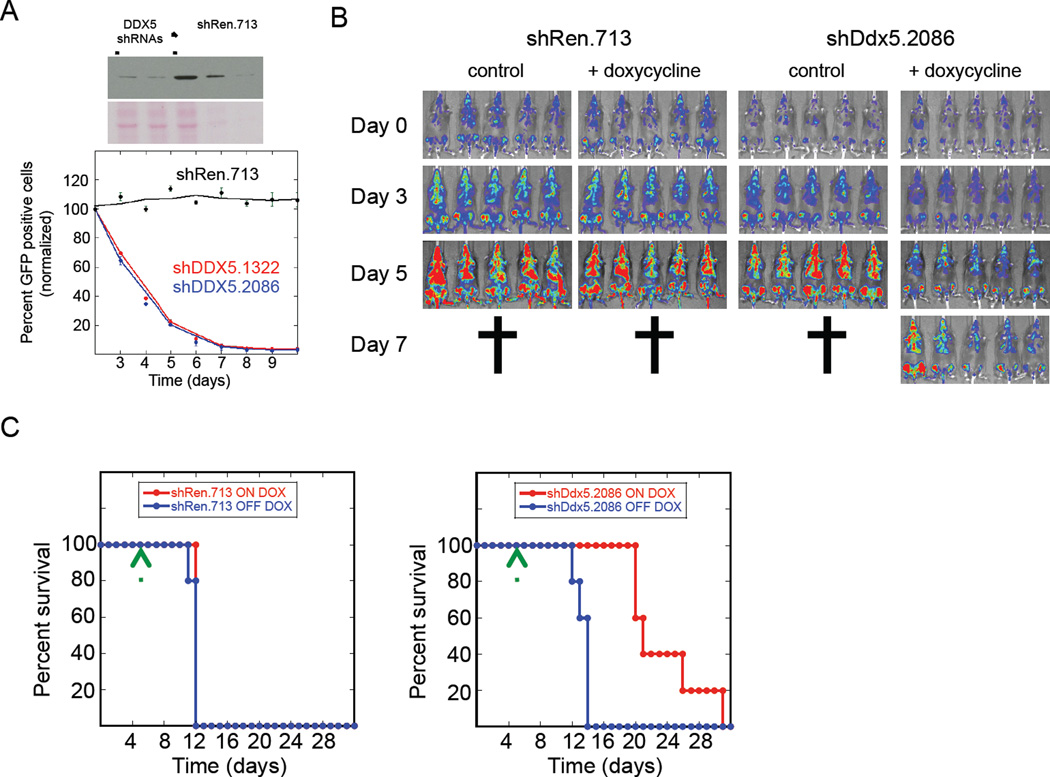
Two independent murine Mll-Af9; NrasG12D AML cell lines were tested here (RN2 and LG1 cell lines), that were derived from the spleens of terminally ill leukemic mice and cultured. The cells were then infected with virus encoding either of 2 different potent DDX5 shRNAs (shDDX5.1322 and shDDX5.2086) or the control shRen.713 shRNA in which shRNA expression was either constitutive (LG1 cell line series) or controlled by a doxycycline-inducible promoter (RN2 cell line series). The competition proliferation assay was applied to measure the effect of DDX5 knockdown on proliferation of LG1 cells. DDX5 knockdown by both DDX5 shRNAs substantially depleted LG1 cells in the cultures (Figure 2A).
Figure 2. DDX5 is required for AML progression in vivo.
(A) Mouse LG1 AML cells were infected with virus encoding GFP and either of two different shRNAs targeting murine DDX5 (shDDX5.1322 or shDDX5.2086) or shRen.713. ShRNA expression in LG1 cells is constitutive following infection. Similar to Figure 1 WCE prepared from LG1 cells transduced with shRen.713 is loaded onto the immuno-blot either undiluted (lane 3), diluted 1-to-4 (lane 4) or 1-to-10 (lane 5) and WCE from LG1 cells transduced with either shDDX5.1322 or shDDX5.2086 are loaded in lanes 1 and 2 respectively. The immunoblot in the upper panel is detecting DDX5 protein expression and the lower panel is the Ponceau S stained membrane to show protein loading in the wells. Cell proliferation was analyzed using the competition proliferation assay as described in Figure 1. Data is presented as the mean from triplicate samples with standard deviations shown. (B) Clonal RN2 AML cells selected for either doxycycline-induced shDDX5.2086 or shRen.713 shRNA expression were transplanted by tail vein injection from leukemic spleens into secondary recipient mice as described in Experimental Procedures. The AML cells constitutively express Firefly luciferase enabling bioluminescence imaging of the transplanted leukemia cells in vivo. Day 0 indicated to the left of the bioluminescence imaging results indicates the time-point 5 days after bone marrow transplantation with AML cells when leukemia was established in the mice and they were separated into either doxycycline treated or untreated groups. (C) Kaplan-Meier curves showing survival of mice transplanted with AML cells either induced or not induced to express the indicated shRNAs. Time zero on these plots indicates the time-point when the AML cells were transplanted into the mice. Doxycycline was begun on day 5 post-transplantation as indicated by the green arrow.
Clonal RN2 cells harboring doxycycline-inducible DDX5 shRNAs were derived and transplanted into sub-lethally irradiated recipient mice (see Experimental Procedures). The RN2 cells constitutively express firefly luciferase to enable bioluminescence imaging of leukemia onset and progression in mice following transplantation. Five days following transplantation a relatively uniform leukemia signal was detected and the mice were separated into doxycycline-free or + doxycycline treatment groups (indicated as day 0 in Figure 2B). Doxycycline induced expression of either the DDX5 experimental or Renilla luciferase control shRNAs in the leukemia cells. Whereas leukemia rapidly progressed to terminal stage within 7 days for doxycycline treated shRen.713 control mice, leukemia progression was significantly attenuated in the + doxycycline mice transplanted with RN2 cells expressing either of the two different DDX5 shRNAs (Figure 2B and Figures S2A and S2B). DDX5 knockdown also significantly increased the length of mouse survival post-transplantation (Figure 2C and Figures S2C). These results indicate that inhibition of DDX5 expression slows progression of established AML in vivo.
Inhibition of DDX5 expression kills AML cells by apoptosis
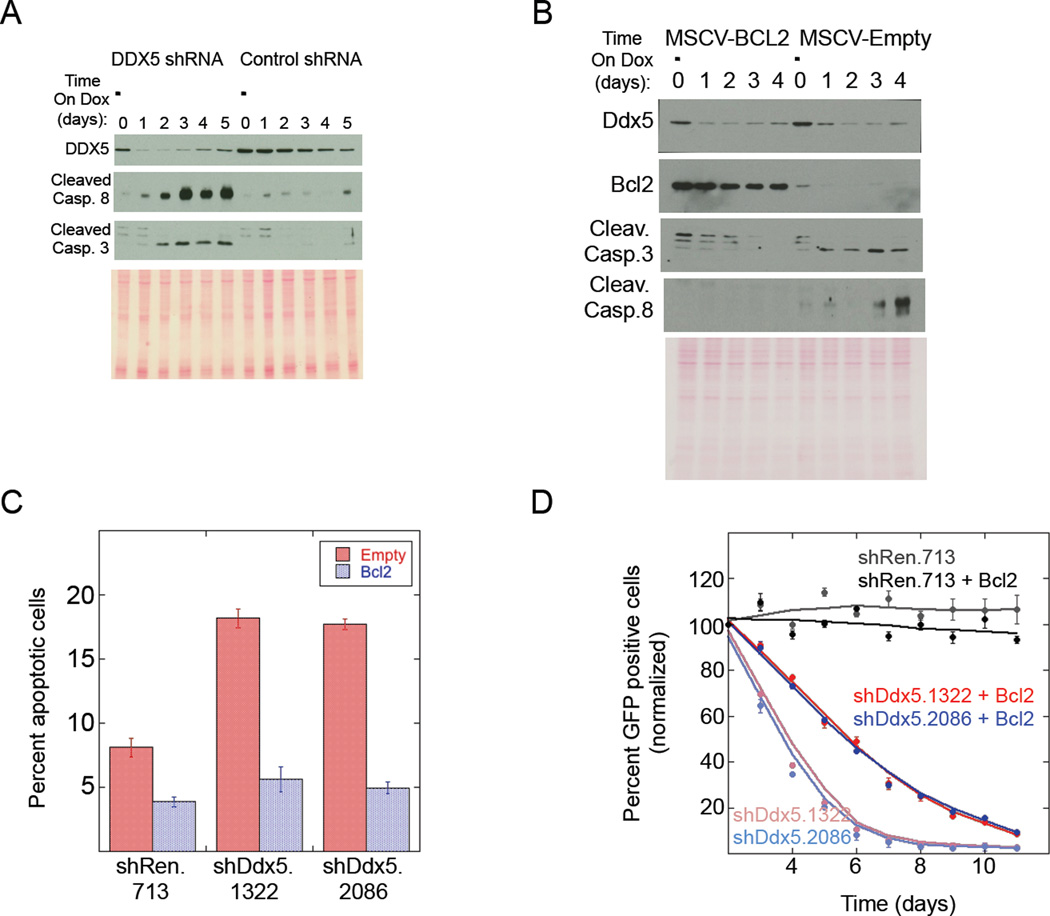
The mechanism by which AML cells are dependent on DDX5 to proliferate was investigated in the RN2 AML cell line since DDX5 knockdown in these cells was inducible and rapid (Figure 3A). Maximal DDX5 depletion was observed within 24hrs following induction of shRNA expression. Within 48hrs of doxycycline-treatment there was strong activation of both Caspase 3 and Caspase 8 indicating that DDX5 knockdown induced apoptosis. This result was specific for cells with DDX5 knockdown compared to the shRen.713 control shRNA. Moreover, RN2 cells with DDX5 knockdown were rapidly selected against in the cultures as evident by the re-appearance of DDX5 signal in the cultures expressing the DDX5 shRNA within 5 days after doxycycline treatment, due to loss or silencing of the DDX5 shRNA. Similar experiments were performed in immortalized MEF cells and despite strong DDX5 depletion in these cells their proliferation was not affected and there was no activation of either Caspase 3 or 8 (Figures S2D and S2E). These results indicate a cell-type specific DDX5 requirement in AML cells to promote cell survival.
Figure 3. DDX5 depletion induces apoptosis in AML cells that can be blocked by BCL2 overexpression.
(A) Immuno-blot analysis of whole cell extracts (WCEs) from doxycycline treated RN2 cultures at increasing time after doxycycline treatment. The DDX5 shRNA tested was shDDX5.2086 and the control shRNA is shRen.713. (B) Immuno-blot analysis similar to (A) except the RN2 cells were induced to express shDDX5.2086 in the presence (MSCV-Bcl2) or absence (MSCV-Empty) of BCL2 overexpression. (C) Plot showing the percent of apoptotic cells (Annexin V positive) in the indicated RN2 cultures +/− BCL2 overexpression. All cultures were treated with doxycycline to induce expression of the indicated shRNAs. Data is presented as the mean from triplicate samples with standard deviations shown. The blue bar shows results for cells overexpressing BCL2 and the red bars show results for cells not overexpressing BCL2. (D) Competition cell proliferation assay for LG1 cells constitutively expressing the indicated shRNAs +/− BCL2 overexpression. Data is presented as the mean from triplicate samples with standard deviations shown.
The apoptotic response induced upon DDX5 knockdown was independent of p53 activation (Figure S2F) but could be rescued by overexpressing BCL2, as shown by measuring Caspase cleavage and Annexin V staining (Figures 3B and 3C). This result implied that inhibition of DDX5 expression was activating Caspase 3 and Caspase 8 via the intrinsic apoptotic pathway. BCL2 stabilizes mitochondria and prevents cytochrome c release to activate apoptosis, however BCL2 should not interfere with Caspase 8 cleavage resulting from death receptor activation. BCL2 overexpression blocked Caspase 8 cleavage, suggesting that this caspase was activated downstream of cytochrome c release from the mitochondria rather than from death receptor activation. Interestingly, BCL2 overexpression did not completely rescue proliferation of the DDX5-depleted AML cells (Figure 3D). Thus DDX5 depletion slowed cell proliferation via a non-BCL2 dependent mechanism in addition to inducing apoptosis via a BCL2-dependent mechanism.
The dependence of the RNA helicase activity of DDX5 to proliferate was investigated, however RNAi resistant Ddx5 transgenes could not be expressed in cancer cell lines (Mazurek et al., 2012). Others have reported overexpression of Ddx5 transgenes expressing an N-terminal epitope tag (Shin et al., 2007) and therefore shDDX5.2008-resistant, Ddx5 transgenes encoding either an HA- or 6myc-N-terminal tag were expressed. The transgenes were either wild type DDX5 or mutant DDX5 that contained amino acid changes that block the RNA helicase activity by abolishing either ATP binding (GNT) or ATP hydrolysis (DQAD) (Jalal et al., 2007). Retroviral gene transfer was used to transduce these N-terminally tagged, RNAi resistant wild-type or mutant Ddx5 transgenes into cancer cell lines. We could detect stable HA- and 6myc-DDX5 expression in HCT116 cells. Expression of HA-DDX5 was stronger than 6myc-DDX5 in these cells (data not shown). Endogenous DDX5 was knocked down by subsequently infecting these derivative HCT116 cell lines with virus encoding shDDX5.2008. Expression of the wild-type and mutant RNAi resistant DDX5 proteins were relatively uniform after knockdown of endogenous DDX5 (Figure S3A). As expected knockdown of endogenous DDX5 impaired proliferation of HCT116 cells (Figure S3B). Expression of RNAi resistant wild-type HA-DDX5 rescued HCT116 cell proliferation. However neither expression of the ATP binding nor ATP hydrolysis HA-DDX5 mutants rescued HCT116 proliferation, suggesting that RNA helicase activity of DDX5 was required for cancer cell proliferation.
We were unable obtain human AML cell lines that stably expressed either the untagged or N-terminal tagged Ddx5 transgenes, suggesting that DDX5 overexpression in AML cells was not tolerated. Furthermore, while we were able to obtain RN2 AML cells resistant to the antibiotic selection marker (puromycinR) co-expressed with the Ddx5 transgenes, immuno-blot analysis failed to reveal HA-tagged DDX5 protein expression (Figure S3C). Despite our inability to detect the HA-DDX5 protein in these cells, increased Ddx5 transcript in cells transduced with the HA-Ddx5 transgenes was clearly detected (Figure S3D). These results indicate that DDX5 protein level is tightly regulated in AML where overexpression or inhibition of DDX5 expression has deleterious consequences to AML cell proliferation.
Inhibition of DDX5 expression induces oxidative stress in AML cells
RNA-Seq analyses of gene expression changes at time-points prior to and concurrent with activation of apoptotic signaling following DDX5 knockdown were performed to gain insight into how DDX5 depletion slows AML cell proliferation and induces apoptosis. Expression changes were measured at both 24hrs and 48hrs following induction of DDX5 shRNA expression in RN2 cells. These were time-points that corresponded to maximal DDX5 knockdown and preceded the 72hr time-point when depletion of RN2 cells with DDX5 knockdown first became evident in cell cultures (Figures 3A and 3D).
Genes exhibiting greater than 1.5-fold change in expression in RN2 cells with DDX5 knockdown with either of the two different DDX5 shRNAs compared to RN2 cells expressing the control shRen.713 shRNA were identified. Poorly expressed genes with RPKM values (reads per kilobase per million reads) of 3 or less were excluded from this analysis. At 24hrs following DDX5 shRNA induction there were 125 differentially expressed genes for the shDDX5.1322 vs. shRen.713 triplicate and 104 differentially expressed genes for the shDDX5.2086 vs. shRen.713 triplicate where the two gene lists shared 61 overlapping genes (Table S1). At 48hrs following induction of DDX5 shRNA expression there were 349 differentially expressed genes for the shDDX5.1322 vs. shRen.713 triplicate and 318 differentially expressed genes for the shDDX5.2086 vs. shRen.713 triplicate where these two gene lists shared 236 overlapping genes (Table S2). Thus considerable overlap in differentially expressed genes was observed following knockdown of DDX5 with either of two different DDX5 shRNAs.
Previously we analyzed gene expression changes in the human colorectal cancer cell line, HCT116 (Mazurek et al., 2012) that overexpressed DDX5 compared to colon epithelial cells (Shin et al., 2007). We compared the genes whose expression were consistently affected by either of the 2 different DDX5 shRNAs at either 24hrs or 48hrs after DDX5 knockdown with the genes whose expression were significantly affected by DDX5 knockdown in HCT116 cells also at 24hrs and 48hrs after DDX5 knockdown and found only 9 overlapping genes at 24hrs (Figure S4A - top) and 15 overlapping genes at 48hrs (Figure S4A - bottom). This modest overlap at both time-points indicated that DDX5 regulates the expression of different genes in different cancer cells.
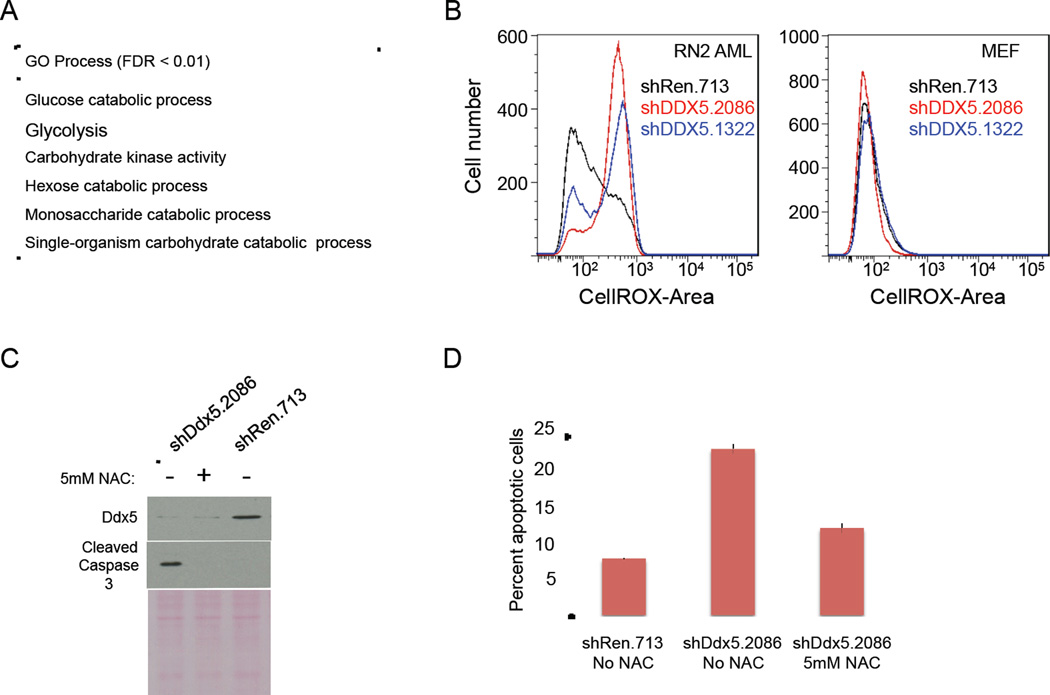
Two different types of analysis were performed on the RNA-Seq data. First processes enriched in the differentially expressed genes resulting from DDX5 knockdown was determined using Gene Ontology (GO) groups with between 10 and 100 genes (~2800 GO groups). For this analysis the “ROC” (Receiver Operator Characteristic) method for ermineJ across the differential expression q-values was applied for each dataset (Gillis et al., 2010). At the 24hr time-point specific enrichment for both DDX5 shRNAs compared to the shRen.713 control were obtained for the GO process “negative regulation of myeloid cell differentiation” (False Discovery Rate; FDR<0.05). At the 48hr time-point with an FDR cut-off of 0.05 twenty GO processes significantly enriched for both DDX5 shRNAs were observed (Table S3). Applying an FDR cut-off of 0.01, enrichment for only metabolic functions were seen suggesting that this is a robust and specifically characteristic observation (Figure 4A). In most cases expression of metabolic genes were downregulated suggesting metabolic stress in AML cells with DDX5 knockdown.
Figure 4. Inhibition of DDX5 expression downregulates the expression of glucose metabolism genes and elevates reactive oxygen species to induce in apoptosis.
(A) Enrichment analysis indicating biological processes enriched for genes differentially expressed as a consequence of DDX5 knockdown in AML cells. Only pathways scoring with an FDR<0.01 in this analysis are shown. (B) Flow cytometry analysis of levels of ROS in AML (left plot) or MEF (right plot) cells +/− DDX5 knockdown using a ROS-activated fluorescent compound (CellROX – BD Biosciences). The black line shows results for cells expressing the control shRen.713 shRNA, the red line shows results for cells expressing the shDDX5.2086 shRNA, and the blue line shows results for cells expressing the shDDX5.1322 shRNA. (C) Immuno-blot analysis of RN2 WCE’s +/− DDX5 knockdown either treated or untreated with 5mM NAC. (D) Flow cytometry analysis of apoptotic cells (Annexin V positive) in AML cell cultures +/− DDX5 knockdown +/− 5mM NAC. Data is presented as the mean from triplicate samples with standard deviations shown.
In addition to the enrichment analysis, co-expression network analysis was performed on the genes differentially expressed following DDX5 knockdown (see Supplemental Experimental Procedures and Figure S5). Enrichment analysis is dependent on prior characterization of gene function and the annotated functions of genes may be biased by prior interest. Co-expression network analysis offers an alternative approach to determine whether differentially expressed genes resulting from DDX5 knockdown have a shared property independent of prior annotation of gene function by determining whether these genes exhibit similar expression profiles (co-expression). Our analysis revealed that the differentially expressed genes resulting from DDX5 knockdown preferentially clustered with a cancer gene co-expression network compared to a brain-based gene co-expression network (Figure S5B, ROC difference ~0.1). This analysis indicated that DDX5 regulated the expression of genes much more relevant to cancer than normal cells such as neurons. Moreover, this entirely orthogonal mode of analysis to the enrichment analysis revealed enrichment for genes involved in monosaccharide metabolic process and hexose metabolic process in the tail end of the distribution of ROC differences in Figure S5B (ROC difference > 0.05) Thus gene expression analysis using two orthogonal approaches pointed to cell metabolism as a process influenced by DDX5 knockdown.
Metabolic stress can alter cellular NADPH production affecting the redox environment in the cell to trigger an increase in reactive oxygen species (ROS) levels and induce an apoptotic response (Andersen and Kornbluth, 2013). DDX5 regulation of cell metabolism genes suggested that DDX5 knockdown might trigger an increase in ROS levels in AML cells to promote apoptosis. Indeed ROS levels were elevated in AML cells after DDX5 knockdown (Figure 4B). Importantly, the elevated ROS level was sufficient to induce apoptosis since treating the AML cells with the exogenous ROS scavenger N-acetyl-L-cysteine (NAC) blocked induction of apoptosis in DDX5-depleted AML cells (Figure 4C and 4D).
In addition to apoptosis, increased ROS levels can also induce AML cells to differentiate (Callens et al., 2010). The effect of DDX5 knockdown on differentiation of AML cells was tested by comparing gene expression changes in the RN2 cells after DDX5 knockdown with gene expression changes in these cells following either withdrawal of expression of the Mll-Af9 oncogene (Zuber et al., 2011a) or BRD4 knockdown (Zuber et al., 2011b), the consequences of which have been shown to induce differentiation of these cells. We found very little overlap for genes affected by DDX5 knockdown (Figures S4B - top and S4B - bottom), which is inconsistent with DDX5 knockdown activating a differentiation gene expression signature. Moreover, DDX5 knockdown did not alter RN2 cell morphology or affect the expression of surface markers indicative of differentiation (Figures S4C and S4D). Thus DDX5 depletion activated apoptosis and did not promote myeloid cell differentiation.
Inhibition of DDX5 expression cooperates with ABT-737 to kill AML cells
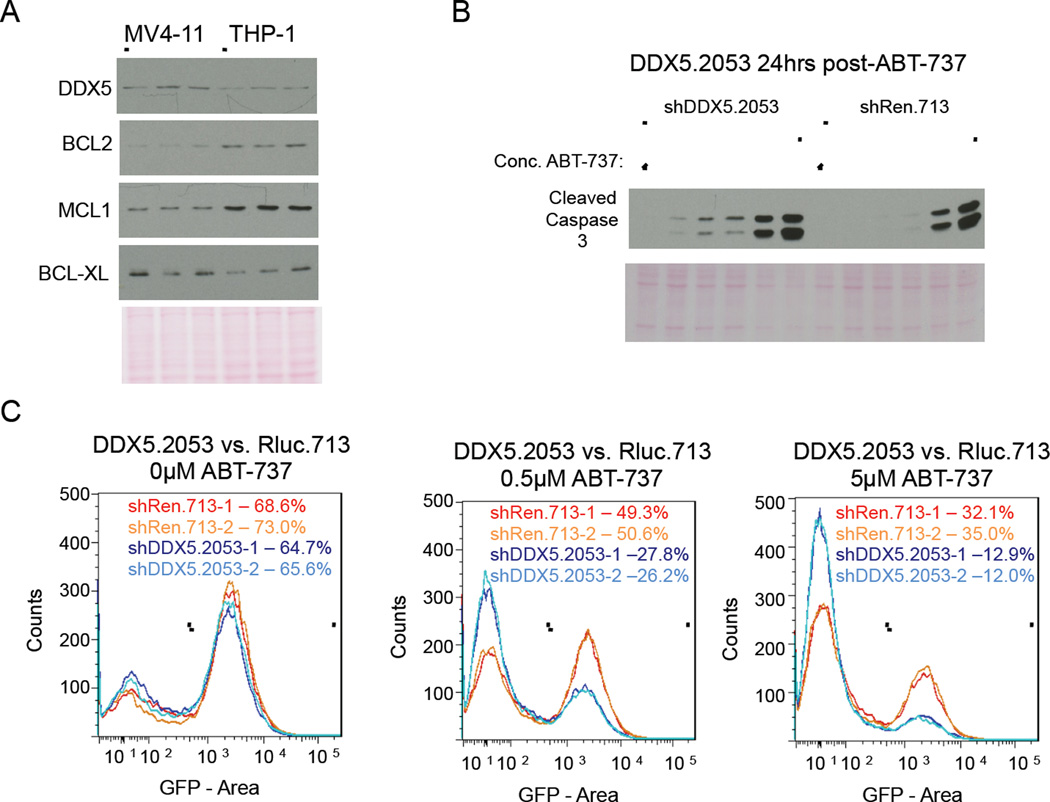
Since BCL2 is frequently overexpressed in AML (Tzifi et al., 2012) we considered whether a DDX5 requirement for AML cell survival might inversely correlate with BCL2 levels. DDX5 knockdown in MV4-11 cells resulted in apoptosis whereas DDX5 knockdown in THP1 cells did not (Figure S6A and Figure 5B, lane 1). Similar to the RN2 AML cell line, DDX5 knockdown in MV4-11 cells induced apoptosis but did not activate a p53 response (Figure S6B). Interestingly, both BCL2 and MCL1 are overexpressed in THP1 cells compared to MV4-11 cells (Figure 5A). It is noteworthy though that DDX5 knockdown still impaired THP1 cell proliferation (Figure 1) mirroring our results with RN2 cells we engineered to overexpress BCL2 (Figure 3D).
Figure 5. Combined inhibition of DDX5 and BCL2 family proteins cooperate to induce apoptosis in THP1 AML cells.
(A) Immuno-blot analysis of BCL2 family proteins in WCEs from the indicated AML cell lines. Note WCEs prepared from 50,000 cells per cell line were loaded onto the immuno-blots and results for triplicate independently prepared WCEs from each cell line are presented. (B) Immuno-blot analysis of Caspase 3 activation for THP1 cells +/− DDX5 knockdown treated for 24hrs with increasing concentration of ABT-737. Concentrations of ABT-737 tested were 0µM, 0.1µM, 0.5µM, 1µM, 5µM, and 10µM. (C) Flow cytometry analysis of live GFP positive cells in THP1 cultures +/− DDX5 knockdown untreated or treated for 24hrs with either low dose (0.5µM) or high dose (5µM) ABT-737. Results from duplicate cultures per RNAi condition are presented. GFP positive cells were gated and the percent of GFP positive cells in each culture are shown.
We next considered whether inhibiting BCL2 function might sensitize THP1 cells to undergo apoptosis following DDX5 knockdown. ABT-737 is a BH3 mimetic compound that blocks the interaction of pro- and anti-apoptotic BCL2 family proteins to result in an apoptotic response in the presence of pro-apoptotic signaling (Cory and Adams, 2005; Oltersdorf et al., 2005). The BCL2 family inhibitor, ABT-737, was titrated onto THP1 cells with or without DDX5 knockdown (shDDX5.2053 versus the negative control shRen.713; Figure 5B). Combining DDX5 knockdown with ABT-737 resulted in apoptosis at a much lower dose of ABT-737 (0.5µM) compared to THP1 cells treated with ABT-737 alone (5µM). Moreover, combining DDX5 knockdown with ABT-737 treatment resulted in substantially fewer GFP-positive live cells within 24hrs of drug treatment compared to ABT-737 treatment alone (Figure 5C). We conclude that DDX5 knockdown induces an apoptotic signal in THP1 cells that is blocked by BCL2.
DDX5 knockdown is well tolerated in normal adult tissues
DDX5 depletion blocks the proliferation of colon and breast cancer cells (Mazurek et al., 2012) and induces apoptosis in T-ALL (Lin et al., 2012) and AML cells. However it is unclear whether normal adult tissues will be similarly sensitive to inhibition of DDX5 expression. A Ddx5 knockout is incompatible with embryonic development (Fukuda et al., 2007) and Ddx5 inactivation by Cre-mediated gene excision in adult mice was reported to lead to apoptotic cells in the bone marrow and altered tissue organization of the large intestine (Nicol et al., 2012). However the long-term consequences of systemic DDX5 suppression on normal physiology has not been thoroughly examined.
Transgenic mice with doxycycline-inducible, systemic expression of a potent DDX5 shRNA were developed to test the effect of DDX5 depletion in adult tissues. This method allows reversible gene knockdown in normal tissues of an adult mouse followed by monitoring for adverse consequences (Dow et al., 2012). The DDX5 shRNA was cloned into the 3’ untranslated region of a Gfp transgene and expression of both GFP and the shRNA was induced in tissues by giving the transgenic mice doxycycline in their food and water. To generate the transgenic DDX5 shRNA mice clonal lines of KH2 mouse embryonic stem (ES) cells (Beard et al., 2006) encoding the reverse Tet-transactivator (rtTA) at the Rosa26 enhancer and shDDX5.2086 in the Col1A1 locus were established and tested for doxycycline-induced GFP expression and DDX5 knockdown in vitro. An ES cell clone was selected that showed tight doxycycline-induced GFP expression and greater than 10-fold DDX5 knockdown (Figure S7A). Using tetraploid embryo complementation, founder mice were derived from the ES cell clone and these mice were subsequently bred (Figure S7B) to obtain mice homozygous for both the Rosa26-rtTA allele and Col1A1-shDdx5.2086 allele (Rosa26-rtTA+/+, Col1A1-shDdx5.2086+/+). The mice were bred in the absence of doxycycline and they yielded pups with the expected Mendelian ratios for the Rosa26-rtTA and Col1A1-shDdx5.2086 alleles. At 8-to-12 weeks of age the mice were separated into 2 groups where one group was given a normal diet and the second group was given water and food containing doxycycline to induce DDX5 shRNA expression.
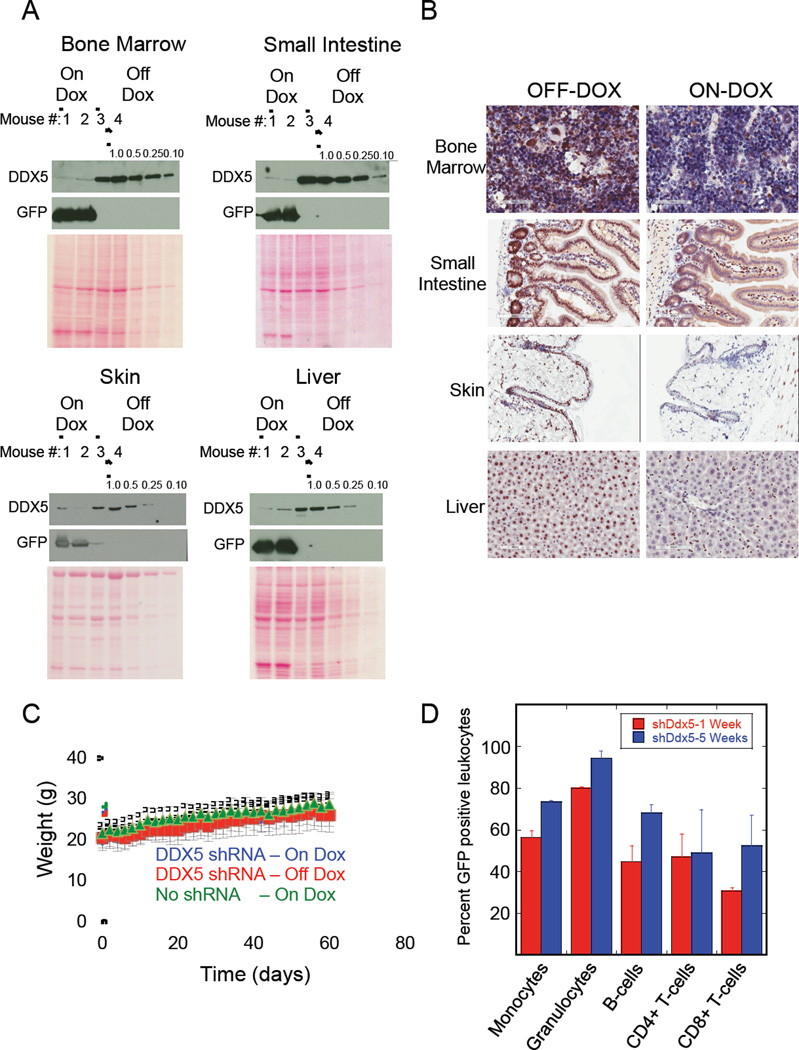
After 2 weeks of doxycycline treatment several mice were sacrificed and their organs dissected for analysis. Immuno-blots showed strong doxycycline-induced GFP expression as well as 4-to-greater than 10 fold knockdown of DDX5 in whole cell extracts prepared from bone marrow, intestine, skin, liver, thymus, and pancreas (Figure 6A and Figure S7C). Immuno-histochemical analysis of DDX5 in these tissues revealed the expected strong nuclear staining for DDX5 that was ubiquitous in the organs dissected from animals fed the doxycycline-free diet (Figure 6B and Figure S7D). This DDX5 signal was largely depleted in most cell types that comprise these tissues dissected from mice fed doxycycline. Western blot analysis of tissue extracts revealed that DDX5 knockdown was particularly strong (greater than 10-fold in the organ as a whole) in the intestine, bone marrow, thymus, and pancreas. In the small intestine DDX5 depletion was evident in the nuclei of cells that comprise both the villi and the Crypts of Lieberkuhn (Figure S8). In liver, DDX5 knockdown was strong in hepatocytes but weak in Kupffer cells. Hence the 4-to-10 fold depletion of DDX5 observed in the immuno-blot analysis of these tissues may be an underestimate of DDX5 knockdown in a particular cell type. DDX5 knockdown in heart and lung was generally weak by both immuno-blot (Figure S7C) and immuno-histochemical analysis (Figure S7D).
Figure 6. DDX5 knockdown in adult mice is well tolerated.
(A) Immuno-blot analysis of the indicated proteins in WCEs prepared from the noted organs of duplicate mice either treated (ON-DOX-lanes 1 and 2) or untreated (OFFDOX-lanes 3 and 4) with doxycycline to induce shRNA expression. WCEs prepared from the organs of the fourth OFF-DOX mouse is loaded on each blot either with equal total protein loading as the WCEs from the other mice (lane 4) or they were diluted either 1-to-2 (lane 5), 1-to-4 (lane 6) or 1-to-10 (lane 7). (B) Representative tissue sections obtained from mice +/− doxycycline stained to detect DDX5 protein. The brown staining marks DDX5 protein in the tissues. For the ON-DOX mice presented in both (A) and (B) the organs were dissected after 2 weeks of continuous doxycycline to induce shRNA expression. (C) Weight measurements of mice either given or not given doxycycline that were measured over 2 months. Data is presented as the mean from multiple mice (n=6 for each genotype/doxycycline group) with standard deviations shown. (D) Flow cytometry analysis of GFP positive DDX5 shRNA expressing peripheral blood leukocytes at either 1 week or 5 weeks of continuous doxycycline administration. Data is presented as the mean from duplicate mice with standard deviations shown.
Mice were maintained on a doxycycline for 2 months and their weights were recorded to determine whether DDX5 depletion caused changes in weight, an indicator of stress. As an additional control we included doxycycline-fed mice that were homozygous for the Rosa26-rtTA allele but negative for the Col1A1-shDdx5.2086 allele (No shRNA -Rosa26-rtTA+/+, Col1A1-shDdx5.2086−/−). Mice with DDX5 depletion continued to gain weight over 2 months of knockdown similar to Rosa26-rtTA+/+, Col1A1-shDdx5.2086+/+ mice not given doxycycline or the control No shRNA mice that were given doxycycline (Figure 6C). We also analyzed peripheral blood leukocytes to determine whether 10-fold DDX5 knockdown in the bone marrow had an adverse effect on white blood cell production. GFP was tested as a surrogate marker of DDX5 shRNA expression in the different leukocyte sub-populations. If DDX5 depletion adversely affected the differentiation of leukocytes then over time of doxycycline treatment it was expected that the GFP positive cells in the affected leukocyte sub-population would be depleted from peripheral blood, presumably because precursor cells that lack GFP expression and DDX5 knockdown would outcompete. The fraction of GFP positive leukocytes in peripheral blood from mice treated with doxycycline for either 1 week or 5 weeks showed no reduction in the percent of GFP positive peripheral blood leukocytes (Figure 6D). The lack of depletion of GFP positive leukocytes indicated normal bone marrow function in the mice despite >10-fold DDX5 knockdown in the bone marrow.
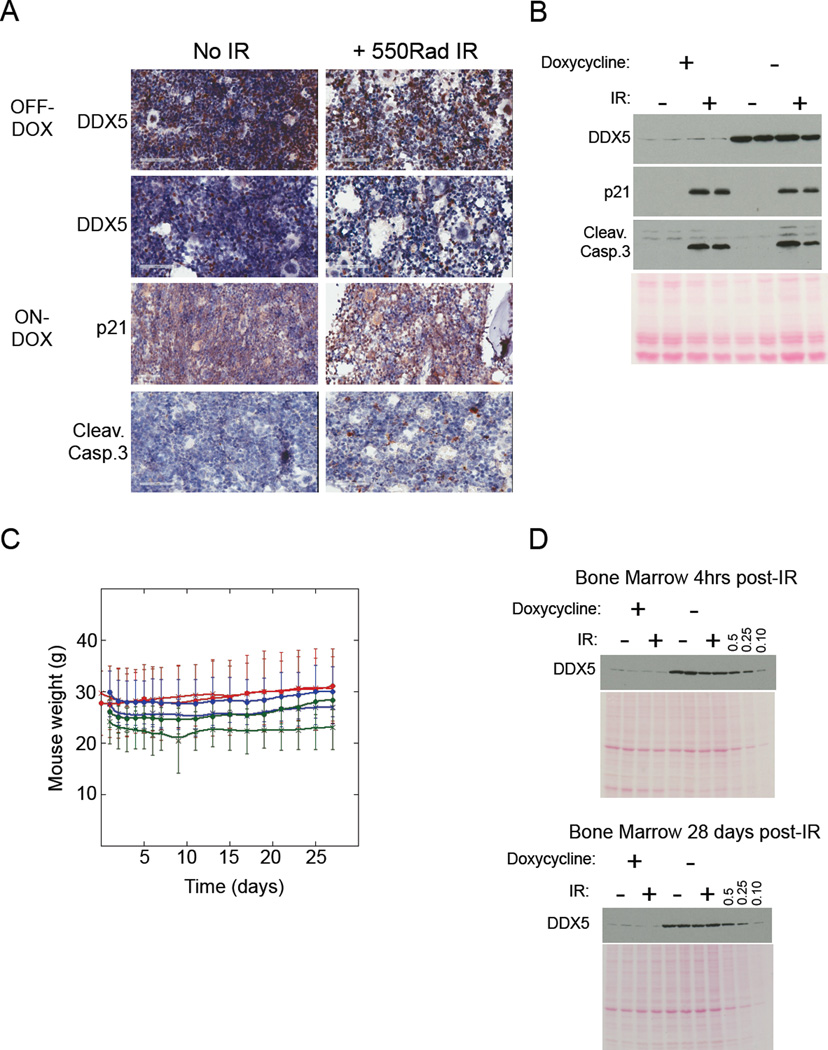
The impact of >10-fold DDX5 knockdown on bone marrow function was further tested by depleting DDX5 in mice given doxycycline for 2 weeks then irradiating the mice with a high IR dose (550Rad). Mice were sacrificed shortly after irradiation and their bone marrows were analyzed for apoptotic cells. DDX5 inactivation was reported to increase the signal for both p21 and cleaved Caspase 3 in the bone marrow following DDX5 ablation (Nicol et al., 2012). However, in our experiments neither immuno-blot nor immuno-histochemical analysis indicated increased p21 or Caspase 3 activation in the bone marrows harvested from mice with DDX5 knockdown in the absence of irradiation (Figure 7A and 7B). As expected irradiation strongly induced both p21 expression and Caspase 3 activation regardless of DDX5 expression status. Importantly, mice with DDX5 knockdown recovered from irradiation similar to littermate controls with normal DDX5 expression (Figure 7C). Moreover, immuno-blot analysis of DDX5 protein abundance in whole cell extracts prepared from bone marrow 28 days following irradiation retained the 10-fold knockdown of DDX5 similar to mice that were not irradiated (Figure 7D). These data indicate that 10-fold DDX5 depletion did not adversely affect normal bone marrow function or adult mouse physiology.
Figure 7. DDX5 depletion does not impair mouse recovery from stress caused by high dose irradiation.
(A) Representative tissue sections obtained from mice +/− doxycycline +/− irradiation with 550Rad IR. Tissue samples were collected 4hrs after irradiation and stained for the indicated proteins. (B) Immuno-blot analysis of WCEs prepared from bone marrows of mice +/− doxycycline +/− irradiation (4hrs post-IR). (C) Weight measurements of mice with the indicated genotypes +/− doxycycline +/− irradiation every other day for 1 month following IR. Results for irradiated mice are indicated by “X” labels and results for non-irradiated mice are indicated with closed squares. Results for shDDX5.2086 mice ondoxycycline are color coded red, results for shDDX5.2086 mice off-doxycycline are color coded blue, and results for No shRNA mice are color coded green. Data is presented as the mean from multiple mice (n=4 for each genotype +/− doxycycline +/− irradiation) with standard deviations shown. (D) Quantitative immuno-blot analysis of DDX5 in WCEs prepared from bone marrow collected from duplicate mice +/− doxycycline +/− irradiation at either 4hrs after IR (upper blot) or 28 days after IR (lower blot). In both blots, bone marrow WCE from the second irradiated mouse that was not given doxycycline was loaded at either equivalent total protein as the other bone marrow WCEs (lane 8) or was diluted either 1-to-2 (lane 9), 1-to-4 (lane 10) or 1-to-10 (lane 11).
DISCUSSION
Current AML therapy consists of an induction phase that typically includes the cytotoxic compound cytarabine in combination with an anthracycline such as daunorubicin or idarubicin. If the induction phase results in complete remission then it is followed with a consolidation therapy phase also consisting of cytotoxic chemotherapy. In addition to killing leukemia cells these agents also kill rapidly dividing normal cells to cause substantial side effects. The limited efficacy of this therapeutic approach is evident in that AML patients 56 years or older have a 2 year survival rate of ~20–25%. (Estey and Dohner, 2006). Our study demonstrates an important activity for DDX5 in the proliferation and survival of acute myeloid leukemia cells in vitro and in vivo, including AML cell lines that are dependent on multiple different oncogenic driver mutations. Importantly, DDX5 depletion was well tolerated in normal adult tissues in vivo suggesting that therapeutic approaches targeting DDX5 might be effective in blocking genetically diverse AML and be well tolerated and safe.
One approach to block DDX5 activity in AML could be with small molecule compounds that inhibit the DDX5 ATP-dependent RNA helicase activity. However, the DDX5 shares strong sequence identity with another closely related DEAD-box RNA helicase DDX17, presenting a significant challenge for identifying DDX5-specific inhibitors. Strategies that disrupt key protein-protein interactions offer an alternative approach. In fact in two cases where the oncogenic activity of DEAD-box proteins or related DEXH-box proteins have been targeted, the compounds antagonize protein-protein interactions required for cancer cell proliferation / survival. Silvesterol inhibits free EIF4A (DDX2A) assembly into the EIF4F complex to reduce translation of proteins required for tumorigenesis (Cencic et al., 2009). Compound YK-4-279 disrupts an interaction between the EWS-FLI1 oncogenic fusion protein and RNA Helicase A (DHX9) and induces apoptosis in Ewing’s Sarcoma Family Tumor cells (Erkizan et al., 2009). Further investigation to identify important protein-protein interactions that underlie the acquired dependence of AML cells and other cancer cells on DDX5 may reveal interactions amenable for targeting by small molecule inhibitors as well as provide more mechanistic insights into why these cancers are sensitive to DDX5 depletion.
Mutations that repress expression of differentiation genes or that increase the expression of genes to support aberrant self-renewal contribute toward AML pathogenesis. One example is the MLL-AF9 fusion protein that is the product of the t(9;11)(p22;q23) chromosomal translocation. Withdrawal of MLL-AF9 expression in a mouse model of MLL-AF9-dependent AML resulted in induction of differentiation and blockage of AML progression in vivo (Zuber et al., 2011a). A similar outcome was observed the BET-family protein BRD4 was blocked either by RNAi or pharmacologically (Zuber et al., 2011b). In contrast, DDX5 depletion induced apoptosis and blocked AML proliferation without inducing differentiation. Thus DDX5 depletion illuminates an alternative vulnerability in AML to targeted therapy and suggests that combined inhibition of BET-family proteins and DDX5 may synergize to kill AML cells. This is underscored by the finding that BCL2 expression was down-regulated by pharmacologic inhibition of BET-family proteins in AML (Dawson et al., 2011) and thus BET-family inhibitors may cooperate with DDX5 inhibition to kill AML cells, similar to BCL2-family inhibitors.
Consistent with our findings in AML cells, DDX5 knockdown in T-cell acute lymphoblastic leukemia (T-ALL) cells also results in impaired cell proliferation and induction of apoptosis (Lin et al., 2012). However in T-ALL DDX5 functions as a transcriptional co-activator of NOTCH-dependent gene expression via its interaction with MAML1. Thus DDX5 knockdown impacts the expression of different genes in different cancer contexts. Indeed we observed very little overlap between genes down-regulated by DDX5 knockdown in the RN2 AML cell line compared to the epithelial colorectal cancer cell line, HCT116. Independent of mechanism, however, the combined results suggest DDX5 as a therapeutic target for some epithelial cancers as well as hematologic malignancies including AML and potentially T-ALL.
The transgenic shRNA approach we applied enables assessment of the therapeutic index for blocking the activity of a candidate drug target such as DDX5. This approach was successfully applied to study the dependence of adult mouse physiology on the expression of essential genes such as Rpa3, encoding the essential single stranded DNA binding protein subunit (McJunkin et al., 2011). Systemic RPA3 knockdown caused dramatic organ degeneration and was fatal within 8-to-10 days following RPA3 shRNA induction.
DDX5 is required for development since Ddx5 knockout mouse embryos fail to develop beyond day E11.5 (Fukuda et al., 2007). Conditional, systemic Ddx5 knockout in adult mice was reported to increase apoptosis in bone marrow, reduce clonogenic survival of blasts derived from bone marrow, and alter organ morphology in the large intestine (Nicol et al., 2012). In contrast, 4-to-10 fold inhibition of DDX5 expression in multiple organs of the transgenic DDX5 shRNA mice, including organs with a high rate of cell proliferation such as bone marrow, intestine, and skin, resulted in no adverse consequences on organ morphology and these mice continued to gain weight normally over the 6 month duration. Importantly, 10-fold DDX5 knockdown in the bone marrow did not induce detectable apoptosis or impair bone marrow function, even after high dose irradiation. The differences in outcomes of DDX5 depletion in the bone marrow between our study and the Nicol et al. study may be due to the remaining 10% DDX5 expression in our transgenic mice or could be due to differences between our methods for blocking DDX5 expression. For example, Cre-mediated recombination may potentially cause DNA damage and produce a phenotype independent of DDX5 depletion. Moreover the altered tissue organization reported by Nicol et al. in the large intestines of mice with Ddx5 knockout appears to have been misinterpreted.
The observations reported here provide a comprehensive procedure to identify and validate an anti-tumor therapeutic target and at the same time assess the impact of the anti-cancer therapy on normal tissues. DDX5 depletion selectively induces stress in AML cells and delays AML progression in vivo but is well tolerated by normal adult physiology. We have also identified a potential mechanism of resistance to anti-DDX5 therapy since BCL2 is frequently overexpressed in AML (Tzifi et al., 2012) and BCL2 overexpression prevents apoptosis induced by DDX5 knockdown. But the results further suggest that combination therapy that blocks both DDX5 and BCL2 may be effective in AML that is refractory to current treatments.
EXPERIMENTAL PROCEDURES
For a detailed description of cell lines, plasmids, antibodies, shRNAs, and protocols see Supplemental Experimental Procedures. Plasmids encoding N-terminal HA-tagged or 6myc-tagged DDX5 that we used to generate RNAi resistant wild-type and mutant HA-tagged DDX5 transgenes presented in Figure S5 were kindly provided by Dr. Ralf Janknecht (Shin et al., 2007).
Preparation of cell lines with either constitutive or inducible shRNA expression
Cell lines with either constitutive or inducible shRNA expression were prepared using retroviral infection. Details including retroviral packaging and cell infections are described in the Supplemental Experimental Procedures.
Competition Cell Proliferation Assay
To measure the effect of DDX5 knockdown on cell proliferation infected AML cells were not selected following infection with virus encoding constitutive expression of the indicated shRNAs. These viruses also encode constitutive GFP expression so the fate of infected GFP positive cells expressing the shRNAs were tracked in unselected cell cultures by analysis on a Guava easyCyte 8HT flow cytometer (Millipore). Infections typically yielded 10-to-20% infected cells for each cell line except LG1 and MEF cells where infection efficiency was 70%-to-90%. The GFP positive populations in each culture were measured each day following infection and recording of the GFP positive populations was begun when GFP expression in each culture was maximum, which was typically 2 days (for LG1 and MEF cells) and 4 days (for human AML cell lines) following infection. This is indicated as day 0 on the plots shown in Figures 1, 2A, and 3D and Supplemental Figures S1A, S1B, and S2D. The percent GFP positive cells observed on each following day was then normalized to this percent GFP positive cells on day 0 for each cell line.
In vivo mouse experiments
The Cold Spring Harbor Laboratory animal care and use committee approved all mouse experiments. The in vivo AML experiment was performed as described (Zuber et al., 2011b). For a detailed description of the in vivo AML experiments and also generation of transgenic DDX5 shRNA mice see Supplementary Experimental Procedures. (Dow et al., 2012; Zuber et al., 2011b). To induce shRNA expression in either the transplanted AML cells in the in vivo AML experiment or the transgenic DDX5 shRNA mice tested in the safety studies doxycycline was provided to the mice in their drinking water (containing 2mg/mL doxycycline (Sigma, D-9891) with 2% Sucrose) and in their food (625mg/kg, Harlan Laboratories). Mice tested in the in vivo AML experiment were given doxycycline food and water 5 days after transplantation with AML cells and were maintained on doxycycline food and water for the duration of the experiment. For the safety studies, transgenic DDX5 shRNA mice were given doxycycline-containing food and water for 2–4 weeks to induce DDX5 knockdown then doxycycline food with doxycycline-free water thereafter to maintain DDX5 knockdown. For the irradiation experiment the mice were given doxycycline-containing food and water for 2 weeks prior to irradiation then were maintained on doxycycline food and water for the 28-day follow-up after irradiation.
Apoptosis and ROS measurements in RN2 cells
For analysis of apoptotic AML cells the cells were stained with Pacific Blue conjugated anti-Annexin V (BioLegend catalog #640917) and 7-AAD (BioLegend catalog #420401) as per the manufacturer’s protocol. Sample analysis was performed on an LSRII flow cytometer (BD Biosciences) using FACSDiva software. Annexin V positive / 7-AAD negative cells in each cell suspension were recorded as the early apoptotic cell fraction. For analysis of ROS levels in AML cells following 3 days of doxycycline induced shRNA 2,000,000 cells per suspension were treated for 30 minutes at 37 degrees C with CellROX Deep Red Reagent (LifeTechnologies catalog #C10422). The cells were then processed for flow cytometry analysis using the manufacturer’s protocol. Analysis was performed on the LSRII flow cytometer and viable cells in each suspension were gated for analysis of CellROX fluorescence.
Cell viability measurements in THP1 cells
Tet-On THP1 cells with doxycycline inducible expression of either shDDX5.2053 or shRen.713 were prepared by infecting Tet-On THP1 cells that stably expressed rtTA (Zuber et al., 2011b) with virus packaged with either the pTRIN-shDDX5.2008 or pTRIN-shRen.713 packaging plasmids. Infected cells were selected in RPMI + 10% FBS + 1mg/mL G418. Expression of the shRNAs in the derivative Tet-On THP1 cell lines was then induced in RPMI + 10% FBS + 1mg/mL G418 + 1µg/mL doxycycline. After 12 days of shRNA expression 1,000,000 Tet-On THP1 cells expressing the different shRNAs were seeded to new wells of 6-well tissue culture plates in 4mL RPMI + 10% FBS + 1mg/mL G418 + 1µg/mL doxycycline + the indicated concentrations of ABT-737 (Selleckchem.com catalog # S1002) dissolved in DMSO. Cultures not treated with ABT-737 were treated with an equivalent volume of DMSO. Samples were prepared for immuno-blot and flow cytometry analysis 24hrs after ABT-737 treatment. For flow cytometry analysis cells in each culture were washed twice in 1x PBS then GFP positive cells suspended in 1x PBS from each culture were measured using an LSRII flow cytometer.
RNA-Seq Experiment
DDX5 was knocked down in RN2 AML cells by doxycycline-induced expression of either shDDX5.1322 or shDDX5.2086. For control, expression of the shRen.713 shRNA was similarly induced in RN2 AML cells. At 24hrs and 48hrs RNA was isolated and barcoded cDNA libraries were prepared as described in Supplemental Experimental procedures. The resulting 18 barcoded cDNA libraries were pooled into 3 pools of 6 cDNA libraries each and then each pool was loaded onto 2 lanes of an Illumina HiSeq 2000 sequencer on which paired-end 101 sequencing runs were performed. Processing and mapping of the reads are described in the Supplemental Experimental Procedures. (Gillis and Pavlidis, 2011). The reported data have been deposited in NCBI’s Gene Expression Omnibus, GEO Series accession number GSE53599 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53599).
Supplementary Material
Highlights.
DDX5 is required for proliferation of genetically diverse AML cells.
DDX5 depletion slows AML progression in vivo.
DDX5 knockdown induces ROS to activate apoptosis in AML cells.
DDX5 inhibition in non-cancer cells is well tolerated in vivo.
ACKNOWLEDGEMENTS
This research was supported by a grant from the National Cancer Institute (CA13106); the Cold Spring Harbor Laboratory Cancer Center Support grant (P30-CA45508) and the Don Monti Memorial Research Foundation. AM was a fellow supported by the Goldring Family Foundation. We thank Raisa Puzis, Aigoul Nourjanova, and Denise Hoppe in the Cold Spring Harbor Histology Shared Resource for preparation of immuno-histochemical slides.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Molecular cell. 2013;49:399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–28. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- Callens C, Coulon S, Naudin J, Radford-Weiss I, Boissel N, Raffoux E, Wang PH, Agarwal S, Tamouza H, Paubelle E, et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. The Journal of experimental medicine. 2010;207:731–750. doi: 10.1084/jem.20091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causevic M, Hislop RG, Kernohan NM, Carey FA, Kay RA, Steele RJ, Fuller-Pace FV. Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene. 2001;20:7734–7743. doi: 10.1038/sj.onc.1204976. [DOI] [PubMed] [Google Scholar]
- Cencic R, Carrier M, Galicia-Vazquez G, Bordeleau ME, Sukarieh R, Bourdeau A, Brem B, Teodoro JG, Greger H, Tremblay ML, et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS One. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EL, Coulson A, Dalgliesh C, Rajan P, Nicol SM, Fleming S, Heer R, Gaughan L, Leung HY, Elliott DJ, et al. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Res. 2008;68:7938–7946. doi: 10.1158/0008-5472.CAN-08-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Dardenne E, Pierredon S, Driouch K, Gratadou L, Lacroix-Triki M, Espinoza MP, Zonta E, Germann S, Mortada H, Villemin JP, et al. Splicing switch of an epigenetic regulator by RNA helicases promotes tumor-cell invasiveness. Nat Struct Mol Biol. 2012;19:1139–1146. doi: 10.1038/nsmb.2390. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Premsrirut PK, Zuber J, Fellmann C, McJunkin K, Miething C, Park Y, Dickins RA, Hannon GJ, Lowe SW. A pipeline for the generation of shRNA transgenic mice. Nat Protoc. 2012;7:374–393. doi: 10.1038/nprot.2011.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkizan HV, Kong Y, Merchant M, Schlottmann S, Barber-Rotenberg JS, Yuan L, Abaan OD, Chou TH, Dakshanamurthy S, Brown ML, et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing's sarcoma. Nat Med. 2009;15:750–756. doi: 10.1038/nm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H, Nakamura T, et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Biol. 2007;9:604–611. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace FV. The DEAD box proteins DDX5 (p68) and DDX17 (p72): multi-tasking transcriptional regulators. Biochim Biophys Acta. 2013;1829:756–763. doi: 10.1016/j.bbagrm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Gillis J, Mistry M, Pavlidis P. Gene function analysis in complex data sets using Ermine. J. Nat Protoc. 2010;5:1148–1159. doi: 10.1038/nprot.2010.78. [DOI] [PubMed] [Google Scholar]
- Gillis J, Pavlidis P. The role of indirect connections in gene networks in predicting function. Bioinformatics. 2011;27:1860–1866. doi: 10.1093/bioinformatics/btr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal C, Uhlmann-Schiffler H, Stahl H. Redundant role of DEAD box proteins p68 (Ddx5) and p72/p82 (Ddx17) in ribosome biogenesis and cell proliferation. Nucleic acids research. 2007;35:3590–3601. doi: 10.1093/nar/gkm058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Tian L, Shen H, Gu Y, Li JL, Chen Z, Sun X, You MJ, Wu L. DDX5 is a positive regulator of oncogenic NOTCH1 signaling in T cell acute lymphoblastic leukemia. Oncogene. 2012;32:4845–4853. doi: 10.1038/onc.2012.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek A, Luo W, Krasnitz A, Hicks J, Powers RS, Stillman B. DDX5 regulates DNA replication and is required for cell proliferation in a subset of breast cancer cells. Cancer Discov. 2012;2:812–825. doi: 10.1158/2159-8290.CD-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin K, Mazurek A, Premsrirut PK, Zuber J, Dow LE, Simon J, Stillman B, Lowe SW. Reversible suppression of an essential gene in adult mice using transgenic RNA interference. Proc Natl Acad Sci U S A. 2011;108:7113–7118. doi: 10.1073/pnas.1104097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol SM, Bray SE, Black HD, Lorimore SA, Wright EG, Lane DP, Meek DW, Coates PJ, Fuller-Pace FV. The RNA helicase p68 (DDX5) is selectively required for the induction of p53-dependent p21 expression and cell-cycle arrest after DNA damage. Oncogene. 2012;32:3461–3469. doi: 10.1038/onc.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Saporita AJ, Chang HC, Winkeler CL, Apicelli AJ, Kladney RD, Wang J, Townsend RR, Michel LS, Weber JD. RNA helicase DDX5 is a p53- independent target of ARF that participates in ribosome biogenesis. Cancer Res. 2011;71:6708–6717. doi: 10.1158/0008-5472.CAN-11-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C, Schnittger S, Klaus M, Kern W, Hiddemann W, Haferlach T. AML with 11q23/MLL abnormalities as defined by the WHO classification: incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood. 2003;102:2395–2402. doi: 10.1182/blood-2003-02-0434. [DOI] [PubMed] [Google Scholar]
- Shin S, Rossow KL, Grande JP, Janknecht R. Involvement of RNA helicases p68 and p72 in colon cancer. Cancer Res. 2007;67:7572–7578. doi: 10.1158/0008-5472.CAN-06-4652. [DOI] [PubMed] [Google Scholar]
- Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv Hematol. 2012;2012:524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortham NC, Ahamed E, Nicol SM, Thomas RS, Periyasamy M, Jiang J, Ochocka AM, Shousha S, Huson L, Bray SE, et al. The DEAD-box protein p72 regulates ERalpha-/oestrogen-dependent transcription and cell growth, and is associated with improved survival in ERalpha-positive breast cancer. Oncogene. 2009;28:4053–4064. doi: 10.1038/onc.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanada M, Naoe T. Acute myeloid leukemia in older adults. International journal of hematology. 2012;96:186–193. doi: 10.1007/s12185-012-1137-3. [DOI] [PubMed] [Google Scholar]
- Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, McCurrach ME, Yang MM, Dolan ME, Kogan SC, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, Shi J, Weissmueller S, Fellmann C, Taylor MJ, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011a;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011b;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.