Abstract
Here we report that 5'-monophosphate (AMP)-activated protein kinase (AMPK) agonist A-769662 inhibited hydrogen peroxide (H2O2)-induced viability loss and apoptosis of human and mouse osteoblast cells. H2O2-induced moderate AMPK activation in osteoblast cells, which was enhanced by A-769662. Inactivation of AMPK by its inhibitor compound C, or by target shRNA-mediated silencing and kinase dead (KD) mutation exacerbated H2O2-induced cytotoxicity in osteoblast cells. A-769662-mediated protective effect against H2O2 was also blocked by AMPK inhibition or depletion. A-769662 inhibited reactive oxygen species (ROS) accumulation by H2O2 in osteoblast cells. Meanwhile, H2O2-induced ATP depletion was inhibited by A-769662, but was aggravated by compound C. Further, H2O2 induced AMPK-dependent and pro-survival autophagy in cultured osteoblast cells, which was enhanced by A-769662. Our results suggested that activation of AMPK by H2O2 is anti-apoptosis and pro-survival in osteoblast cells, probably due to its anti-oxidant, pro-autophagy and ATP preservation abilities, and A-769662-mediated cell-protective effect in osteoblast cells requires AMPK activation. Our study suggests that A-769662 might be further investigated as a novel anti-osteonecrosis agent.
Keywords: osteonecrosis, AMPK, A-769662, oxidative stress and apoptosis
1. Introduction
In the pathological condition of osteonecrosis, bones become fragile and will lead to bone fracture if not treated properly. Osteoblasts are important for bone formation and remodeling. Studies have shown that osteonecrosis is associated with oxidative stress in osteoblasts. Elevated reactive oxygen species (ROS) (i.e., hydrogen peroxide (H2O2)) induce osteoblasts dysfunction and apoptosis, serving as an important contributor of osteonecrosis [1,2]. Different groups have added H2O2 to cultured osteoblasts to create a cellular model of osteonecrosis [1,2].
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a heterotrimer kinase composed of α, β and γ subunits. It is a member of metabolite-sensing kinase family, which plays important roles in metabolic balance [3,4]. Recent studies suggest that AMPK is also important for cell survival under stress conditions. For example, AMPK activation regulates nicotinamide adenine dinucleotide phosphate (NADPH) homeostasis to promote cell survival [5]. Further, AMPK-dependent tuberous sclerosis complex 2 (TSC2) phosphorylation is important for cell survival under energy starvation conditions [6]. Resveratrol, an AMPK activator, improves endurance of mice on a high-calorie diet [7]. Other studies, however, showed that activation of AMPK is somehow pro-apoptotic [8,9,10,11]. AMPK could be activated by H2O2 [11,12,13]. However, the defensive role of AMPK in H2O2-induced cell apoptosis is still debatable.
Recent studies have confirmed other functions of AMPK activation. For example, AMPK is important for regulation of autophagy, which is an evolutionarily conserved caspase-independent process of degradation and recycling of long-lived proteins and cytoplasmic-damaged organelles [14,15,16]. Autophagy exerts its pro-survival ability through apoptosis inhibition [14,17]. AMPK promotes autophagy activation through phosphorylation of its downstream target Raptor and consequent inhibition of mammalian target of rapamycin (mTOR) [18,19], as well as through directly activating Ulk1, the autophagy initiator [20,21].
AICAR (5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside) is a well-characterized AMPK activator, which becomes N 1-(β-d-5'-phosphoribofuranosyl)-5-aminoimidazole-4-carboxamide (ZMP) after taken by the cell to mimic the effect of AMP [22]. A-769662 is another validated AMPK activator that stimulates AMPK via allosteric activation of AMPKα at Thr-172 [23]. A recent study by Meester et al., demonstrated that pharmacological activation of AMPK by A-769662 inhibits mesenchymal stem cells (MSCs) proliferation via the regulation of p27 expression [24]; Ducommun et al. showed that AMPK activator A-769662 inhibited lipogenesis in primary hepatocytes, co-treatment with AICAR caused profound lipogenesis inhibition through synergistic activation of AMPK [25].
In the current study, we aimed to understand the potential role of AMPK in H2O2-induced apoptosis of osteoblasts, and to investigative the underlying mechanisms. Here, we mainly focused on the effect of A-769662 on H2O2-induced osteoblast cell apoptosis.
2. Results
2.1. A-769662 Inhibits H2O2-Induced Osteoblast Cell Death
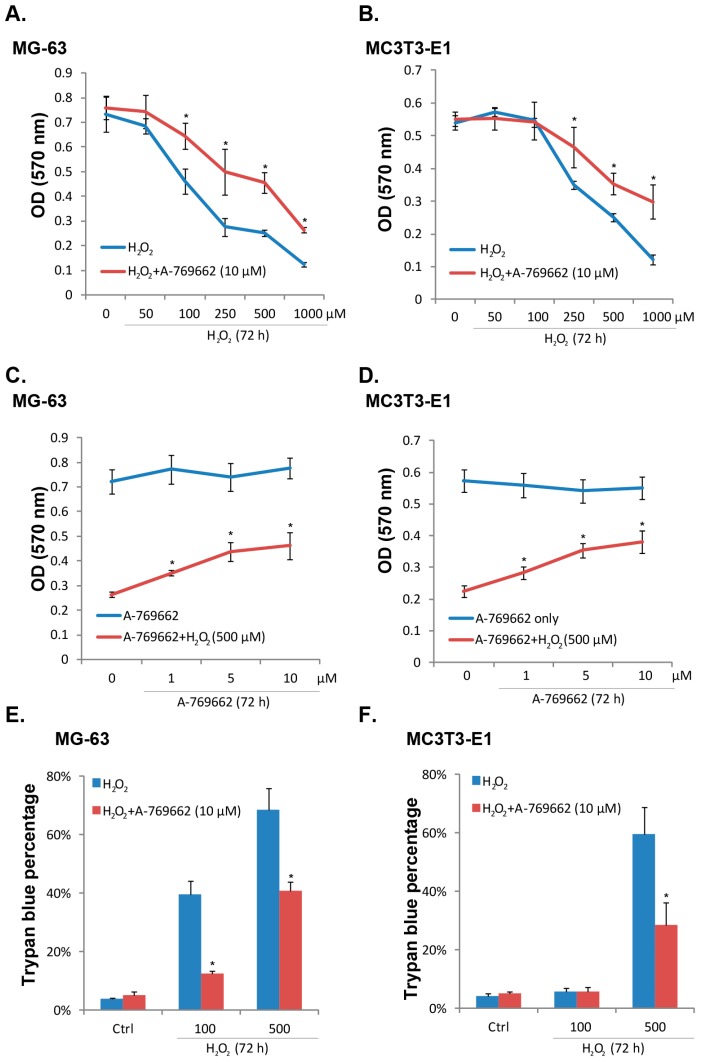
As shown in Figure 1A,B, hydrogen peroxide (H2O2, 50–1000 μM) dose-dependently inhibited the viability of human and mouse osteoblast cells (MG-63 and MC3T3-E1 lines). Significantly, co-administration of A-769662 (10 μM) inhibited viability loss by H2O2 in both cell lines (Figure 1A,B). Meanwhile, the effect of A-769662 was dose-dependent (Figure 1C,D), and A-769662 at 10 μM showed best cell-protective effect (Figure 1C,D). Meanwhile, as demonstrated in Figure 1E,F, H2O2-induced death of osteoblasts, shown by the increased number of trypan blue stained cells, was also inhibited by A-769662 (10 μM). Thus, A-769662 inhibits H2O2-induced osteoblast cell death.
Figure 1.
A-769662 inhibits H2O2-induced osteoblast cell death. Cultured human and mouse osteoblast-like cell lines (MG-63 and MC3T3-E1) were pre-treated with A-769662 (10 μM) for 1 h, followed by hydrogen peroxide (H2O2, 50–1000 μM) stimulation, cells were further cultured for 72 h, and MTT assay was performed to test cell viability (A) and (B); trypan blue staining was utilized to test cell death (E) and (F); MG-63 and MC3T3-E1 cells were pre-treated with different concentration of A-769662 (1, 5 and 10 μM) for 1 h, followed by H2O2 (500 μM) stimulation, cells were further cultured for 72 h, and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay was performed to test cell viability (C) and (D). Values in this figure were expressed as mean ± SD, experiments were repeated 3 times and similar results were obtained (same for all figures). * p < 0.05 vs. group without A-769662.
2.2. A-769662 Suppresses H2O2-Induced Osteoblast Cell Apoptosis
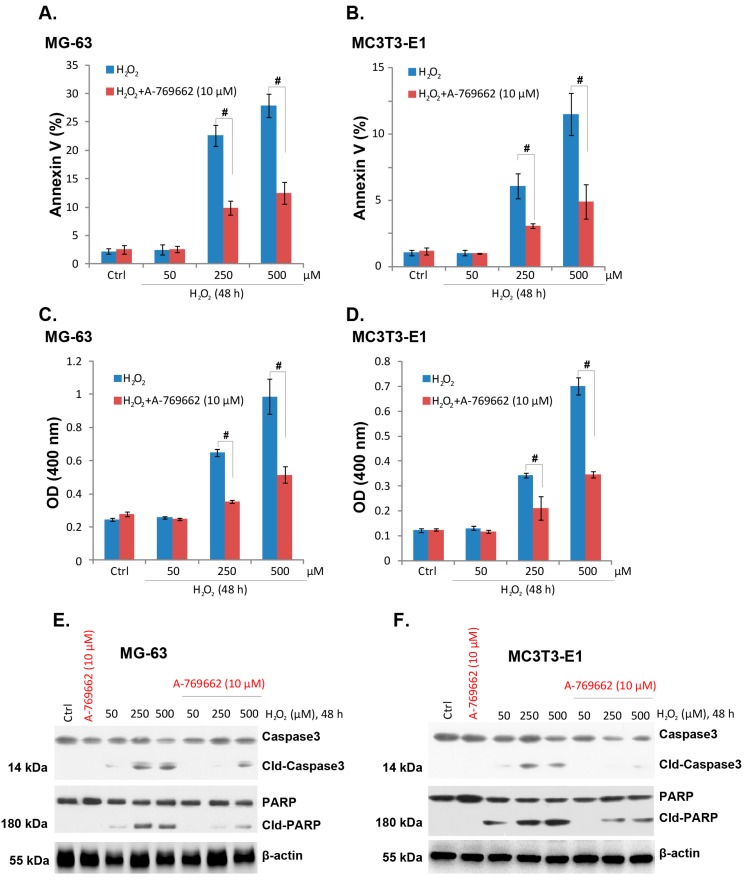
Results in Figure 1 showed that A-769662 suppressed H2O2-induced osteoblast cell death. Next, we tested whether A-769662 affected osteoblast cell apoptosis. As mentioned, cell apoptosis was tested by histone-DNA apoptosis enzyme linked immunosorbent assay (ELISA) assay and Annexin V FACS assay. Results in Figure 2A–D demonstrated that H2O2 (50–500 μM) induced cell apoptosis in both lines, as the histone DNA ELISA optical density (OD) and the percentage of Annexin V cells increased significantly after H2O2 treatment (Figure 2A–D). Notably, co-administration with A-769662 (10 μM) dramatically suppressed H2O2-induced cell apoptosis in both cell lines (Figure 2A,D). H2O2-induced cleavage of PARP and caspase-3 was also inhibited by A-769662 (Figure 2E,F). These results indicated that A-769662 significantly suppressed H2O2-induced osteoblast cell apoptosis.
Figure 2.
A-769662 suppresses H2O2-induced osteoblast cell apoptosis. MG-63 and MC3T3-E1 cells were pre-treated with A-769662 (10 μM) for 1 h, followed by hydrogen peroxide (H2O2, 50–500 μM) stimulation, cells were further cultured for 48 h, and Annexin V fluorescence-activated cell sorting (FACS) assay (A) and (B) and histone-DNA ELISA assay (C) and (D) were performed to test cell apoptosis; the expressions of PARP (cleaved and regular), caspase-3 (cleaved and regular) and β-actin were tested by western blots (E) and (F). # p < 0.05.
2.3. A-769662-Induced Pro-Survival Effect against H2O2 Requires AMPK Activation
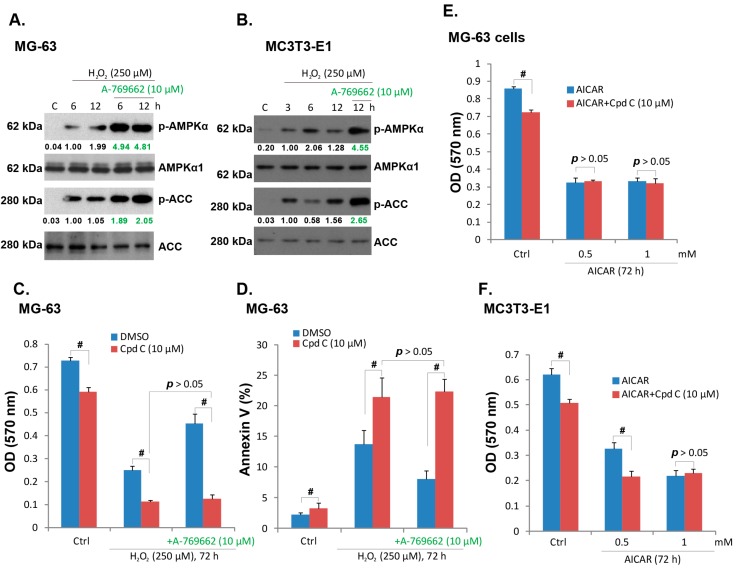
As discussed, A-769662 is a well-known AMPK agonist [26]. Thus we tested the involvement of AMPK in A-769662-induced protective effect against H2O2. Western blot results in Figure 3A,B showed that H2O2 induced a moderate AMPK activation, and the expressions of phospho (p)-AMPKα and p-acetyl CoA carboxylase (ACC) increased after H2O2 stimulation in both MG-63 and MC3T3-E1 cells. Notably, co-administration with A-769662 (10 μM) dramatically enhanced AMPK activation (AMPKα/ACC phosphorylation) by H2O2 (Figure 3A,B). Compound C (Cpd C), the AMPK inhibitor, aggravated H2O2-induced MG-63 cell damage, with increased cell viability loss and Annexin V positive cells observed (Figure 3C,D). Importantly, A-769662-induced protective effect against H2O2 was abolished by compound C (Figure 3C,D). These results indicated that A-769662-induced protective effect in osteoblast cells requires AMPK activation. Note that compound C alone also slightly inhibited MG-63 cell viability (Figure 3C,D), indicating that basal AMPK activation might be important for cell survival. Interestingly, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), another well-known AMPK agonist [27], dramatically inhibited viability of MG-63 and MC3T3-E1 cells alone (Figure 3E,F). Such an effect of AICAR appeared not dependent on AMPK activation, as compound C failed to rescue it (Figure 3E,F). These results indicate that AICAR induces AMPK-independent osteoblast cell death, a phenomenon that is seen in other cell lines [28].
Figure 3.
A-769662-induced pro-survival effect against H2O2 requires AMP-activated protein kinase (AMPK) activation. MG-63 and MC3T3-E1 cells were pre-treated with A-769662 (10 μM) for 1 h, followed by hydrogen peroxide (H2O2, 250 μM) stimulation, cells were further cultured for indicated time points, and western blots were performed to test phospho(p)- and total (t)-AMPKα, p- and t- ACC (A) and (B); MG-63 and MC3T3-E1 cells were pre-treated with AMPK inhibitor compound C (Cpd C, 10 μM) for 2 h, followed by hydrogen peroxide (H2O2, 250 μM) or H2O2+ A-769662 (10 μM) stimulation, cells were further cultured, cell viability (C) and Annexin V percentage (D) were tested as described; MG-63 and MC3T3-E1 cells were pre-treated with compound C (Cpd C, 10 μM) for 2 h, followed by AICAR (0.5 and 1 mM) stimulation, cells were further cultured for 72 h when cell viability was examined (E) and (F). # p < 0.05.
2.4. A-769662 Alleviates Reactive Oxygen Species (ROS) Accumulation and ATP Depletion Caused by H2O2
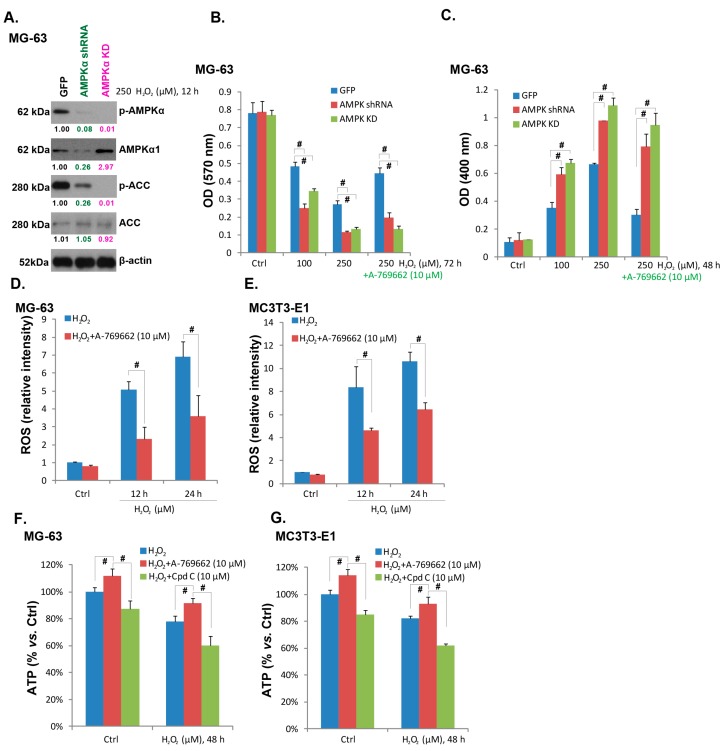
To further confirm that AMPK activation is required for A-769662-induced pro-survival effect, we generated stable MG-63 cell lines expressing AMPKα shRNA (AMPKα knockdown cell line) or kinase dead (KD) AMPKα (T172A, AMPKα KD cell line). As shown in Figure 4A, AMPKα stable knockdown or KD mutation almost blocked AMPK activation by H2O2. H2O2-induced MG-63 cell viability loss (Figure 4B) and apoptosis (Figure 4C) were exacerbated in AMPKα-knockdown or mutated cells, confirming that activation of AMPK by H2O2 is a pro-survival signaling in osteoblasts. Meanwhile, A-769662-induced pro-survival effect against H2O2 was almost blocked with AMPKα knockdown or mutation (Figure 4B,C), further supporting the involvement of AMPK activation in the process. A recent study by Jeon et al. showed the antioxidant ability of AMPK [5], and AMPK activation suppresses oxidative stress through inhibiting NADPH depletion and promoting NADPH synthesis [5]. As shown in Figure 4D,E, A-769662 alleviated ROS accumulation in H2O2-treated osteoblasts, further confirming its anti-oxidant ability. Meanwhile, H2O2 stimulation decreased cellular ATP content in both cell lines (Figure 4E,F), which was partly recued by A-769662, but was exacerbated by Compound C (Figure 4E,F). These results suggested that A-769662 alleviates ROS accumulation and ATP depletion caused by H2O2, which may serve as the main mechanisms of its cell-protective efficiency.
Figure 4.
A-769662 alleviates ROS accumulation and ATP depletion caused by H2O2. The expressions of AMPKα (p- and t-), ACC (p- and t-) and β-actin in hydrogen peroxide (H2O2, 250 μM) stimulated MG-63 cells transfected with GFP-shRNA, AMPKα shRNA lentivirus or AMPKα kinase dead (KD) plasmid (A); Above cells were also subjected to H2O2 stimulation (100 and 250 μM) in the presence or absence of A-769662 (10 μM), cell viability and apoptosis were analyzed by MTT assay (B) and histone-DNA apoptosis ELISA assay respectively (C); The effect of A-769662 (10 μM, 1 h pretreatment) on H2O2-induced ROS accumulation was tested as described (D) and (E); The effect of A-769662 (10 μM, 1 h pretreatment) or compound C (Cpd C, 10 μM, 1 h pretreatment) on cellular ATP content in H2O2-stimulated MG-63 and MC3T3-E1 cells were analyzed (F) and (G). # p < 0.05.
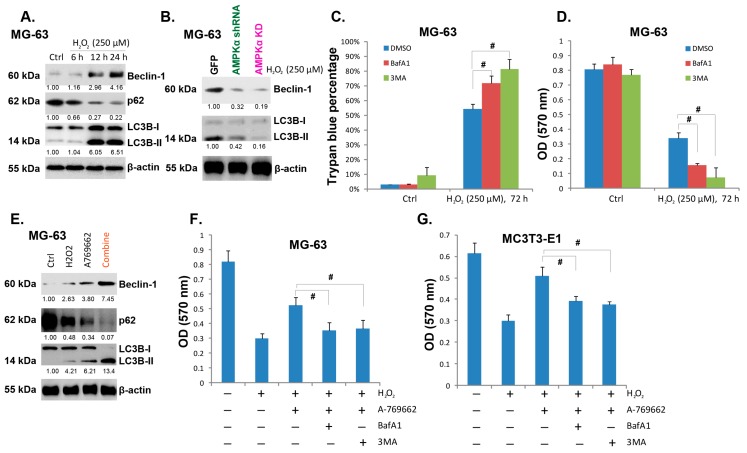
2.5. A-769662 Facilitates H2O2-Induced Autophagy Activation
As discussed, one main consequence of AMPK activation is autophagy, which is also cyto-protective. Here, we found that H2O2 activated autophagy is MG-63 cells, which was demonstrated by p62 degradation, LC3-II production and Beclin-1 expression (Figure 5A). AMPK activation was required for H2O2-induced autophagy induction in MG-63 cells, and AMPK shRNA depletion or kinase-dead (KD) mutation inhibited autophagy activation by H2O2 (Figure 5B). H2O2-induced autophagy appeared cytoprotective, as inhibition of autophagy by two inhibitors (3-MA and BafA1) exaggerated H2O2-induced viability loss and cell death (Figure 5C,D). On the other hand, the AMPK activator A-769662 facilitated H2O2-induced autophagy activation (Figure 5E), while A-769662-induced pro-survival effect against H2O2 was alleviated by the autophagy inhibitors in both MG-63 and MC3T3-E1 cells (Figure 5F,G). Together, these data suggest that H2O2 induces AMPK-dependent and cyto-protective autophagy in cultured osteoblasts. A-769662-induced pro-survival effect against H2O2 is mediated, at least in part, by its ability to activate autophagy (Figure 5).
Figure 5.
A-769662 facilitates H2O2-induced autophagy activation. MG-63 cells were treated with hydrogen peroxide (H2O2, 250 μM), cells were further cultured for indicated time points, and western blots were performed to test LC3B, Beclin-1, p62 and β-actin (A); The expressions of LC3B, Beclin-1, p62 and β-actin in H2O2 (250 μM) stimulated MG-63 cells transfected with GFP-shRNA, AMPKα shRNA lentivirus or AMPKα kinase dead (KD) plasmid were tested (B); MG-63 cells were pre-treated with 3-methyladenine (3-MA, 1 mM), or Bafilomycin A1 (BafA1, 0.1 μM) for 1 h, followed by H2O2 (250 μM) stimulation for 72 h, cell viability (C) and trypan blue assay (D) were performed; MG-63 cells were treated with H2O2 (250 μM), A-769662 (10 μM) or both for 24 h, the expressions of LC3B, Beclin-1, p62 and β-actin were tested (E); The viability of MG-63 or MC3T3-E1 cells with indicated treatment for 72 h was tested by MTT assay (F and G). # p < 0.05.
3. Discussion
Here we reported that A-769662 inhibited H2O2-induced death and apoptosis in both human and mouse osteoblast cells. AMPK inhibition by its inhibitor compound C, by targeted shRNA-mediated silencing or kinase dead (KD) mutation exacerbated H2O2-induced death and apoptosis of osteoblast cells. A-769662-induced cell-protective effect was diminished when AMPK was inactivated or depleted. We found that A-769662 inhibited ROS accumulation and ATP depletion by H2O2, which might mediate its pro-survival effect. Further, H2O2 induced AMPK-dependent and cyto-protective autophagy in cultured osteoblast cells, which was enhanced by A-769662. Based on these results, we suggested that activation of AMPK by H2O2 might serve as a negative regulator to promote osteoblast cell survival.
Recent studies have shed lights on how AMPK mediates cell survival. AMPK activation attenuates oxidative stress-mediated cell death through promoting NADPH synthesis and limiting its consumption [5]. Thus, in addition to its function in ATP homeostasis, the AMPK/acetyl-CoA carboxylase (ACC) signaling axis works as an anti-oxidant to maintain NADPH homeostasis, and to decrease ROS accumulation, rescuing cells from oxidative stresses [5]. In the current study, we found that H2O2-induced oxidative stress in osteoblast cells was inhibited A-769662, but was aggravated by compound C, suggesting that AMPK activation and A-769662 might have the similar anti-oxidant effect.
In almost all eukaryotic cells, AMPK constitutes a molecular hub for cellular metabolic control. When cells facing metabolic stress (i.e., ischemia/hypoxia and glucose deprivation), AMP/ATP ratio will increase to activate AMPK [6]. TSC2 and its phosphorylation by AMPK protect cells from energy deprivation-induced apoptosis [6]. In the current study, we also observed that ATP level was decreased when osteoblast cells stimulated with H2O2, which might be the key mechanism to activate AMPK. Activated AMPK then tried to maintain ATP level and recue cells. Forced activation of AMPK by A-769662 could then increase cellular ATP level and to maintain cell survival.
Autophagy begins with formation of double membrane vesicles (also known as autophagosomes) in the cytoplasm, which will “eat” cytoplasmic materials and fuse with lysosome, where the contents will be “recycled” by acidic lysosomal hydrolases. In this process, LC3B-I is cleaved and converted to LC3B-II, which is associated with Beclin-1 and other components to form pre-autophagosomal structures. In the current study, we observed LC3B-II and Beclin-1 increase in osteoblast cells with H2O2 and/or A769662 stimulation, indicating autophagy activation. The fact that autophagy inhibitors (3-MA and BafA1) enhanced H2O2-induced cytotoxicity suggests that autophagy activation is cyto-protective in osteoblast cells, and A-769662-mediated pro-survival effect could be associated with its ability to promote autophagy activation.
4. Materials and Methods
4.1. Chemicals and Regents
A-769662 was purchased from Cayman Chemical (Ann Arbor, MI, USA). Compound C and AICAR were obtained from Calbiochem (Darmstadt, Germany). 3-methyladenine (3-MA), Bafilomycin A1 (BafA1) and H2O2 were purchased from Sigma (Shanghai, China).
4.2. Antibodies
Anti-AMPKα1, acetyl-CoA carboxylase (ACC), rabbit and mouse horseradish peroxidase (HRP)-conjugated IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho(p)-AMPKα (Thr 172), p-ACC (Ser 79), light chain 3 B (LC3B), Beclin-1, p62 and β-actin were purchased form Cell Signaling Tech. (Denver, MA, USA).
4.3. Cell Culture
Human osteoblast-like cell line MG63 and mouse osteoblast cell line MC3T3-E1 were grown in Dulbecco’s modified Eagle’s medium-high glucose (DMEM) and α-MEM, respectively, supplemented with 10% fetal bovine serum (FBS), 50 units/mL penicillin and 50 μg per streptomycin in a humidified atmosphere of 5% CO2. Cultures were trypsinized upon confluence and sub-cultured into 12-, 6-, or 96-well plates for further experiments.
4.4. Cell Viability Assay
Methyl thiazolyl tetrazolium (MTT) assays were performed to assess the cell viability. Briefly, MC3T3-E1 and MG63 cells were planted into 96-well plates. After incubation overnight, the medium was replaced with fresh medium with or without H2O2 at indicated concentrations for various time points. Some of wells were also pre-treated with indicated inhibitors. Six wells were included in each concentration. At the end of each treatment, 20 μL of MTT (5 mg/mL, Sigma) was added for 4 h. Then the medium was discarded carefully and 150 μL of DMSO was added. Absorbance was recorded at 570 nm with the Universal Microplate Reader (Bio-Tek instruments, Winooski, VT, USA ) using wells without cells as blanks.
4.5. Western Blot and Data Analysis
After treatment, cells were lysed immediately in the lysis buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol, 0.01% bromophenol blue) for 5 min at 95 °C. Cell lysates were analyzed by SDS/PAGE and transferred electrophoretically to poly-vinylidene fluoride (PVDF) membranes (Bio-Rad Corp, Hercules, CA, USA). Blots were probed with specific antibodies and immuno-reactive proteins were revealed by the enhanced chemiluminescence (ECL) kit (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The band intensity of each blot was quantified by Image J software before normalizing with the corresponding loading controls, and the value was expressed as fold or percentage change vs. the band labeled “1.00”.
4.6. Cell Apoptosis Assay by Annexin V Staining
After treatment, the apoptosis of osteoblasts was detected by the Annexin V fluorescence-activated cell sorting (FACS). Briefly, one million cells with indicated treatments were stained with FITC-Annexin V and propidium iodide (PI) (Beyotime, Shanghai, China). Both early (annexin V+/PI−) and late (annexin V+/PI+) apoptotic cells were sorted by the fluorescence activated cell sorter (FACS) machine (Becton Dickinson FACS Calibur, San Jose, CA, USA). The percentage of apoptotic cells was recorded.
4.7. Cell Apoptosis Assay by Histone-DNA Enzyme-Linked Immunosorbent Assay (ELISA) PLUS Kit
Osteoblasts’ apoptosis was also quantified by histone-DNA ELISA PLUS (Roche Applied Science, Shanghai, China) according to the manufacturer’s protocol. Briefly, the cytoplasmic histone/DNA fragments from cells were extracted and bound to the immobilized anti-histone antibody. Subsequently, the peroxidase-conjugated anti-DNA antibody was added for the detection of immobilized histone/DNA fragments. After addition of substrate for peroxidase, the spectrophotometric absorbance of the samples was determined using a plate reader at a test wavelength of 400 nm.
4.8. ROS Assay
Cellular ROS level was measured by a DCFH-DA fluorescent dye (Molecular Probes/Invitrogen Eugene, OR, USA). After treatment, the osteoblasts were incubated with 10 μM of DCFH-DA at 37 °C for 20 min and then washed twice with PBS. The cells were then analyzed for fluorescence using the flow cytometer. Cellular ROS level in the treatment group was normalized to that of the control group.
4.9. Trypan Blue Staining of “Dead” Cells
The number of dead cells (trypan blue positive) after indicated treatment was counted, and the percentage (%) of “dead” cells was calculated by the number of the trypan blue positive cells divided by the total number of the cells.
4.10. Stable AMPKα Knockdown through Lentiviral Infection
MG-63 cells were seeded in a six-well plate in growth medium. The lentiviral particles containing human AMPKα1/2 shRNA (10 μL/mL, Santa Cruz Biotech, Santa Cruz, CA, USA) were added to the cells, after 12 h, the lentiviral particles containing medium was replaced by fresh growth medium, and cells were further cultured for another 24 h. Puromycin (1 μg/mL) was added to select resistant stable colonies. The expression of AMPKα in stable cells was detected by western blots. Control cells were infected with GFP-shRNA containing lentiviral particles (Kaiji Biotech, Nanjing, China).
4.11. AMPK Kinase Dead (KD) Mutation and Stable Cell Selection
As previously reported [29], the human AMPK-α1 cDNA was amplified from MG-63 cell cDNA, and was sub-cloned into the BamHI site of pcDNA3.1 (Invitrogen, Shanghai, China). The kinase dead (KD) mutant of AMPK-α1 (AMPK-α1-T172A) was created by mutating the Thr172 residue into Ala as previously reported [30,31]. KD-AMPK-α1 cDNA was transfected to the MG-63 cells through Lipofectamine 2000 protocol [29], stable cells were selected through neomycin (1 μg/mL) as described.
4.12. Measurement of Intracellular ATP Content
As reported [32], after treatment, the intracellular ATP content in osteoblasts was measured using the Molecular Probes’ ATP Determination Kit (Kaiji, Nanjing, China) according to the manufacturer’s directions [32]. This kit is based on the bioluminescence detection of ATP, using recombinant firefly luciferase and its substrate, luciferin. Total chemiluminescence was collected by a luminometer. The amount of ATP from tested cells was quantified by comparison to a calibration curve using ATP as the standard. The value in the treatment group was normalized to that of control group. All experiments were performed in triplicate.
4.13. Statistical Analysis
Statistical analyses were performed by analysis of variance (ANOVA), and p < 0.05 was considered statistically significant.
5. Conclusions
In conclusion, our study indicated that A-769662 significantly protects against H2O2-induced apoptosis of osteoblast cells. A-769662 might be further investigated as a novel anti-osteonecrosis agent.
Acknowledgments
This work is supported by the National Science Foundation of China.
Author Contributions
Yalong Zhu, Jianhua Zhou, Rongguang Ao and Baoqing Yu carried out the experiments. Yalong Zhu, Jianhua Zhou, Rongguang Ao and Baoqing Yu participated in the design of the study and performed the statistical analysis. Yalong Zhu, Jianhua Zhou, Rongguang Ao and Baoqing Yu conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Park B.G., Yoo C.I., Kim H.T., Kwon C.H., Kim Y.K. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology. 2005;215:115–125. doi: 10.1016/j.tox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Fatokun A.A., Stone T.W., Smith R.A. Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: The effects of glutamate and protection by purines. Bone. 2006;39:542–551. doi: 10.1016/j.bone.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 3.Wang S., Song P., Zou M.H. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin. Sci. 2012;122:555–573. doi: 10.1042/CS20110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon S.M., Chandel N.S., Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoki K., Zhu T., Guan K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 7.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M.B., Zhang Y., Wei M.X., Shen W., Wu X.Y., Yao C., Lu P.H. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal. 2013;25:1993–2002. doi: 10.1016/j.cellsig.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Chen M.B., Wu X.Y., Gu J.H., Guo Q.T., Shen W.X., Lu P.H. Activation of AMP-activated protein kinase contributes to doxorubicin-induced cell death and apoptosis in cultured myocardial H9c2 cells. Cell Biochem. Biophys. 2011;60:311–322. doi: 10.1007/s12013-011-9153-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen M.B., Shen W.X., Yang Y., Wu X.Y., Gu J.H., Lu P.H. Activation of AMP-activated protein kinase is involved in vincristine-induced cell apoptosis in B16 melanoma cell. J. Cell Physiol. 2011;226:1915–1925. doi: 10.1002/jcp.22522. [DOI] [PubMed] [Google Scholar]
- 11.Chen L., Xu B., Liu L., Luo Y., Yin J., Zhou H., Chen W., Shen T., Han X., Huang S. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab. Investig. 2010;90:762–773. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z., Shen X., Shen F., Zhong W., Wu H., Liu S., Lai J. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70. Mol. Cell. Biochem. 2013;377:35–44. doi: 10.1007/s11010-013-1568-z. [DOI] [PubMed] [Google Scholar]
- 13.Liangpunsakul S., Wou S.E., Zeng Y., Ross R.A., Jayaram H.N., Crabb D.W. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1173–G1181. doi: 10.1152/ajpgi.90349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 15.Codogno P., Meijer A.J. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12:1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 16.Klionsky D.J., Emr S.D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine B., Yuan J. Autophagy in cell death: an innocent convict? J. Clin. Investig. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura N., Tokunaga C., Dalal S., Richardson C., Yoshino K., Hara K., Kemp B.E., Witters L.A., Mimura O., Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 19.Luo Z., Saha A.K., Xiang X., Ruderman N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W., Vasquez D.S., Joshi A., Gwinn D.M., Taylor R., et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell R.R., 3rd, Bergeron R., Shulman G.I., Young L.H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 23.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 24.De Meester C., Timmermans A.D., Balteau M., Ginion A., Roelants V., Noppe G., Porporato P.E., Sonveaux P., Viollet B., Sakamoto K., et al. Role of AMP-activated protein kinase in regulating hypoxic survival and proliferation of mesenchymal stem cells. Cardiovasc. Res. 2014;101:20–29. doi: 10.1093/cvr/cvt227. [DOI] [PubMed] [Google Scholar]
- 25.Ducommun S., Ford R.J., Bultot L., Deak M., Bertrand L., Kemp B.E., Steinberg G.R., Sakamoto K. Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am. J. Physiol. Endocrinol. Metab. 2014;306:E688–E696. doi: 10.1152/ajpendo.00672.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George R.E., Sanda T., Hanna M., Frohling S., Luther W., 2nd, Zhang J., Ahn Y., Zhou W., London W.B., McGrady P., et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulda S. The PI3K/Akt/mTOR pathway as therapeutic target in neuroblastoma. Curr. Cancer Drug Targets. 2009;9:729–737. doi: 10.2174/156800909789271521. [DOI] [PubMed] [Google Scholar]
- 28.Matsui T., Sano K., Tsukamoto T., Ito M., Takaishi T., Nakata H., Nakamura H., Chihara K. Human neuroblastoma cells express alpha and beta platelet-derived growth factor receptors coupling with neurotrophic and chemotactic signaling. J. Clin. Investig. 1993;92:1153–1160. doi: 10.1172/JCI116684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B., Wang X.B., Chen L.Y., Huang L., Dong R.Z. Belinostat-induced apoptosis and growth inhibition in pancreatic cancer cells involve activation of TAK1-AMPK signaling axis. Biochem. Biophys. Res. Commun. 2013;437:1–6. doi: 10.1016/j.bbrc.2013.05.090. [DOI] [PubMed] [Google Scholar]
- 30.Matthew E.M., Hart L.S., Astrinidis A., Navaraj A., Dolloff N.G., Dicker D.T., Henske E.P., El-Deiry W.S. The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions. Cell Cycle. 2009;8:4168–4175. doi: 10.4161/cc.8.24.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsarouhas V., Yao L., Samakovlis C. Src-kinases and ERK activate distinct responses to Stitcher receptor tyrosine kinase signaling during wound healing in Drosophila. J. Cell Sci. 2014;127:1829–1839. doi: 10.1242/jcs.143016. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Li X.J., Chen Z., Zhu X.X., Wang J., Zhang L.B., Qiang L., Ma Y.J., Li Z.Y., Guo Q.L., et al. Wogonin induced calreticulin/annexin A1 exposure dictates the immunogenicity of cancer cells in a PERK/AKT dependent manner. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050811. [DOI] [PMC free article] [PubMed] [Google Scholar]