Abstract
The Behavioral Approach System (BAS) hypersensitivity theory of bipolar disorder (BD; Alloy & Abramson, 2010; Depue & Iacono, 1989) suggests that hyperreactivity in the BAS results in the extreme fluctuations of mood characteristic of BD. In addition to risk conferred by BAS hypersensitivity, cognitive and personality variables may play a role in determining risk. We evaluated relationships among BAS sensitivity, risk taking, and an electrophysiological correlate of approach motivation, relative left-frontal electroencephalography (EEG) asymmetry. BAS sensitivity moderated the relationship between risk taking and EEG asymmetry. More specifically, individuals who were high in BAS sensitivity showed left-frontal EEG asymmetry regardless of their level of risk-taking behavior. However, among individuals who were moderate in BAS sensitivity, risk taking was positively associated with asymmetry. These findings suggest that cognitive and personality correlates of bipolar risk may evidence unique contributions to a neural measure of trait-approach motivation. Clinical implications of these findings are discussed.
Keywords: behavioral approach system, risk taking, left-frontal EEG asymmetry, bipolar disorder
Bipolar disorder (BD) is a serious mental illness that has been estimated to affect between 1% (for bipolar I) and 4% (for all bipolar spectrum disorders [BSDs]) of the U.S. population (Merikangas & Pato, 2009). Individuals with BSDs are at overall high risk for poor outcomes, with impaired occupational functioning (Judd et al., 2008), as well as fewer social interactions and worse cognitive functioning than individuals with no psychiatric illness, even when in clinical remission (Rosa et al., 2010).
Previous research has highlighted the role of two neurobiological motivational systems, the Behavioral Activation System (BAS) and Behavioral Inhibition System (BIS), in the development of healthy and pathological functioning. The BAS is hypothesized to underlie approach behavior, such as reward seeking and goal-striving behaviors, as well as emotions such as elation (Gray, 1987, 1990) and anger (Carver, 2004; Harmon-Jones, 2003). Conversely, the BIS is hypothesized to underlie inhibitory behavior and aversion, including sensitivity to punishment and anxiety (Gray, 1987, 1990). BD is thought to result from dysregulation of the BAS, especially in relation to stressful (both positive and negative) life events (Alloy & Abramson, 2010; Depue & Iacono, 1989; Depue, Krauss, & Spoont, 1987; Urošević, Abramson, Harmon-Jones, & Alloy, 2008). Among individuals with BD, goal-striving and achievement-related life events may trigger an overactivation of the BAS, resulting in a manic or hypomanic episode. Negative life events involving losses and definite failures to attain goals may trigger an excessive down regulation of the BAS, resulting in a depressive episode (Alloy & Abramson, 2010; Alloy, Abramson, et al., 2009; Depue & Iacono, 1989; Depue et al., 1987; Urošević et al., 2008).
The BAS hypersensitivity theory of BD has received substantial empirical support (see Alloy & Abramson, 2010; Johnson, Edge, Holmes, & Carver, 2012; Urošević et al., 2008, for reviews). Alloy et al. (2006) showed that individuals with high BAS sensitivity were approximately six times more likely to have a lifetime history of BD than individuals with moderate BAS sensitivity, but were no more likely to report a history of unipolar depression, suggesting that BAS hypersensitivity may be uniquely related to BD as compared with mood disorders in general. Also, individuals with BSDs exhibit higher BAS sensitivity than healthy controls (Hayden et al., 2008; Meyer, Johnson, & Winters, 2001). Among those with BD, higher BAS sensitivity predicts shorter time to onset of a hypomanic or manic episode (Alloy et al., 2008) and progression to more severe disorders (e.g., from cyclothymia to bipolar II and from cyclothymia and bipolar II to bipolar I; Alloy, Urošević, et al., 2012). Moreover, among adolescents with no prior history of BD, BAS hypersensitivity predicted first lifetime onset of a BSD, even controlling for family history of BD and initial symptoms (Alloy, Bender, et al., 2012).
Research also has suggested that BAS hypersensitivity among individuals with BD is not limited to specific mood states, but rather is a trait exhibited even in euthymic states. Among individuals with bipolar I, BAS sensitivity predicted increases in manic symptoms over time (Meyer et al., 2001) but did not correlate with current manic symptoms. Similar findings also have been observed in individuals at risk for BD who do not meet DSM-IV criteria for a diagnosis (Carver & Johnson, 2009; Meyer & Hofmann, 2005; Meyer, Johnson, & Carver, 1999).
Following from the BAS conceptualization of BD, it has been hypothesized that individuals with BD may experience difficulty in exerting cognitive control over their responses to appetitive or goal-relevant stimuli, resulting in higher levels of impulsivity. Individuals with BSDs exhibit higher self-reported impulsivity across mood episodes (Alloy, Bender, et al., 2009; Holmes et al., 2009; Swann, Anderson, Dougherty, & Moeller, 2001; Swann, Pazzaglia, Nicholls, Dougherty, & Moeller, 2003), as well as more impulsive responses on behavioral measures of impulsivity in both manic (Swann et al., 2001, 2003) and euthymic states (Adida et al., 2011; Swann et al., 2003). Given that self-report measures of impulsivity are thought to primarily measure trait impulsiveness, whereas behavioral measures are thought to index state levels of impulsivity, these findings suggest that individuals on the bipolar spectrum may exhibit higher trait levels of impulsivity, with superimposed state-dependent fluctuations (see Najt et al., 2007, for a review). Impulsivity also has been shown to predict progression from less severe BSDs (cyclothymia and bipolar II) to bipolar I disorder (Alloy, Urošević, et al., 2012), and is associated with a variety of poor outcomes including substance use comorbidity (Alloy, Bender, et al., 2009), poorer quality of life (Victor, Johnson, & Gotlib, 2011), and overall functional impairment (Jiménez et al., 2012). Importantly, it is thought that impulsivity may work in concert with reward sensitivity to drive illness course (Alloy, Urošević, et al., 2012; Molz et al., 2013).
Impulsivity may manifest behaviorally as risk taking, which is one of the core symptoms of manic and hypomanic episodes (American Psychiatric Association, 2013). Individuals with BD score higher on behavioral measures of risk taking in both manic (Adida et al., 2008; Murphy et al., 2001) and euthymic states (Holmes et al., 2009). BD also has been associated with a variety of clinically significant risky behaviors such as risky sexual behavior (Di Nicola et al., 2010; Meade et al., 2011), problem gambling (Di Nicola et al., 2010; McIntyre et al., 2007), compulsive shopping (Di Nicola et al., 2010; Karakus & Tamam, 2011), and substance abuse (e.g., Alloy, Bender, et al., 2009; Grant et al., 2004; Richardson, 2011). Risk taking also has been shown to be positively associated with BAS sensitivity (Brunborg, Johnsen, Mentzoni, Molde, & Pallesen, 2011; Suhr & Tsanadis, 2007). These findings further highlight the role of risk taking as an important feature of BD related to BAS dysregulation.
Electrophysiological data have further supported the BAS hypersensitivity model of BD. Harmon-Jones et al. (2008) demonstrated that individuals with BSDs showed increased relative left-frontal cortical activation in response to a hard behavioral task where they had the opportunity to win money, but not when they had the opportunity to lose money. This cortical asymmetry is a well-known correlate of approach motivation (e.g., Balconi & Mazza, 2010; Miller & Tomarken, 2001; Sobotka, Davidson, & Senulis, 1992). After positive mood induction, bipolar individuals experiencing mood episodes exhibited higher left-frontal activation than remitted bipolar individuals and healthy controls (Hayden et al., 2008). Further research has suggested that left-frontal cortical asymmetry is a trait characteristic of individuals high in BAS, regardless of diagnostic status, given that high-BAS individuals show increased left-frontal cortical electroencephalography (EEG) activity in a resting state (Coan & Allen, 2003; Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997), and increased left-frontal EEG asymmetry is associated with bias toward reward-related cues among healthy individuals (Pizzagalli, Sherwood, Henriques, & Davidson, 2005).
Applying electrophysiological techniques to objectively measure BAS dysregulation and associated cognitive control deficits may be important in the study of BD. The evaluation of electrophysiological markers in individuals at risk for BD could enable researchers and clinicians to more objectively assess and understand vulnerability factors in BSDs. Given that asymmetry is understood to be an index of approach motivation, it is as yet unclear whether the well-documented relationship between risk for BD and asymmetry is driven solely by BAS (e.g., reward sensitivity) or if other elements of approach tendency (e.g., risk taking) also play a role. Identifying whether risk taking also might be related to approach motivation may be important both for better identification of individuals at high risk for excessive approach tendencies and risk for BD and for the future development of psychosocial interventions, as risk taking may be amenable to change through cognitive-behavioral techniques.
The current study investigated the relationships among BAS sensitivity, impulsivity/risk taking, and left-frontal EEG asymmetry. We hypothesized that higher levels of risk taking would be related to increased relative left-frontal EEG asymmetry in a resting state. This study also explored whether higher risk for BD, as measured by BAS sensitivity, moderated this relationship. We hypothesized that the proposed relationship would be stronger for individuals at high risk for BD based on high BAS sensitivity than for those at low risk, with moderate BAS sensitivity.
Method
PARTICIPANTS
The present study was approved by the Temple University Institutional Review Board. Participants were 55 adolescents and young adults (ages 14–21; mean age 18.39 years, SD = 1.51 years, 36 females) recruited from the greater Philadelphia region, who were a subset of individuals participating in the Teen Emotion and Motivation (TEAM) project (Alloy, Bender, et al., 2012). The sample was racially diverse (27.3% African American, 52.7% White, 9.1% Asian, 1.8% Native American, 3.6% other, 5.5% no response/prefer not to answer). In the screening phase of Project TEAM (Phase I), 9,991 students (ages 14–19) were recruited from Philadelphia public high schools and universities and completed two measures of BAS sensitivity—the Carver and White Behavioral Inhibition System/Behavioral Activation System scales (BIS/BAS; Carver & White, 1994) and the Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPSRQ; Torrubia, Ávila, Moltó, & Caseras, 2001). To create groups that were distinct in their level of BAS sensitivity, only participants scoring in the 40th to 60th percentile on both the Reward subscale of the SPSRQ and total BAS score (moderate BAS sensitivity, n = 404), and participants scoring in the 85th to 100th percentile on both measures (high BAS sensitivity, n = 776), were invited to participate in the study. Participants with moderate, rather than low, BAS sensitivity were used as the low-risk comparison group for three reasons: (a) because a moderate-BAS group is closer to the mean on the BAS sensitivity dimension and, thus, more normal from a statistical perspective; (b) to provide a more conservative test of the BAS hypersensitivity theory; and (c) because low BAS sensitivity is associated with vulnerability to unipolar depression (e.g., Depue & Iacono, 1989; Depue et al., 1987).
Following Phase I, participants in the moderate-and high-BAS groups were contacted and invited to return for the second phase (Phase II) of screening. For participants under age 18, parents provided written consent and adolescents provided written assent; participants age 18 and older provided their own written consent. Participants then were interviewed using an expanded Schedule for Affective Disorders and Schizophrenia–Lifetime diagnostic interview (SADS-L; Endicott & Spitzer, 1978) by interviewers who were unaware of participants’ risk status. In total, 390 participants (244 high BAS and 146 moderate BAS) completed this second phase of screening. Because the purpose of the TEAM study was to follow participants with high risk of BD (based on BAS risk group) prospectively to predict first onset of BSD, participants who met DSM-IV-TR (American Psychiatric Association, 2000) criteria for BD (n = 22) were excluded. Participants who met DSM-IV criteria for a psychotic disorder (n = 7) or those who were not fluent in English (n = 5) were also excluded from further participation.
Participants who were not excluded during Phase II screening were invited to complete a Time 1 assessment, including a behavioral measure of risk taking, described below. Following completion of these baseline measures, a random subset of participants was then invited to participate in an EEG study. In all, 100 participants completed the EEG session; however, 45 were excluded on the basis of excessive artifact due to technical difficulties. Participants whose data were included in the present study did not differ from those who were excluded on the basis of age, gender, race, BAS risk group, or level of hypomanic symptoms on the day of EEG recording. Participants were also excluded from analysis if they were left-handed (n = 4) or were excluded as outliers based on the magnitude of the left-frontal EEG asymmetry, described below (n = 2). Overall, 28 participants from the high-BAS group and 21 participants from the moderate-BAS group were included in the final analysis. Participants in the high- and moderate-BAS groups did not differ from one another in terms of age, sex, race, or ethnicity (Table 1). As expected, participants in the high-BAS group had significantly higher scores on their total BAS score, all BAS subscale scores, and the Sensitivity to Reward subscale of the SPSRQ. The time between the Time 1 assessment and the EEG session was highly variable and normally distributed and ranged from 7 days to 832 days (M =190.31 days, SD = 173.64 days), but did not differ between high- and moderate-BAS participants. Participants in the EEG study did not differ from the overall sample on any demographic characteristics or BAS risk group (all ps > .30). Participants were compensated $40 for the completion of the EEG session, and both participants and research assistants administering the EEG session were blind to the participants’ risk group.
Table 1.
Demographic and Personality Characteristics of the Study Sample
| High BAS (n = 28) | Moderate BAS (n = 21) | t/χ2 | |
|---|---|---|---|
| Age | 18.52 (1.16) | 18.57 (1.50) | 0.14 |
| Sex | 64.3% female | 66.7% female | .03 |
| Race | 53.6% Caucasian | 47.6% Caucasian | 1.55 |
| 28.6% African American | 26.3% African American | ||
| 10.7% Asian/Pacific Islander | 9.5% Asian/Pacific Islander | ||
| 3.6% Other | 4.8% Native American | ||
| 3.6% No response/declined to respond | 4.8% Other | ||
| 9.5% No response/declined to respond | |||
| Ethnicity | 7.1% Hispanic or Latino | 9.5% Hispanic or Latino | .09 |
| BIS | 19.36 (4.03) | 19.79 (2.88) | 0.40 |
| BAS-Total | 46.08 (2.54) | 38.15 (.99) | 13.16* |
| BAS-Reward | 18.50 (1.20) | 16.65 (1.66) | 4.48* |
| BAS-Drive | 13.73 (1.56) | 10.60 (1.14) | 7.53* |
| BAS-Fun Seeking | 13.89 (1.52) | 10.90 (1.59) | 6.60* |
| SPSRQ-SR | 8.36 (1.28) | 4.84 (1.68) | 8.14* |
| Time to EEG | 182.59 (178.94) | 210.95 (175.86) | 0.55 |
| Left-frontal EEG asymmetry | .02 (.10) | −.02 (.08) | 1.31 |
Note. BIS/BAS = Behavioral Inhibition System/Behavioral Activation System; SPSRQ-SR = Sensitivity to Punishment/Sensitivity to Reward Questionnaire–Sensitivity to Reward subscale; time to EEG = days from Time 1 session until EEG session.
p < .001.
MEASURES
Behavioral Approach System Sensitivity
Participants completed two measures in Phase I, which were combined to create a composite BAS sensitivity score. The Carver and White (1994) BIS/BAS scale is a widely used measure of trait sensitivity of the behavioral activation and behavioral inhibition systems. It consists of twenty 4-point Likert items (1 = strongly disagree, 4 = strongly agree). Although there is only one BIS subscale, the BAS scale consists of three subscales: BAS Drive (related to goal striving behavior), BAS Fun Seeking (related to desire for and pursuit of rewards and novelty), and BAS Reward Responsiveness (related to positive responses after rewarding stimuli). In the present study, we used a total BAS score (BAS-T), which was a sum of all three BAS subscales. In the screening sample, the BAS-T scale had acceptable internal consistency, α = .80, as did the BIS scale, α = .72. Total BAS score has been shown to have acceptable test–retest reliability (Meyer et al., 2001).
The SPSRQ (Torrubia et al., 2001) is another widely used measure designed to tap the BAS-related construct of reward/approach motivation as well as the BIS-related construct of sensitivity to punishment/aversive conditioning. The SPSRQ consists of 48 “yes” or “no” items and is composed of Sensitivity to Punishment (SP; measuring BIS) and Sensitivity to Reward (SR) subscales (measuring BAS). In the screening sample, both the SP and SR subscales had acceptable internal consistency, αSP =.84 and αSR = .76, and the BAS-T scale and SR subscale were significantly correlated (r = 0.40). Participants were assigned to either the high- or moderate-BAS sensitivity group based on both the total BAS score from the BIS/BAS scales and the Reward subscale of the SPSRQ. Participants were dichotomized to either a high- or moderate-BAS sensitivity group, rather than utilizing a continuous measure of BAS, to conservatively test the prediction that only very high levels of BAS confer risk for BD.
Risk Taking
The Balloon Analogue Risk Task (BART; Lejuez et al., 2002), a measure of risk taking, was completed at Time 1. The BART is a computerized behavioral task assessing the tendency to make risky decisions (Lejuez et al., 2002; Reynolds, Ortengren, Richards, & de Wit, 2006), which was designed as a more ecologically valid alternative to assessing risky behavior than existing self-report questionnaires. As in real-world situations, risk taking on the BART results in higher rewards to a certain point, after which continued risk taking is likely to result in a poor outcome. Consequently, this paradigm is ideal for use with a bipolar risk sample, as BD is thought to be associated with risk taking in response to potentially rewarding cues.
In the BART, participants are presented with an image of a small balloon attached to a “pump” button, which participants click during the task to “fill” the balloon. Participants are instructed that they are able to earn points for every time they click the “pump,” but that at some point, each balloon would explode. Participants decide how many times to click the pump; if the participant does not reset his or her balloon and add the points to his or her bank before that balloon’s individual maximum capacity is reached, the balloon “pops” and the points accrued for that individual balloon are lost. Participants complete a total of 30 trials. The main variable of interest is the adjusted average number of pumps (BART-Avg), which is the average number of pumps per balloon not counting the balloons that exploded (because those that exploded naturally constrained the number of pumps that could be made). BART-Avg scores have been shown to correlate with several measures of impulsivity and sensation seeking, and account for a significant proportion of variance in delinquent behaviors and risky substance use and sexual behavior in adolescents (Aklin, Lejuez, Zvolensky, Kahler, & Gwadz, 2005; Lejuez et al., 2007) and adults (Lejuez et al., 2002; Skeel, Pilarski, Pytlak, & Neudecker, 2008), even after accounting for self-reported impulsivity and arousal seeking (Lejuez et al., 2002).
Internal State Scale (ISS)
The ISS (Bauer, Crits-Christoph, Ball, & Dewees, 1991) is a 15-item scale including four subscales: Activation (e.g., “I feel sped up inside,”), Depression (e.g., “I feel like nothing will ever work out for me,”), Perceived Conflict (e.g., “I feel like people are out to get me,”), and well-being (e.g., “Today I feel like a capable person”). The ISS was administered directly prior to resting EEG acquisition to assess current symptoms of hypomania. The Activation subscale of the ISS is highly correlated with symptoms of mania (Bauer et al., 1991), although some recent evidence has shown only moderate associations with bipolar status (Udachina & Mansell, 2007). The ISS Activation subscale has shown good internal consistency in previous research, α = .92 (Bauer et al., 1991). In the current study, internal consistency for the Activation subscale was also good, α = .81.
Handedness
Handedness was assessed with Chapman and Chapman’s (1987) self-report questionnaire, which requires participants to identify which hand they use to do a variety of daily tasks (e.g., write, use a hammer, or stir a can of paint). The questionnaire allows the participant to select either the right hand, left hand, or either hand, and also asks the participant if anyone in his or her family is left-handed. Participants who reported left-handedness (score > 33) on this questionnaire (n = 4) were excluded from the final data analyses due to potential neurological differences between right- and left-handers.
Electroencephalography
EEG recordings were acquired using a 14-channel electrode Lycra cap connected to a SynAmps (Charlotte, NC) amplifier. Twelve homologous electrode sites (two midfrontal, two lateral frontal, two temporal, two central, two parietal, and two occipital) were used to collect EEGs, which were recorded using Neuroscan 2.0 software (Charlotte, NC). Electrooculograms (EOGs) were collected from the right and left temple, and above and below the left eye. Electrodes were placed on the right and left earlobes. An offline, digitally derived averaged earlobe reference was computed. All electrode impedances were below 5 kΩ, and all homologous electrode pairs’ impedances were within 1 kΩ of one another. EEGs were collected during four 1-minute blocks of resting with eyes open and four 1-minute blocks of resting with eyes closed; the order of blocks was counterbalanced among participants.
EEG ANALYSIS
Offline analysis of EEGs was conducted using Neuroscan 4.2 (Charlotte, NC) software. All EEGs were visually inspected by trained researchers and cleared of muscle artifact, abnormal ocular artifact, and other sources of signal disruption. Vertical EOGs were analyzed to determine average peak shape and amplitude, and a regression-based algorithm to remove the effects of vertical ocular artifact on EEGs was applied. EEG epochs of 1,024 ms were extracted through a Hamming window and overlapped 75% to minimize loss of data. The overlapped data were analyzed using a fast Fourier transform to extract power spectra, and then averaged across resting condition (eyes open vs. eyes closed). Alpha-band activity (8–12 Hz) was log transformed to normalize the distribution of the data, and an asymmetry metric was calculated as in previous research (e.g., Harmon-Jones et al., 2008) by subtracting log-normalized left-hemisphere alpha activity from log-normalized right-hemisphere alpha activity. As alpha power is inversely related to cortical activity, higher scores on the asymmetry index reflect relatively greater left-hemisphere activation (see Coan & Allen, 2004, for a review). Previous work using left-frontal EEG asymmetry in the resting state in relationship to BAS has focused on midfrontal sites (e.g., Coan & Allen, 2003; Harmon-Jones & Allen, 1997); therefore, primary hypotheses were tested using the midfrontal electrodes (F3 and F4). However, other research has demonstrated stronger effects of BAS sensitivity in the lateral frontal regions (F7 and F8; Harmon-Jones et al., 2008); consequently, hypotheses also were explored using these electrodes. Upon inspection of the EEG asymmetry data, it was found that two participants were extreme outliers (left-frontal EEG asymmetry scores > 2.5 SD below the mean); thus, these participants were excluded from further analysis,1 leaving the total sample at 49. Overall left-frontal EEG asymmetry values in the current sample were consistent with values found among healthy control subjects in previous work (Nusslock et al., 2011).
Results
Bivariate correlations between primary study variables indicated that left-frontal EEG asymmetry correlated .29 (p < .05, one-tailed) with risk taking from the BART and .19 (ns) with risk-group status. Risk-taking and risk-group status did not correlate with each other (r = .05). To investigate whether risk factors for BD predicted greater relative left midfrontal and lateral-frontal EEG asymmetries, we conducted hierarchical linear regressions. Regressions controlled for the amount of time from initial assessment to completion of the EEG session, as well as current hypomanic symptoms as assessed by the ISS Activation subscale. First, demographic characteristics were evaluated as predictors of EEG asymmetry. At both midfrontal and lateral sites, sex, race, and age did not significantly predict EEG asymmetry (all ps > .10). Inasmuch as the sample was selected based on BAS risk for BD, the regression analysis examining the effects of risk taking controlled for BAS risk status. BAS risk group was dummy coded, with 0 denoting the moderate BAS group and 1 denoting the high BAS group.
Main effects of the predictors on midfrontal asymmetry are presented in Table 2. Hierarchical linear regressions demonstrated that risky behavior on the BART positively predicted midfrontal EEG asymmetry at the trend level, β = .26, t(41) = 1.79, p = .08, such that more pumps (more risky behavior) marginally predicted more left, relative to right, cortical activity. Risk group also marginally predicted midfrontal EEG asymmetry after controlling for ISS Activation during EEG acquisition, β = .26, t(44) = 1.70, p < .10. There were no significant main effects at lateral sites.
Table 2.
Effects of BAS Risk Group and BART Risk Taking on Left-Midfrontal EEG Asymmetry
| Risk Group
|
BART Avg
|
BART × Risk Group
|
||||
|---|---|---|---|---|---|---|
| Measure | β | t | β | T | β | t |
| ISS Activation | −.10 | −.60 | −.14 | −.93 | −.18 | −1.20 |
| Time to EEG | .22 | 1.52 | .17 | 1.16 | .21 | 1.46 |
| Risk Group | .26 | 1.70* | .25 | 1.68 | .28 | 1.93* |
| BART | .26 | 1.79* | .72 | 2.90*** | ||
| BART × Risk Group | −.56 | −2.24** | ||||
| Adjusted R2 | .05 | .10 | .18 | |||
Note. BART = Balloon Analogue Risk Taking; control variables included time to EEG session and ISS Activation.
p < .05, one-tailed;
p < .05, two-tailed;
p < .01, two-tailed.
To determine whether BAS risk group moderated the effect of risk taking on midfrontal EEG asymmetry, we followed the procedures described by Aiken and West (1991) to test the interactions using hierarchical linear regressions. Prior to analysis, BART scores were centered around their means, and the centered BART scores were used to predict EEG asymmetry, controlling for time between the initial assessment and the EEG session, as well as ISS Activation during EEG acquisition. Then, the interaction between BAS risk group and risk taking was entered in Step 3 of the hierarchical regression analyses (Table 2).
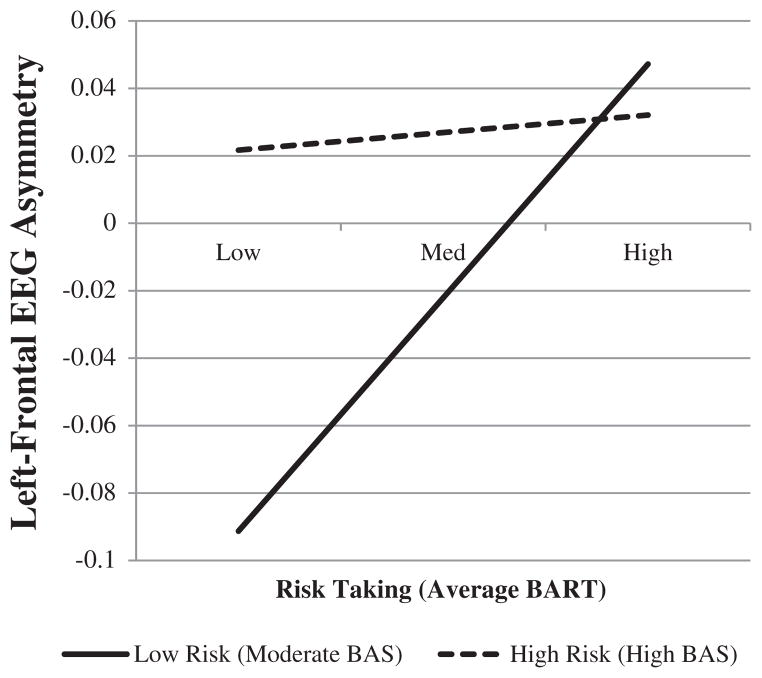
At midfrontal sites, risk taking on the BART significantly interacted with BAS risk group to predict left-midfrontal EEG asymmetry, controlling for ISS Activation and time to the EEG session, β = −.56, t(40) = −2.24, p = .03. To investigate the form of this interaction (Figure 1), we followed the procedures outlined by Aiken and West (1991) and examined the simple effect of BART risk taking at each level of BAS risk. Among individuals moderate in BAS sensitivity, BART risk taking significantly predicted left-midfrontal EEG asymmetry, β = .01, t(40) = 2.90, p < .01, whereas among high BAS sensitivity participants, there was no significant effect of BART risk taking on EEG asymmetry, β < .01, t(40) = .33, p = .74. There were no significant interactions at the lateral frontal sites.
FIGURE 1.
Risk taking on the BART interacts with BAS risk group to predict left-frontal EEG asymmetry controlling for time to EEG session and ISS Activation. BART = Balloon Analogue Risk Task; risk = BAS risk group.
Discussion
Our findings suggest that risk taking alone only marginally predicts greater left- relative to right-midfrontal cortical activity. However, we also found that among individuals at low risk for BD based on moderate BAS sensitivity, risk taking strongly predicted EEG asymmetry, whereas this effect was nonsignificant among individuals at high risk for BD based on high BAS sensitivity. Individuals at high risk for BD (high BAS sensitivity) had high levels of left- relative to right-frontal activity across all levels of risk taking. Conversely, individuals at low risk for BD who were lower on risk taking actually showed greater right- relative to left-midfrontal activation, whereas individuals with low bipolar risk who were high on risk taking showed greater left- relative to right-midfrontal activity. Taken together, these findings suggest that risk-taking tendencies may be an important qualifier of the well-documented relationship between BAS sensitivity and a neurophysiological marker of appetitive motivation, such that risk-taking tendencies may be associated with high left-frontal asymmetry even in the absence of strong BAS sensitivity.
These results are consistent with current conceptualizations of bipolar risk. Recent reports have suggested that BAS sensitivity is distinct from, but interacts with, impulsivity in predicting approach-related behaviors and psychopathology. In one such recent study, Alloy, Bender, and colleagues (2009) demonstrated that BAS sensitivity and impulsivity each independently prospectively predicted bipolar status and increases in substance abuse problems. A factor analysis by Quilty and Oakman (2004) found that BAS sensitivity and impulsivity were distinct but related constructs, and suggested that impulsivity may be a single component of a broad class of approach-related personality traits. Given that risky behavior is thought to result from impulsivity, particularly urgency and sensation seeking (e.g., Cyders et al., 2007; Deckman & DeWall, 2011; Zapolski, Cyders, & Smith, 2009), a logical extension might be that risk-taking traits may reflect another such class of approach-related personality traits that is related to, but distinct from, BAS sensitivity.
The findings of the current study go beyond previous work by examining multiple indicators of risk for BD in the same study. The finding that risk taking is associated for some individuals with a well-established neural correlate of approach motivation highlights the necessity of a conceptualization of BD that incorporates not only self-report but also biological and behavioral correlates of risk. This study also examined the interaction of risk taking and BAS sensitivity in predicting frontal EEG asymmetry, which is an important first step toward investigating how different components of risk for BD may combine. Importantly, the current study controlled for current hypomanic symptoms, providing a conservative statistical test of the influence of BD risk.
However, this study is not without limitations. First, the number of participants who completed the EEG study was small compared with the number of participants who enrolled in the overall study. Although these participants did not differ from the overall sample on any demographic characteristics or BAS risk group, it is possible that individuals who participated in the EEG session were different on unmeasured cognitive, emotional, or neurobiological variables. Second, the present study included individuals high and moderate on BAS sensitivity. Although this provides an excellent and conservative test of the effects of high BAS sensitivity, it is possible that these findings might not extend to individuals who have low BAS sensitivity. Indeed, we believe that our failure to observe more than a marginal relationship between BAS sensitivity and EEG asymmetry was likely due to the restricted range of BAS scores in our sample. Additionally, hypomanic and depressive symptoms were not specifically assessed at the time of EEG acquisition, so although the use of ISS Activation scores is useful in statistically controlling for activated mood state, it is not possible to know if participants were experiencing symptomatology at the time of the EEG without conducting a diagnostic interview.
Based on the findings of this study, it appears an important correlate of bipolar risk, risk taking, may relate to left-frontal EEG asymmetry, above the effect of BAS sensitivity. Future research should seek to elucidate these relationships in individuals with existing diagnoses of BD, and could potentially evaluate the utility of left-frontal EEG asymmetry as a mediator between behavioral and self-report risk factors and prospective BSDs. Further, future investigations should seek to parse the specific contributions of BAS sensitivity and risk taking from a potential underlying positive emotionality dimension, which could be contributing to the relationship between these factors and asymmetry.
The current findings may have future applications in the diagnosis and treatment of BSDs. Although a substantial literature supports the roles of BAS sensitivity and risk taking as important components of bipolar risk, the current study is unique in that it demonstrates independent contributions of each of these risk factors toward a neurophysiological correlate of approach motivation. Existing psychotherapies for the treatment of BD have demonstrated some efficacy in preventing relapse, increasing medication compliance, and reducing affective symptoms (Lam, Hayward, Watkins, Wright, & Sham, 2005; Miklowitz et al., 2007; Szentagotai & David, 2010). However, although these treatments have demonstrated efficacy primarily for depressive symptoms, effective prophylaxis or attenuation of manic symptoms has been more elusive (Gregory, 2010; Miklowitz, 2008; Scott, 2006). Psychotherapy for BD might be improved by specifically targeting psychological mechanisms thought to underlie manic symptoms, such as BAS sensitivity, overly positive cognitive style, or goal dysregulation (Johnson & Fulford, 2009; Lam, Wright, & Sham, 2005; Leahy, 2005; Nusslock, Abramson, Harmon-Jones, Alloy, & Coan, 2009; Scott et al., 2006). The current findings suggest that high risk taking may contribute to high approach motivation even in the absence of high BAS. Thus, it also may be critical to implement strategies aimed at reducing risk taking as part of a broader aim of achieving a balanced level of approach motivation and preventing mania. Consistent with this proposal, a small study (n = 10) of an intervention specifically aimed at prevention of mania (GOALS; Johnson & Fulford, 2009) has suggested that targeting elements of reward sensitivity and impulsive action relevant to goal-setting behavior may show promise in preventing mania.
Given that reward sensitivity and tendency toward risk taking are both traits thought to precede first onset of BSDs, these findings suggest that left-frontal EEG asymmetry may have potential as a biomarker of bipolar risk or a bipolar prodrome. Consistent with this notion, Nusslock et al. (2012) found that greater left-frontal EEG asymmetry predicted conversion to bipolar I disorder (onset of mania) among individuals with softer bipolar spectrum conditions. Further, this metric potentially could be used to evaluate which individuals with risk factors such as BAS sensitivity or risk taking may have a higher risk of developing BSD. Future research will be necessary to determine the specificity of these findings to BSDs compared with other diagnoses also thought to involve dysregulation of the BAS (e.g., substance use disorders) and the effect of comorbid conditions on left-frontal EEG asymmetry***.
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH77908 to Lauren B. Alloy.
Footnotes
Inclusion of these outliers led the significant interaction reported below to no longer attain significance.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Contributor Information
Chelsea L. Black, Temple University
Kim E. Goldstein, Temple University
Denise R. LaBelle, Temple University
Christopher W. Brown, Temple University
Eddie Harmon-Jones, University of New South Wales.
Lyn Y. Abramson, University of Wisconsin–Madison
Lauren B. Alloy, Temple University
References
- Adida M, Clark L, Pomietto P, Kaladjian A, Besnier N, Azorin J, Goodwin GM. Lack of insight may predict impaired decision making in manic patients. Bipolar Disorders. 2008;10(7):829–837. doi: 10.1111/j.1399-5618.2008.00618.x. http://dx.doi.org/10.1111/j.1399-5618.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biological Psychiatry. 2011;70(4):357–365. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behaviour Research and Therapy. 2005;43(2):215–228. doi: 10.1016/j.brat.2003.12.007. http://dx.doi.org/10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19(3):189–194. doi: 10.1177/0963721410370292. http://dx.doi.org/10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Hogan ME. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith JM, Neeren AM, Nusslock R. Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motivation and Emotion. 2006;30(2):143–155. http://dx.doi.org/10.1007/s11031-006-9003-3. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, Whitehouse WG, Harmon-Jones E. Behavioral Approach System (BAS)—relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. Journal of Abnormal Psychology. 2009;118(3):459–471. doi: 10.1037/a0016604. http://dx.doi.org/10.1037/a0016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, Harmon-Jones E. Bipolar spectrum–substance use co-occurrence: Behavioral Approach System (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. Journal of Personality and Social Psychology. 2009;97(3):549–565. doi: 10.1037/a0016061. http://dx.doi.org/10.1037/a0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Abramson LY. High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology. 2012;121:339–351. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, Hogan M. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology. 2012;121(1):16–27. doi: 10.1037/a0023973. http://dx.doi.org/10.1037/a0023973(10.1037/a0023973.supp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. ed. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Balconi M, Mazza G. Lateralisation effect in comprehension of emotional facial expression: A comparison between EEG alpha band power and Behavioural Inhibition (BIS) and Activation (BAS) Systems. Laterality: Asymmetries of Body, Brain and Cognition. 2010;15(3):361–384. doi: 10.1080/13576500902886056. http://dx.doi.org/10.1080/13576500902886056. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Crits-Christoph P, Ball WA, Dewees E. Independent assessment of manic and depressive symptoms by self-rating: Scale characteristics and implications for the study of mania. Archives of General Psychiatry. 1991;48(9):807–812. doi: 10.1001/archpsyc.1991.01810330031005. [DOI] [PubMed] [Google Scholar]
- Brunborg GS, Johnsen BH, Mentzoni RA, Molde H, Pallesen S. Individual differences in evaluative conditioning and reinforcement sensitivity affect bet-sizes during gambling. Personality and Individual Differences. 2011;50(5):729–734. http://dx.doi.org/10.1016/j.paid.2010.12.026. [Google Scholar]
- Carver CS. Negative affects deriving from the Behavioral Approach System. Emotion. 2004;4(1):3–22. doi: 10.1037/1528-3542.4.1.3. http://dx.doi.org/10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL. Tendencies toward mania and tendencies toward depression have distinct motivational, affective, and cognitive correlates. Cognitive Therapy and Research. 2009;33(6):552–569. doi: 10.1007/s10608-008-9213-y. http://dx.doi.org/10.1007/s10608-008-9213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6(2):175–183. doi: 10.1016/0278-2626(87)90118-7. http://dx.doi.org/10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the Behavioral Activation and Inhibition Systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. http://dx.doi.org/10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67(1–2):7–49. doi: 10.1016/j.biopsycho.2004.03.002. http://dx.doi.org/10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychological Assessment. 2007;19(1):107–118. doi: 10.1037/1040-3590.19.1.107. http://dx.doi.org/10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Deckman T, DeWall CN. Negative urgency and risky sexual behaviors: A clarification of the relationship between impulsivity and risky sexual behavior. Personality and Individual Differences. 2011;51(5):674–678. http://dx.doi.org/10.1016/j.paid.2011.06.004. [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. In: Porter LW, editor. Annual review of psychology. Vol. 40. Palo Alto, CA: 1989. pp. 457–492. Annual Reviews. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss SP, Spoont MR. A two-dimensional threshold model of seasonal bipolar affective disorder. In: Öhman A, editor. Psychopathology: An interactional perspective. San Diego, CA: Academic Press; 1987. pp. 95–123. [Google Scholar]
- Di Nicola M, Tedeschi D, Mazza M, Martinotti G, Harnic D, Catalano V, Janiri L. Behavioural addictions in bipolar disorder patients: Role of impulsivity and personality dimensions. Journal of Affective Disorders. 2010;125 doi: 10.1016/j.jad.2009.12.016. http://dx.doi.org/10.1016/j.jad.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The schedule for affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou P, Dufour MC, Compton W, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. http://dx.doi.org/10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition and Emotion. 1990;4(3):269–288. http://dx.doi.org/10.1080/02699939008410799. [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- Gregory VLJ. Cognitive-behavioral therapy for mania: A meta-analysis of randomized controlled trials. Social Work in Mental Health. 2010;8(6):483–494. http://dx.doi.org/10.1080/15332981003744388. [Google Scholar]
- Harmon-Jones E. Anger and the Behavioral Approach System. Personality and Individual Differences. 2003;35(5):995– 1005. http://dx.doi.org/10.1016/S0191-8869(02)00313-6. [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urošević S, Turonie LD, Fearn M. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry. 2008;63(7):693–698. doi: 10.1016/j.biopsych.2007.08.004. http://dx.doi.org/10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–163. doi: 10.1037//0021-843x.106.1.159. http://dx.doi.org/10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Bodkins M, Brenner C, Shekhar A, Nurnberger JI, Jr, O’Donnell B, Hetrick WP. A multimethod investigation of the Behavioral Activation System in bipolar disorder. Journal of Abnormal Psychology. 2008;117(1):164–170. doi: 10.1037/0021-843X.117.1.164. http://dx.doi.org/10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- Holmes MK, Bearden CE, Barguil M, Fonseca M, Monkul ES, Nery FG, Glahn DC. Conceptualizing impulsivity and risk taking in bipolar disorder: Importance of history of alcohol abuse. Bipolar Disorders. 2009;11(1):33–40. doi: 10.1111/j.1399-5618.2008.00657.x. http://dx.doi.org/10.1111/j.1399-5618.2008.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E, Arias B, Castellví P, Goikolea JM, Rosa AR, Fañanás L, Benabarre A. Impulsivity and functional impairment in bipolar disorder. Journal of Affective Disorders. 2012;136(3):491–497. doi: 10.1016/j.jad.2011.10.044. http://dx.doi.org/10.1016/j.jad.2011.10.044. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The Behavioral Activation System and mania. Annual Review of Clinical Psychology. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. http://dx.doi.org/10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Fulford D. Preventing mania: A preliminary examination of the GOALS program. Behavior Therapy. 2009;40(2):103–113. doi: 10.1016/j.beth.2008.03.002. http://dx.doi.org/10.1016/j.beth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. Journal of Affective Disorders. 2008;108(1–2):49–58. doi: 10.1016/j.jad.2007.06.014. http://dx.doi.org/10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Karakus G, Tamam L. Impulse control disorder comorbidity among patients with bipolar I disorder. Comprehensive Psychiatry. 2011;52(4):378–385. doi: 10.1016/j.comppsych.2010.08.004. http://dx.doi.org/10.1016/j.comppsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, Sham P. Sense of hyper-positive self and response to cognitive therapy in bipolar disorder. Psychological Medicine: Journal of Research in Psychiatry and the Allied Sciences. 2005;35(1):69–77. doi: 10.1017/s0033291704002910. http://dx.doi.org/10.1017/S0033291704002910. [DOI] [PubMed] [Google Scholar]
- Lam DH, Hayward P, Watkins ER, Wright K, Sham P. Relapse prevention in patients with bipolar disorder: Cognitive therapy outcome after 2 years. American Journal of Psychiatry. 2005;162(2):324–329. doi: 10.1176/appi.ajp.162.2.324. http://dx.doi.org/10.1176/appi.ajp.162.2.324. [DOI] [PubMed] [Google Scholar]
- Leahy RL. Clinical implications in the treatment of mania: Reducing risk behavior in manic patients. Cognitive and Behavioral Practice. 2005;12(1):89–98. http://dx.doi.org/10.1016/S1077-7229(05)80043-4. [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and validity of the youth version of the balloon analogue risk task (BART-Y) in the assessment of risk taking behavior among inner-city adolescents. Journal of Clinical Child and Adolescent Psychology. 2007;36(1):106–111. doi: 10.1080/15374410709336573. http://dx.doi.org/10.1207/s15374424jccp3601_11. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. http://dx.doi.org/10.1037/1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, McElroy SL, Konarski JZ, Soczynska JK, Wilkins K, Kennedy SH. Problem gambling in bipolar disorder: Results from the Canadian Community Health Survey. Journal of Affective Disorders. 2007;102(1–3):27–34. doi: 10.1016/j.jad.2006.12.005. http://dx.doi.org/10.1016/j.jad.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Meade CS, Fitzmaurice GM, Sanchez AK, Griffin ML, McDonald LJ, Weiss RD. The relationship of manic episodes and drug abuse to sexual risk behavior in patients with co-occurring bipolar and substance use disorders: A 15-month prospective analysis. AIDS and Behavior. 2011;15(8):1829–1833. doi: 10.1007/s10461-010-9814-9. http://dx.doi.org/10.1007/s10461-010-9814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Pato M. Recent developments in the epidemiology of bipolar disorder in adults and children: Magnitude, correlates, and future directions. Clinical Psychology: Science and Practice. 2009;16(2):121–133. http://dx.doi.org/10.1111/j.1468-2850.2009.01152.x. [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment. 1999;21(4):275–292. doi: 10.1023/A:1022119414440. http://dx.doi.org/10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psycho-pathology and Behavioral Assessment. 2001;23(3):133–143. doi: 10.1023/A:1010929402770. http://dx.doi.org/10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TD, Hofmann BU. Assessing the dysregulation of the Behavioral Activation System: The hypomanic personality scale and the BIS-BAS scales. Journal of Personality Assessment. 2005;85(3):318–324. doi: 10.1207/s15327752jpa8503_08. http://dx.doi.org/10.1207/s15327752jpa8503_08. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: State of the evidence. American Journal of Psychiatry. 2008;165(11):1408–1419. doi: 10.1176/appi.ajp.2008.08040488. http://dx.doi.org/10.1176/appi.ajp.2008.08040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, Sachs GS. Psychosocial treatments for bipolar depression. Archives of General Psychiatry. 2007;64(4):419–427. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Tomarken AJ. Task-dependent changes in frontal brain asymmetry: Effects of incentive cues, outcome expectancies, and motor responses. Psychophysiology. 2001;38(3):500–511. http://dx.doi.org/10.1017/S0048577201991164. [PubMed] [Google Scholar]
- Molz AR, Black CL, Shapero BG, Bender RE, Alloy LB, Abramson LY. Aggression and impulsivity as predictors of stress generation in bipolar spectrum disorders. Journal of Affective Disorders. 2013;146(2):272–280. doi: 10.1016/j.jad.2012.07.022. http://dx.doi.org/10.1016/j.jad.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES, Sahakian BJ. Decision-making cognition in mania and depression. Psychological Medicine: Journal of Research in Psychiatry and the Allied Sciences. 2001;31(4):679–693. doi: 10.1017/s0033291701003804. http://dx.doi.org/10.1017/S0033291701003804. [DOI] [PubMed] [Google Scholar]
- Najt P, Perez J, Sanches M, Peluso MAM, Glahn D, Soares JC. Impulsivity and bipolar disorder. European Neuropsychopharmacology. 2007;17(5):313–320. doi: 10.1016/j.euroneuro.2006.10.002. http://dx.doi.org/10.1016/j.euroneuro.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Abramson L, Harmon-Jones E, Alloy L, Coan J. Psychosocial interventions for bipolar disorder: Perspective from the behavioral approach system (BAS) dysregulation theory. Clinical Psychology: Science and Practice. 2009;16(4):449–469. doi: 10.1111/j.1468-2850.2009.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urošević S, Goldstein K, Abramson LY. Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. Journal of Abnormal Psychology. 2012;121(3):592–601. doi: 10.1037/a0028973. http://dx.doi.org/10.1037/a0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness. A source-localization study. Psychological Science. 2005;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. http://dx.doi.org/10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Quilty LC, Oakman JM. The assessment of behavioural activation—the relationship between impulsivity and behavioural activation. Personality and Individual Differences. 2004;37(2):429–442. http://dx.doi.org/10.1016/j.paid.2003.09.014. [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40(2):305–315. http://dx.doi.org/10.1016/j.paid.2005.03.024. [Google Scholar]
- Richardson T. Correlates of substance use disorder in bipolar disorder: A systematic review and meta-analysis. Mental Health and Substance Use. 2011;4(3):239–255. [Google Scholar]
- Rosa AR, Reinares M, Michalak EE, Bonnin CM, Sole B, Franco C, Vieta E. Functional impairment and disability across mood states in bipolar disorder. Value in Health. 2010;13(8):984–988. doi: 10.1111/j.1524-4733.2010.00768.x. http://dx.doi.org/10.1111/j.1524-4733.2010.00768.x. [DOI] [PubMed] [Google Scholar]
- Scott J. Psychotherapy for bipolar disorders—efficacy and effectiveness. Journal of Psychopharmacology. 2006;20(2):46–50. doi: 10.1177/1359786806063078. http://dx.doi.org/10.1177/1359786806063078. [DOI] [PubMed] [Google Scholar]
- Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Hayhurst H. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: Randomised controlled trial. British Journal of Psychiatry. 2006;188(4):313–320. doi: 10.1192/bjp.188.4.313. http://dx.doi.org/10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- Skeel RL, Pilarski C, Pytlak K, Neudecker J. Personality and performance-based measures in the prediction of alcohol use. Psychology of Addictive Behaviors. 2008;22(3):402–409. doi: 10.1037/0893-164X.22.3.402. http://dx.doi.org/10.1037/0893-164X.22.3.402. [DOI] [PubMed] [Google Scholar]
- Sobotka SS, Davidson RJ, Senulis JA. Anterior brain electrical asymmetries in response to reward and punishment. Electroencephalography and Clinical Neuro-physiology. 1992;83(4):236–247. doi: 10.1016/0013-4694(92)90117-z. http://dx.doi.org/10.1016/0013-4694(92)90117-Z. [DOI] [PubMed] [Google Scholar]
- Suhr JA, Tsanadis J. Affect and personality correlates of the Iowa gambling task. Personality and Individual Differences. 2007;43(1):27–36. http://dx.doi.org/10.1016/j.paid.2006.11.004. [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the Behavioral Approach and Inhibition Systems. Psychological Science. 1997;8(3):204–210. http://dx.doi.org/10.1111/j.1467-9280.1997.tb00413.x. [Google Scholar]
- Swann AC, Anderson JC, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Research. 2001;101(2):195–197. doi: 10.1016/s0165-1781(00)00249-3. http://dx.doi.org/10.1016/S0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. Journal of Affective Disorders. 2003;73(1–2):105–111. doi: 10.1016/s0165-0327(02)00328-2. http://dx.doi.org/10.1016/S0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: A quantitative meta-analysis. Journal of Clinical Psychiatry. 2010;71(1):66–72. doi: 10.4088/JCP.08r04559yel. http://dx.doi.org/10.4088/JCP.08r04559yel. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31(6):837–862. http://dx.doi.org/10.1016/S0191-8869(00)00183-5. [Google Scholar]
- Udachina A, Mansell W. Cross-validation of the Mood Disorders Questionnaire, the Internal State Scale, and the Hypomanic Personality Scale. Personality and Individual Differences. 2007;42(8):1539–1549. http://dx.doi.org/10.1016/j.paid.2006.10.028. [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the Behavioral Approach System (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review. 2008;28(7):1188–1205. doi: 10.1016/j.cpr.2008.04.004. http://dx.doi.org/10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor SE, Johnson SL, Gotlib IH. Quality of life and impulsivity in bipolar disorder. Bipolar Disorders. 2011;13(3):303–309. doi: 10.1111/j.1399-5618.2011.00919.x. http://dx.doi.org/10.1111/j.1399-5618.2011.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TCB, Cyders MA, Smith GT. Positive urgency predicts illegal drug use and risky sexual behavior. Psychology of Addictive Behaviors. 2009;23(2):348–354. doi: 10.1037/a0014684. http://dx.doi.org/10.1037/a0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]