Abstract
In 2008, the European Monitoring Center for Drugs and Drug Addiction (EMCDDA) detected unregulated, psychoactive synthetic cannabinoids (SCBs) in purportedly all-natural herbal incense products (often known as K2 or Spice) that were being covertly abused as marijuana substitutes. These drugs, which include JWH-018, JWH-073 and CP-47,497, bind and activate the cannabinoid receptors CB1R and CB2R with remarkable potency and efficacy. Serious adverse effects that often require medical attention, including severe cardiovascular, gastrointestinal and psychiatric sequelae, are highly prevalent with SCB abuse. Consequently, progressively restrictive legislation in the US and Europe has banned the distribution, sale and use of prevalent SCBs, initiating cycles in which herbal incense manufacturers replace banned SCBs with newer unregulated SCBs. The contents of the numerous, diverse herbal incense products was unknown when SCB abuse first emerged. Furthermore, the pharmacology of the active components was largely uncharacterized, and confirmation of SCB use was hindered by a lack of known biomarkers. These knowledge gaps prompted scientists across multiple disciplines to rapidly (1) monitor, identify and quantify with chromatography/mass spectrometry the ever-changing contents of herbal incense products, (2) determine the metabolic pathways and major urinary metabolites of several commonly abused SCBs and (3) identify active metabolites that possibly contribute to the severe adverse effect profile of SCBs. This review comprehensively describes the emergence of SCB abuse and provides a historical account of the major case reports, legal decisions and scientific discoveries of the ″K2/Spice Phenomenon″. Hypotheses concerning potential mechanisms SCB adverse effects are proposed in this review.
Keywords: CP-47, 497, JWH-018, JWH-073, substance abuse CB1R, CB2R, GPCR, synthetic marijuana
Synthetic cannabinoid abuse
Unregulated adulterants of herbal incense products
Synthetic cannabinoids (SCBs) are chemicals that produce several marijuana-like effects in humans. For this reason, SCBs have in recent years become a commonly abused class of drugs. Like marijuana, SCBs are most often administered by inhalation (Vandrey et al., 2011). After chemical synthesis, SCBs can be ingested or vaporized and inhaled (Drugs-Forum, 2009, 2010a; Hu et al., 2011). More often, they are dissolved in a volatile solvent, such as ethanol or acetone, and then sprayed or otherwise mixed with an assortment of plant leaves, such as Indian Warrior, Lion’s Ear, Dog Rose and/or Marshmallow leaves, which are themselves purported to have psychotropic effects upon smoking (Vardakou et al., 2010). The mixture is spread out in a thin layer under a fan, which facilitates evaporation of the solvent, leaving the SCBs distributed onto the plant mixture (Figure 1A). These synthetic Cannabis “blends” can then be smoked or vaporized like marijuana. Over the past few years, hundreds of synthetic Cannabis “blends” have emerged as commercially available, quasi-legal, unregulated “herbal incense” under names such as K2, K3, Spice, Smoke and Dream, just to name a few (Harris & Brown, 2012). K2, which is named after the second highest mountain on Earth, is one of the original, best-known brands of SCB-laced “herbal incense”; therefore, these products are often collectively referred to by the drug community (and hereafter in this review) as “K2”. While the highly decorative packaging of K2 is usually labeled “Not for human consumption” (Figures 1B and C), distributors and consumers understand that these products are to be used like marijuana primarily to attain a subjectively pleasant cannabimimetic “high”. This label has kept these products from being subjected to the Federal Analogue Act of 1986, which states “A controlled substance analogue shall, to the extent intended for human consumption, be treated, for the purposes of any Federal law as a controlled substance in schedule I”. (emphasis added) (United States, 1986). Consequently, in the absence of specific regulations of these substances, such as scheduling and bans, this labeling essentially transfers all responsibility for the user’s safety from the manufacturers and distributors to the consumer, who is often unaware of the product’s potential danger.
Figure 1.
Representations of SCB-laced herbal incense commonly called K2 (A), typical packaging of K2 and similar products (B), and labeling of K2 packages that explicitly states “not for consumption” (C). Figures (A) and (B) are courtesy of the DEA. Figure (C) is courtesy of the Arkansas Department of Health, Public Health Laboratory.
Adverse effects of SCB use
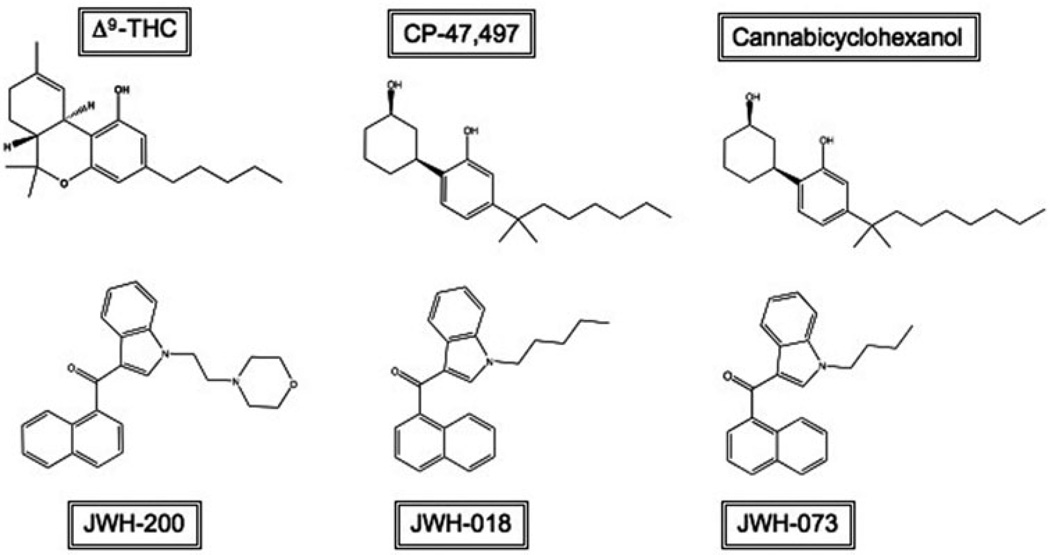
Because clandestine chemists steadily produce novel cannabimimetic designer drugs to replace SCBs as they are banned, there are tens, and perhaps even hundreds, of psychoactive SCBs on the market, the most infamous of which are JWH-018, JWH-073, JWH-200, CP-47,497 and cannabicyclohexanol (Figure 2). This vast selection, along with lack of oversight, quality control regulation and accountability of manufacturers and distributors, results in a highly variable and unpredictable composition of K2 products (Dresen et al., 2010; Hudson et al., 2010). For instance, uneven distribution of SCBs to the plant mixture can result in drug “hot spots”, making dosing unpredictable and difficult, increasing the risk of overdose. Even experienced, habitual users are vulnerable to overdose due not only to these “hot spots”, but also to unlabeled or inaccurately labeled packages and the frequent composition changes that are made to blends without notice or warning to consumers (Dresen et al., 2010). Furthermore, few studies have been done to assess the pharmacological or toxicological effects of even the most well-known SCBs. The lack of scientific knowledge regarding SCBs contained in K2 products is in sharp contrast to information concerning Cannabis, which is the most abused illicit drug worldwide (WHO, 2012). For example, Cannabis has been used relatively safely for medicinal, religious and industrial purposes for thousands of years (Zuardi, 2006) and is currently the subject of intense research for potential medicinal, as well as detrimental, effects. As K2 use has emerged, it has become apparent that in addition to many marijuana-like effects, its use is also associated with many severe adverse effects that are not commonly observed with marijuana use. These include tachycardia, hypertension, nausea, vomiting, convulsions, agitation, hallucinations and psychosis (Every-Palmer, 2011; Hermanns-Clausen et al., 2012; McQuade et al., 2012), with severe cases resulting in hospitalization (Cohen et al., 2012; Tung et al., 2012). More recently, separate reports of rhabdomyolysis, respiratory depression and kidney failure after K2 use have been published (Bhanushali et al., 2013; Durand et al., 2013; Jinwala & Gupta, 2012). K2 use has also been implicated in cases of acute myocardial infarction in three otherwise healthy teenagers who, within a three-month period, independently presented to the same emergency department with chest pains 3–7 days after using K2 (Mir et al., 2011). Further troubling are reports of dependence and withdrawal symptoms associated with chronic use of K2 (Vandrey et al., 2011; Zimmermann et al., 2009) (Drugs-Forum, 2010b, 2011). Unlike marijuana, whose popularity is likely due in part to its perceived harmlessness, K2 use has been associated with several deaths (Boone, 2012; Mayo, 2011, Savini, 2011; Thalji, 2012). Some of these deaths were suicides, committed shortly after onset of a K2-induced episode of severe, acute psychosis and/or panic attack (AAPCC, 2011; Patton et al., 2013; Schecter & Brian, 2011). In one case, the SCB AM2201 was confirmed to be the causative agent of a psychotic episode that lead to the death of a teenage boy who had no prior history of mental illness and whose post-mortem toxicological blood screen was negative for other drugs of abuse (Patton et al., 2013).
Figure 2.
The psychoactive constituent of marijuana, Δ9-THC, is depicted here with the first generation of SCBs commonly found in K2 products. The five SCBs shown were the first to be controlled by the US Federal Government after the emergence of K2 products.
Cannabinoid biology
The cannabinoid receptors
Comparing the signaling properties of SCBs found in K2 products with those of cannabinoids found in marijuana may provide information needed to determine why adverse effects are more often associated with use of K2 when compared to marijuana. The effects of the primary psychoactive cannabinoid of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC, Figure 2), are mediated primarily by activation of two G-protein coupled receptors (GPCRs), Cannabinoid 1 Receptors (CB1Rs) and Cannabinoid 2 Receptors (CB2Rs), which are located throughout the body. CB1Rs are highly expressed in the brain, particularly the cerebellum, with lower expression in the hippocampus, hypothalamus, cerebral cortex, striatum and brainstem (Regard & Coughlin, 2008a); thus, CB1Rs mediate the CNS effects of Δ9-THC and other cannabinoids. These effects include psychoactivity, characterized by acute euphoria, relaxation, sensory and perceptional distortions and disruption of motor coordination, as well as appetite stimulation and analgesia (Mechoulam & Parker, 2012). In neurons, CB1Rs are located on presynaptic terminals and modulate neuronal activity by retrograde signaling (Straiker & Mackie, 2005). In addition to CNS effects, CB1R activity also promotes energy conservation in peripheral tissues by modulating energy storage and utilization in adipose tissue, liver, muscle, gastrointestinal tract, pancreas and the adrenal, thyroid and parathyroid glands (Kunos & Tam, 2011; Regard & Coughlin, 2008a). As such, CB1R antagonists have been intensely investigated for development as therapeutics for obesity and obesity-related diseases (Fulp et al., 2012; Rivera et al., 2012; Shim et al., 2012). CB1Rs are also expressed in reproductive organs such as testes (Regard & Coughlin, 2008a), uterus (Brighton et al., 2011), fallopian tubes (Horne et al., 2008) and placenta (Habayeb et al., 2008). Several studies have thus shown that proper endocannabinoid system (ECS) function is important for successful reproduction (Lewis et al., 2012; Sun & Dey, 2012), as well as early embryonic and fetal neural development (Psychoyos et al., 2012; Psychoyos & Vinod, 2012). Indeed, prenatal exposure to marijuana has been shown to be negatively correlated with scholastic achievement at age 14 (Goldschmidt et al., 2012).
The second most well-characterized cannabinoid receptor, CB2R, is located primarily on lymphocytes and leukocytes, and in tissues such as the spleen, bone marrow, lung, thymus, liver and pancreas (Galiegue et al., 1995; Regard & Coughlin, 2008b), and is heavily involved in regulating immune function. In general, CB2R activation is immunosuppressive, inhibits production of pro-inflammatory cytokines, enhances production of anti-inflammatory cytokines, induces apoptosis of immune cells and suppresses macrophage chemotaxis (Basu & Dittel, 2011). Activation of CB2Rs is thought to underlie the anti-inflammatory and immunosuppressive effects of marijuana (Lombard et al., 2007; Singh et al., 2012). As also observed with CB1Rs, CB2R stimulation produces analgesic effects (Sanchez Robles et al., 2012). Because of CB2R effects on cell-to-cell signaling and cell migration, drugs acting at CB2Rs are being investigated for possible modulation of cancer cell proliferation, metastasis and angiogenesis (Liu et al., 2012; Vidinsky et al., 2012). CB2Rs are generally not expressed in the CNS, but can be upregulated in selected regions of the brain or spinal cord in response to injury and disease (Ashton et al., 2007; Walter et al., 2003). As such, in recent years the CB2R have become a research target for novel drug development to treat amyotrophic lateral sclerosis (ALS) (Shoemaker et al., 2007), stroke (Zarruk et al., 2012), multiple sclerosis (Lou et al., 2011), Parkinson’s disease (Price et al., 2009) and Alzheimer’s disease (Martin-Moreno et al., 2012).
Cannabinoid signaling
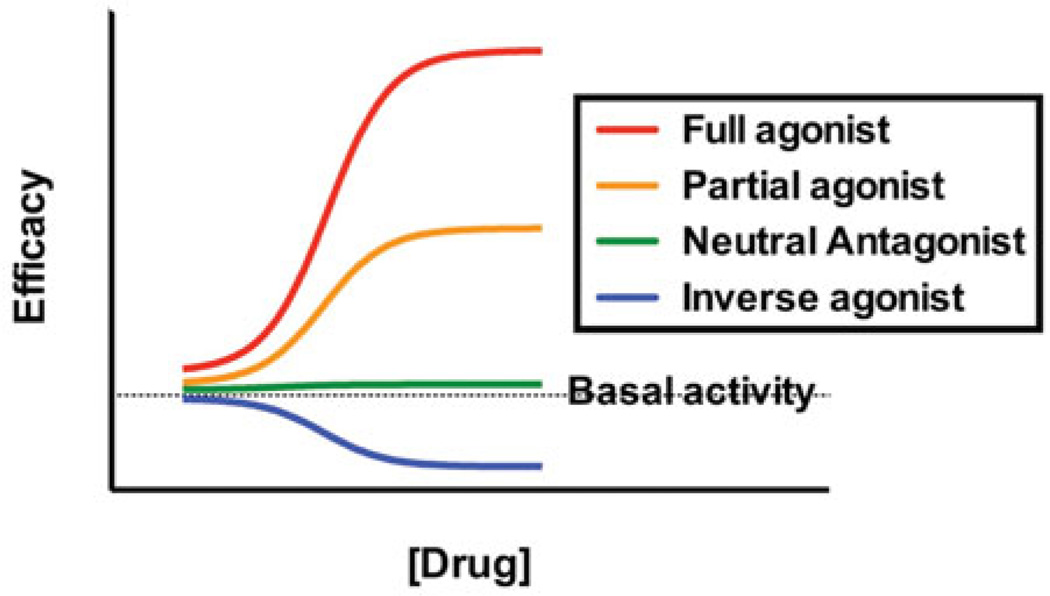
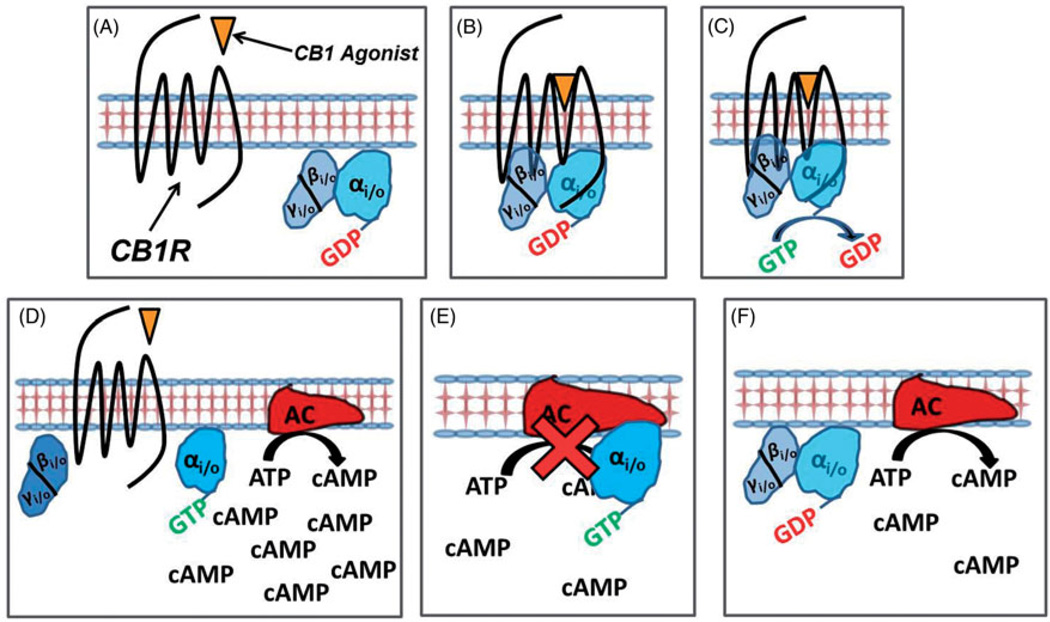
Exogenous cannabinoids (e.g. Δ9-THC, cannabidiol, JWH-018 and CP-47,497) and endogenous cannabinoids [e.g. endocannabinoids; anandamide (AEA) and 2-archido-noylglycerol (2-AG)] bind to cannabinoid receptors with moderate to high affinity (Pertwee et al., 2010). After binding to CB1 or CB2Rs, cannabinoid ligands may modulate receptor activity, and thus downstream signaling cascades, in one of the three ways: (1) by activating the receptor to increase activity (partial and full agonists), (2) by blocking other receptor ligands without altering receptor activity (neutral antagonist) or (3) by reducing constitutive activity of the receptor (inverse agonist) (Figure 3). Most cannabinoids, including Δ9-THC and the SCBs found in K2 products, are partial or full cannabinoid receptor agonists; that is, they bind to and activate CB1Rs and CB2Rs with partial to maximal efficacy. While able to couple to heterotrimeric Gs and Gq/11 proteins under certain circumstances (Glass & Felder, 1997; Lauckner et al., 2005; Maneuf & Brotchie, 1997; McIntosh et al., 2007), cannabinoid receptors couple primarily to Gi/o proteins (Condie et al., 1996, Glass & Northup, 1999). Heterotrimeric G-proteins are composed of alpha (a), beta (b) and gamma (γ) subunits. In the inactive state, these subunits are bound together and form a heterotrimeric protein, with the α-subunit bound to guanosine diphosphate (GDP) (Figure 4A). An agonist binding to a GPCR promotes a conformation change in the receptor that induces an interaction between the receptor and G-protein (Figure 4B). This interaction catalyzes the exchange of GDP for GTP, activating the heterotrimeric G-protein (Figure 4C). This causes the dissociation of the G-protein units into an active monomer Gα–GTP and the dimer Gβγ (Figure 4D). The activated, dissociated subunits then interact with several downstream cellular components (e.g. effectors) to produce multiple signaling cascades (Lodish et al., 2012). For example, CB1R-activated Gαi/o subunits inhibit activity of the enzyme adenylyl cyclase (an integral membrane protein), which catalyzes adenosine triphosphate (ATP) to the second messenger, cyclic adenosine monophosphate (cAMP) (Figure 4E). This in turn lowers intracellular concentrations of cAMP, ultimately decreasing transcription of genes in cAMP-response elements (CRE) (Lodish et al., 2012). CB1R-activated Gαi/o subunits also modulate gene expression by activating the mitogen-activated protein kinase (MAPK) cascade (Bosier et al., 2010; Bouaboula et al., 1995). Several ion channels are also modulated by CB1R-activated Gβγ subunits. Activated Gβγ subunits inhibit the opening of multiple subtypes of voltage-gated Ca2+ channels and activate G-protein coupled inwardly-rectifying potassium channels (GIRKs) (Bosier et al., 2010; Mackie et al., 1995; McAllister et al., 1999), both of which hyperpolarize neurons and decrease the probability of action potential elicitation. Neuronal hyperpolarization inhibits presynaptic neurotransmitter release, particularly of excitatory glutamate and inhibitory gamma amino butyric acid (GABA) (Farkas et al., 2010; Huang et al., 2001; Kovacs et al., 2012); thus, CB1R cannabinoid agonists produce an inhibitory effect on neuronal function. Importantly, CB1-mediated suppression of the inhibitory action of GABAergic neurons that project to the nucleus accumbens removes much inhibition of dopaminergic neurons in this brain region, ultimately resulting in a local increase of dopamine and activation of the mesolimbic dopamine pathway (Fadda et al., 2006; Szabo et al., 2002). These actions contribute to the rewarding properties and abuse liability associated with cannabinoid use. In contrast to CB1Rs, CB2Rs do not regulate the activity of Q-type calcium or inward-rectifying potassium ion channels (Felder et al., 1995). This is only one of the many examples of the complex consequences of cannabinoid signaling. The exact pathways altered by cannabinoid receptor activation depend on many different factors, including cell type, tissue type, receptor subtype (CB1Rs or CB2Rs), homo- or heterodimerization with other receptors (Mackie, 2005), and, because of ligand-biased signaling (Bosier et al., 2010; Glass & Northup, 1999; Shoemaker et al., 2005), which particular ligand is activating the receptor.
Figure 3.
Levels of pharmacological efficacy exhibited by drugs acting at G-protein coupled receptors.
Figure 4.
Agonism of CB1R activates the Gi/o protein, which inhibits adenylyl cyclase activity, lowering intracellular cyclic AMP concentrations.
Medicinal purposes of cannabinoids
The endogenous cannabinoid system (ECS) is widely dispersed throughout the body (Regard & Coughlin, 2008a,b); therefore, even subtle alterations of the ECS have widespread consequences. In addition to the acute psychoactive effects reviewed previously, chronic use of marijuana has also been associated with development of dependence and addiction (Allsop et al., 2012), memory and learning disruptions (Montgomery et al., 2012; Taffe, 2012) and lowering of IQ scores (Meier et al., 2012). Teenagers and young adults appear to be especially vulnerable to these effects, most likely because they are undergoing robust neural development and synaptic restructuring (Gonzalez et al., 2012; Grant et al., 2012). Despite these reported negative effects, marijuana continues to be highly demanded for both its recreational and potential medicinal benefits, spurring great political and social controversy. Marijuana historically has well-established anti-inflammatory, analgesic, anti-emetic and orexogenic effects (Grotenhermen & Muller-Vahl, 2012; Zuardi, 2006), but is currently severely restricted by the US federal government as a Schedule I substance, meaning that it has no accepted medical use. Alternatively, synthetic Δ9-THC is an FDA-approved medication known as dronabinol and sold under the brand name Marinol®. Because of its anti-emetic, anti-nausea and appetite stimulating properties, dronabinol is prescribed for indications including AIDs-related anorexia, chemotherapy-induced nausea and vomiting in cancer patients who have been unresponsive to conventional antiemetic therapies (Unimed Pharmaceuticals, 2004). Another cannabinoid medication under investigation in the USA is Sativex® (GW Pharmaceuticals, Salisbury, UK). It is an oromucosally-administered Cannabis-derived 1:1 combination of Δ9-THC and the phytocannabinoid cannabidiol that appears to alleviate cancer pain (GW Pharmaceuticals, 2012a), as well as the pain, spasticity and bladder dysfunction associated with multiple sclerosis (GW Pharmaceuticals, 2012b). Although cannabinoids in general are promising therapeutics for the indications of pain, cachexia and inflammation, no apparent therapeutic value has yet been discovered for SCBs found in K2 products. While current attention of SCBs focuses on their abuse liability and troubling adverse effects, it should be kept in mind that future research may reveal that these drugs do indeed possess therapeutic effects at lower non-toxic doses, may be used in combination with other medications, or may serve as a scaffold for chemical synthesis of useful therapeutic analogues.
Possible mechanisms underlying the adverse effects reported for K2 products
It is currently unclear why K2 use produces severe adverse effects not commonly observed with marijuana. It is possible that these effects are mediated by actions of SCBs present in K2 at non-cannabinoid receptors. In one study, the full CB1/CB2 agonist SCB CP-55,940 increased serotonin receptor 5-HT2A expression and responsiveness in the rat hypothalamic paraventricular nucleus. Furthermore, rats treated with this SCB exhibited significantly less time in the open arm of an elevated plus maze than control rats, indicating that CP-55,940 induced anxiety (Franklin et al., 2013). A subsequent study by the same group showed that CP-55,940-induced 5-HT2A upregulation was CB2R- and ERK-1/2-dependent (Franklin & Carrasco, 2013). While CP-55,940 is not a common constituent of K2 products, these studies elucidate a signaling pathway that potentially mediates anxiety and psychosis induced by SCBs with full agonist activity at CBRs. Similarly, studies should be conducted to examine the affinity and efficacy of prevalent SCBs acting at likely cellular targets other than CBRs, including adrenergic, serotonergic and glutaminergic receptors. Adverse effects produced by SCBs are unlikely to result from impurities incurred during SCB preparation (Ginsburg et al., 2012). However, differences in the content of non-psychoactive substances present in the marijuana plant versus K2 products offer another possible explanation for differences in the clinical presentation of their adverse symptoms. The marijuana plant naturally produces many pharmacologically active phytocannabinoids, including cannabidiol, tetrahydrocannabivaran and cannabichromene (Pertwee, 2008). These and other phytocannabinoids are currently under investigation for a wide variety of therapeutic applications, including treatment of Cannabis withdrawal (Crippa et al., 2012), inflammation (Valdeolivas et al., 2012), epilepsy (Hill et al., 2010), obesity (Riedel et al., 2009), cancer (Ligresti et al., 2006) and schizophrenia (Leweke et al., 2012). Furthermore, as discussed above, cannabidiol is already being used in conjunction with Δ9 -THC for treatment of cancer pain and multiple sclerosis. Marijuana also contains several terpenoids that likely potentiate the effects of other phytocannabinoids present in Cannabis (Russo, 2011). While terpenoids are not psychoactive, recent evidence suggests that various phytocannabinoids and terpenoids may dampen the activity of Δ9 -THC, “softening” its effects. This is supported, in principle, by the report that marijuana strains with higher cannabidiol content are associated with significantly lower degrees of psychosis (Schubart et al., 2011), combining Δ9 -THC with cannabidiol reduces the adverse effects of Δ9 -THC (Englund et al., 2012) and intraveneous injection of pure Δ9 -THC readily induces psychotic symptoms in humans (Morrison et al., 2009). Consistent with this hypothesis, no known non-psychoactive “modulating” cannabinoids have been detected in seized K2 products. Importantly, it also appears that most SCBs examined to date (e.g. JWH-018, JWH-073 and CP-47,497-C8) possess higher potency and efficacy than Δ9 -THC at CB1Rs (Atwood et al., 2010, 2011; Brents et al., 2011, 2012; Straiker & Mackie, 2005) and CB2Rs (Rajasekaran et al., 2013). It is therefore possible that SCBs, especially taken in the various combinations found in K2 products, achieve levels of CB1 and CB2R activation high enough to produce severe, clinically observable physiological and psychological disturbances when compared to Δ9 -THC. Very clearly, these studies show quantitative differences in the way these ligands activate downstream pathways. Another difference in the effects elicited by Δ9 -THC and SCBs may lie in their qualitative receptor signaling; more simply, which specific pathways are activated or inhibited by individual SCBs. Because of the wide variety of chemical structures identified as cannabinoid agonists, it is highly plausible that structurally distinct ligands bind to CB1Rs in a unique ways. Receptor conformations resulting from the binding of individual ligands, or multiple, simultaneously binding ligands, may dictate the type, strength and number of G-proteins activated by the receptor. The variety of possible cannabinoid receptor-ligand complexes, along with the multiple downstream pathways that may be differentially activated or inhibited, provide ample opportunity for functional selectivity, which indeed has been well established in cannabinoid receptor signaling (Bosier et al., 2008). In functional selectivity, one or more pathways are activated preferentially over others, usually as a result of ligand biasing and/or allosteric modulation (Goupil et al., 2012). It is plausible that the phytocannabinoid/terpenoid “entourage” that accompanies Δ9 -THC when marijuana is smoked may modulate Δ9 -THC signaling in this way. Functional selectivity diversifies the net effects that are possible from activating only a single receptor subtype and therefore may help to explain the differences observed clinically between marijuana use and K2 use.
In addition to the potential differences in pharmacodynamics (e.g. actions occurring due to receptor activation), the active components of marijuana and SCBs are likely metabolized differently and thus have distinct pharmacokinetic profiles. While little is known concerning the pharmacokinetics of SCBs (see “Detection of SC Abuse in Biological Samples” section following), it is well established that Δ9 -THC is metabolized by CYP2C9 to a predominant single biologically active metabolite, 11-OH-Δ9 -THC (Maurer et al., 2006). This metabolite is inactivated by subsequent carboxylation and then glucuronidated prior to excretion. Minor Δ9 -THC metabolites are produced that include hydroxylations and carboxylations at various positions of the parent structure, but these have no known biological activity. In marked contrast, several major metabolites of JWH-018 and JWH-073 exhibit greater CB1R affinity, potency and efficacy than Δ9 -THC, both in vitro and in vivo (Brents et al., 2011, 2012). Importantly, these metabolites also retain in vitro pharmacological activity at CB2R, with some requiring lower fractional receptor occupancy to inhibit adenylyl cyclase activity than their respective parent (Rajasekaran et al., 2013). These data suggest that some metabolites may promote enhanced receptor-effector coupling, resulting in greater signaling efficiency than their parent compounds. Altogether, these studies indicate that some active metabolites of JWH-018 and JWH-073 likely contribute to the effects observed after using these drugs by activating cannabinoid receptors.
Drug–drug interactions, such as synergy, should be considered when multiple drugs are simultaneously used, as is common with K2 abuse. A greater-than-additive effect (i.e. synergy) may increase risk of adverse effects. In vivo rodent experiments performed in our laboratory indicate that effect-dependent drug–drug interactions occur between JWH-018 and JWH-073 (Brents et al., 2013). For example, co-administration of these two SCBs in mice was synergistic for some effects (e.g. Δ9-THC discrimination and analgesia), less-than-additive (i.e. antagonistic) for other effects (e.g. food-maintained response rate suppression) and simply additive for other effects (e.g. hypothermia). Future studies should examine potential drug–drug interactions within the acute adverse effect profile to determine if SCB synergy contributes substantially to the clinical presentation following K2 use.
Detection, identification and monitoring of SCBs in K2 products
History of initial detection of 1st generation SCBs in K2 products
The abuse of SCBs was first detected in December 2008 by German scientists in the European Union’s early-warning system (Surugiu & Minca, 2012) and reported by the European Monitoring Center for Drugs and Drug Addiction (EMCDDA, 2009). At that time, the aminoalkylindole JWH-018, the endocannabinoid oleamide and the cyclohexylphenols CP-47,497 and its active C8 homologue (also known as cannabicyclohexanol) were spotlighted as the primary psychoactive agents present in some K2 products (Auwarter et al., 2009; Steup, 2008). These products were deceptively marketed as completely natural, herbal incense highs as early as 2004 when they first became available for purchase from internet retailers and European “headshops” (Griffiths et al., 2010; Psychonaut Web Mapping Research Group, 2009). These findings prompted Germany to ban JWH-018 and CP-47,497 in January 2009. Monitoring of these herbal mixtures throughout 2009 showed that shortly after this ban was implemented, fewer herbal preparations contained JWH-018 and CP-47,497. In an obvious attempt to circumvent the ban, manufacturers simply replaced the outlawed substances with new uncontrolled SCBs, including JWH-073 and JWH-250, as well as the non-cannabinoid substances O-desmethyltramadol, caffeine and nicotine (Dresen et al., 2010; Lindigkeit et al., 2009). K2 products purchased online from January to May 2009 were detected to contain at least four previously unreported SCBs of the JWH-xxx series (Hudson et al., 2010). Cannabicyclohexanol, along with CP-47,497, oleamide, JWH-018 and JWH-073, were detected in K2 products purchased in Japan between June 2008 and June 2009 (Uchiyama et al., 2010). In late 2008 and early 2009, the US Customs and Border Patrol seized multiple shipments of herbal incense that was laced with the Schedule I cannabinoid HU-210 (CBP.gov, 2009). This was the first confirmed report of SCBs-containing products in the USA. It became evident that by 2009, the market for SCB-containing herbal products had been dispersed throughout much of the world.
One challenge in the control of these apparently harmful psychotropic products is the rapid screening and identification of novel SCBs in K2 products, for which there are no genuine standards. As mentioned previously, the first two analyses of K2 products were conducted in Germany in December 2008. One study (Steup, 2008) employed HPLC (high-performance liquid chromatography) and GC-MS (gas chromatographymass spectrometry) to identify JWH-018 in the herbal incense products “Spice Gold”, “Yukatan Fire” and “Arctic Synergie”. In the second report (Auwarter et al., 2009), ethanolic extractions of “Spice Silver”, “Spice Gold”, “Spice Diamond”, “Smoke”, “Sence”, “Skunk” and “Yucatan Fire” were analyzed by GC-MS and found to contain no illegal drug or known pharmaceutical agent, but did contain JWH-018, CP-47,497 and cannabicyclohexanol. Interestingly, two authors of the study performed self-experiments by smoking Spice Diamond, followed by urine and blood collection to test for the presence of Δ9-THC by immunological methods. This test detected no Δ9-THC, but more importantly also demonstrated that cannabicyclohexanol and JWH-018 (the SCBs present in Spice Diamond) were not cross-reactive with Δ9-THC antibodies used in the assay. In this study, C18 solid-phase extraction, trimethylsilylation and GC-EI/MS were performed to analyze the blood samples for the presence of the SCBs. Only cannabicyclohexanol was detected by employing this analysis. The authors reported effects of smoking Spice Diamond to be similar to those reported anecdotally on the Internet for K2 products, characterized mainly by alterations in perception and mood, moderate cognitive impairment, xerostomia, an increase in pulse rate and reddened conjunctivae. These effects began within 10 minutes of administration and lasted about six hours, with some after-effects felt the next day (Auwarter et al., 2009).
Within the next five months, another group proactively synthesized and analytically characterized authentic references for both naphthoyl and naphthyl analogues of JWH-018 with alkyl chains ranging from 3 to 7 carbons long (Lindigkeit et al., 2009). This group reported 1H and 13C NMR spectral data and GC-MS data for 10 different aminoalkylindoles, including JWH-018, JWH-073 and JWH-175. Using these standards, all ten SCs were identified in 11 different batches of various brands of herbal incense (in mg of SCs per g of incense), some purchased before the initial German bans were instituted (in December 2008) and some purchased afterward (in March 2009). As mentioned above, this study was the first to demonstrate that JWH-073 began to replace the banned SCB JWH-018 in K2 products.
Monitoring of K2 since initial detection of SCBs
Several subsequent studies have been conducted to monitor the composition of K2 products throughout 2010, 2011 and 2012 (Ernst et al., 2011; Hudson et al., 2010; Kneisel et al., 2012; Valoti et al., 2012; Westphal et al., 2012; Zuba et al., 2011). In most studies, SCBs were first extracted from K2 products using methanol or ethanol, then dried and reconstituted into solvents such as ethyl acetate, methanol or acetonitrile, separated by liquid or gas chromatography, and then vaporized, ionized and detected by mass spectrometry. The dominant SCBs detected in K2 products during 2009 and 2010 were CP-47,497, cannabicyclohexanol, JWH-018 and JWH-073 (Zuba et al., 2011), while JWH-081 and JWH-398 were first detected during that time period by Hudson, et al., using accurate high resolution mass spectrometry (Hudson et al., 2010). The presence of JWH-122, the 4-methyl-naphthoyl analogue of JWH-018, was reported in 2011 in concentrations estimated to be 82 mg/g, more than ten times the concentration of CP-47,495 and JWH-018 previously reported by Lindigkeit et al. (Ernst et al. (2011) compared with Lindigkeit et al. (2009)), indicating that replacement drugs were potentially lower in potency than the original SCBs. A year later, the 4-methylnaphthoyl analogue of JWH-073 emerged in Germany (Westphal et al., 2012) and Italy (Valoti et al., 2012). The naphthoylpyrrole JWH-307, as well as 3-(1-adamantoyl)-1-pentylindole (Kneisel et al., 2012), were first detected in K2 products in 2012 (Ernst et al., 2012). A report from New Zealand identified 1-butyl-3-(4-methox-ybenzoyl)indol for the first time (Couch & Madhavaram, 2012), and also reported the presence of significant concentrations (1 mg/g) of the potent benzodiazepine phenazepam in K2 products. Interestingly, while JWH-018 had initially disappeared from the German market after its ban in 2009, in 2012 it was once again detected in much higher concentrations than before (150 mg/g, compared to 2.3 mg/g reported previously (Lindigkeit et al., 2009)) (Ernst et al., 2012).
The year 2012 also brought about the reporting of several new methods to detect SCBs in K2 products. For instance, it was reported that mass defect filtering can be combined with high resolution mass spectrometry, in a non-targeted approach, to detect previously unreported structures that are very similar to well-known SCBs found in K2 products (Grabenauer et al., 2012). This is a valuable strategy to detect unknown SCBs because the most common strategy of clandestine chemists is to make very slight, instead of radical, changes to the structures of established SCBs to produce new, unregulated cannabinoids. Another method, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), was validated as a screen to rapidly quantify the content of SCBs in K2 products (Gottardo et al., 2012). MALDI-TOF MS allows for simpler high-throughput, simultaneous screening of multiple substances in complex mixtures, and is thus more ideal for analyzing K2 products than traditional extractions followed by GC- or LC-MS (liquid chromatographymass spectrometry). Similarly, methods detailing the use of nano-LC, which is the miniaturization of the traditional LC separation techniques, to analyze SCBs present in K2 products have been reported (Merola et al., 2012). Other novel methods to detect SCBs include direct analysis in real time mass spectrometry (DART-MS) using collision-induced dissociation (CID) (Musah et al., 2012) and electrospray ionization quadrupole-time-of-flight tandem mass spectrometry (ESI-QTOF-MS) (Sekula et al., 2012). Ideally, these newer, simpler, more efficient methods will soon be used to help rapidly, selectively and sensitively screen for evidence of SCB use in human biological samples, including blood, oral fluids, hair and urine.
Presence and legislative control of SCB abuse in the USA
While K2 products were first noticed, identified and studied in Europe, use of these substances has risen dramatically in the USA since 2010. A major agency that monitors drug trends in the USA is the National Forensic Laboratory Information System (NFLIS), under direction of the Drug Enforcement Administration (DEA). The NFLIS is a nationwide network of local and state forensic laboratories that collects and reports data regarding the quality and quantity of drugs seized by law enforcement in the USA. The first mention of K2 by the NFLIS was a request for information regarding “Spice” published in its 2008 annual report (Office of Diversion Control, 2009). In 2009, the NFLIS identified only 15 cases of SCB-containing products. This number jumped dramatically to 2977 cases in 32 states the following year, with most of these reports originating from the Midwest (50%) or the South (38%) (Office of Diversion Control, 2011). In 2009, labs in the NFLIS detected only 2 SCBs: JWH-018 and JWH-073, representing 86.67% and 13.33% of reports, respectively. K2 products seized in 2010 were more diverse with respect to SCB content, with JWH-018, JWH-250 and JWH-073 detected in 63.39%, 14.04% and 8.77%, and 10 other SCBs sharing the remainder of the reports (Office of Diversion Control, 2011). The 2011 midyear report listed two SCBs, JWH-018 and JWH-250, in the top 25 most frequently identified drugs of abuse (Office of Diversion Control, 2012), indicating that SCBs have now established a prominent place in the street pharmacopeia.
A surrogate marker of the health effects of K2 use in the USA is the number of K2-associated calls made to US poison control centers. These types of data are gathered and reported by the American Association of Poison Control Centers (AAPCC). In October 2009, US poison control centers began receiving calls that were made by, or on behalf of, people who encountered distress after using K2 products (Bronstein et al., 2011). Calls of this type jumped from 2906 in 2010 to 6959 in 2011, and appear to have decreased to 5205 in 2012 (AAPCC, 2013). Two independently-conducted surveys performed in 2011 revealed that K2 products were used by 11.4% of high school students in the previous year (Johnston, 2011) and by 8% of college students in their lifetime (Hu et al., 2011), indicating that a surprisingly high proportion of young people are at risk for the serious adverse effects associated with K2 use. These harmful effects of K2 products can include seizures, permanent cardiovascular damage, anxiety attacks, aggression, psychosis, paranoia, dependence and suicide (Every-Palmer, 2011; Harris & Brown, 2012; Mir et al., 2011; Patton et al., 2013; Tofighi & Lee, 2012). Furthermore, several cases of significant SCB-induced impairment while operating a motor vehicle have been reported (Musshoff et al., 2013). In response to the initial reports of these alarming observations, many local and state municipalities began controlling both herbal incense products and SCBs by issuing bans. To date, 41 states have legislatively banned SCBs to differing extents (National Conference of State Legislatures, 2012), with the first bans enacted in 2010 by 10 states: Alabama, Georgia, Illinois, Kansas, Kentucky, Louisiana, Michigan, Mississippi, Missouri and Tennessee. Most of the 2010 bans included, at a minimum, the manufacture, sale or possession of the first generation SCBs that were often present in K2 products at the time of legislation, such as JWH-018, JWH-073, CP-47, 497 and cannabicyclohexanol. All of these states, as well as 31 others, added bans in 2011 and 2012 that expanded the list of outlawed SCBs or banned the entire general class of these compounds.
The evident harm caused by K2 products (Seely et al., 2012), along with analytical studies initially identifying CP-47,497, cannabicyclohexanol, JWH-018, JWH-073 and JWH-200 as the primary psychoactive components, prompted the DEA to temporarily categorize these five compounds as Schedule I substances on March 1, 2011 (Department of Justice, 2011). By 2012, the first generation of SCBs were replaced by new compounds in this class with similar structures, such as AM-2201, JWH-122, JWH-203, JWH-210 and RCS-4, apparently indicating that K2 manufacturers have remained one step ahead of SCB regulation (Musah et al., 2012). On 9 July 2012, President Obama signed into law the Synthetic Drug Abuse Prevention Act of 2012, which permanently categorizes several chemical structural classes of cannabinoids as Schedule I substances (United States, 2012). Fifteen SCBs were specifically named in the Act, including the 2-(3-hydroxycyclohexyl)phenols CP-47,497 and cannabicyclohexanol (also called “CP-47,497 C8-homolog”); the 3-(1-naphthoyl)indoles JWH-018 (also called “AM678”), JWH-073, JWH-019, JWH-200, JWH-081, JWH-122, JWH-398 and AM-2201; the 3-benzolylindoles AM694 and SR-19 (also called “RCS-4”); and the 3-phenylacetylindoles SR-18 (also called “RCS-8”), JWH-203 and JWH-250.
Detection of SCB abuse by analysis of biological samples
In vitro determination of SCB metabolites
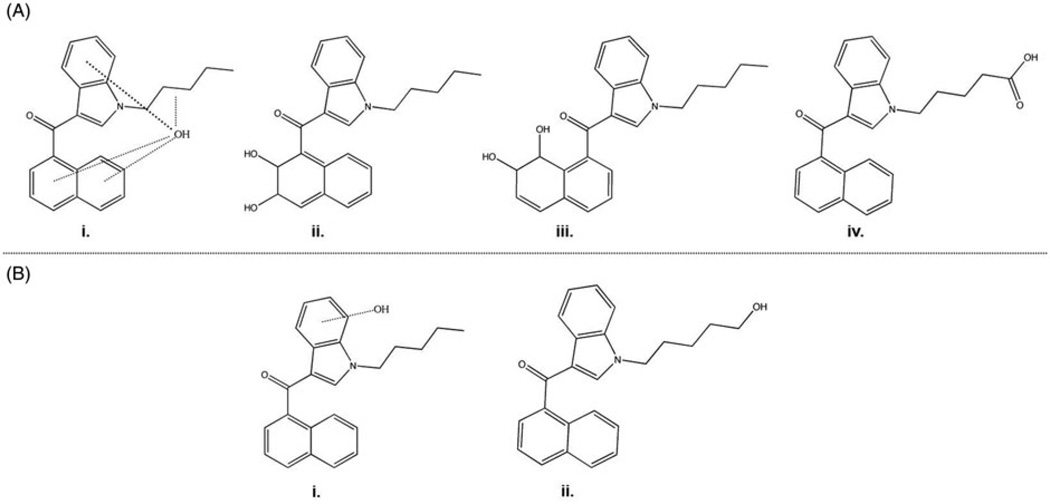
Enforcement of the legislation described above requires the routine, quick and accurate detection of illicit SCB use. For this purpose, screening for SCB metabolites in biological specimens extends the time window that use of K2 products can be definitively confirmed, compared to screening for the presence of the parent drug(s) only. Metabolites are also detectable by urinalysis, a relatively non-invasive biological sampling. This concrete, immediate need has driven several groups to focus on determining the pharmacokinetics of the most prominent SCBs, particularly JWH-018 and JWH-073. Multiple approaches have been taken to determine the specific metabolites that are produced after SCB use, the relative proportions and enzymes involved in SCB metabolism. One in vitro approach is to incubate the parent drug with rodent or human liver microsomes, which contain a crude mixture of the metabolic enzymes that are present in the liver, then extract and measure the resultant metabolites using liquid or gas chromatography followed by mass spectrometry (Chimalakonda et al., 2011, 2012; ElSohly et al., 2011; Wintermeyer et al., 2010; Zhang et al., 2002, 2006). For deeper insight into which enzyme(s) are involved in metabolism, a parent drug may be incubated with individual recombinant enzymes, such as particular cytochrome P450 (CYP450) or uridine diphosphate-glucuronosyltransferase (UDP-UGT) isoforms. From these studies, enzyme kinetics (Vmax and Km), metabolic products of that enzyme, and whether or not the enzyme is inhibited or induced by the parent drug or metabolite(s) can be determined (Chimalakonda et al., 2011). Two of the first SCBs for which the in vitro metabolic pathways were determined were the aminoalkylindoles WIN-55,212-2 and JWH-015. In both studies, SCBs were incubated with pooled rat liver microsomes up to four hours before incubation products were analyzed by HPLC-MS/MS. Metabolism was similar for both SCBs and included primarily the formation of dihydrodiols of the naphthalene ring, as well as monohydroxylations, trihydroxylations and dehydrogenations (Zhang et al., 2002, 2006). Additionally, JWH-015 underwent N-dealkylation (Zhang et al., 2006). The emergence of K2 use prompted similar analyses to be performed employing human liver microsomes (HLM) to analyze the commonly abused SCB JWH-018 (Chimalakonda et al., 2011; ElSohly et al., 2011; Wintermeyer et al., 2010). The findings for JWH-018 were in close agreement with those for the structurally similar JWH-015 reported earlier (Zhang et al., 2006). For JWH-018 this includes monohydroxylations of the indole moiety, the alkyl side chain and the naphthalene ring (Figure 5Ai, and the formation of dihydrodiols of the naphthalene ring (Figures 5Aii and 5Aiii), as well as the more minor metabolites described for JWH-015 above. In contrast, incubation of microsomes with JWH-018, but not JWH-015, produced a carboxylation of the alkyl side chain (Figure 5Aiv). Dehydration of the alkyl side chain occurred more readily and in more combinations with other oxidative modifications in JWH-018 compared to JWH-015. Some studies of the in vitro metabolism of other SCBs have since been reported, (JWH-073 (Chimalakonda et al., 2011, ElSohly et al., 2011), CP-47,497 (Jin et al., 2012), AM-2201 and UR-144 (Sobolevskyet al., 2012)), but are beyond the scope of this review.
Figure 5.
Major JWH-018 metabolites as determined by in vitro metabolism studies (A) and urinalysis after confirmed JWH-018 use (B).
Determination of human urinary metabolites of JWH-018
A straightforward, practical approach to understanding SCB pharmacokinetics is to analyze biological specimens such as urine, blood and hair obtained from individuals after administration of SCBs. Ideally, these studies are controlled and planned well in advanced of specimen collection, with the exact substance, dose and time course known (Auwarter et al., 2009). Realistically, samples potentially containing SCBs and SCB metabolites are often obtained during probationary drug urine screenings, toxicological screening upon presentation to emergency departments, or community drug abstinence programs in which attempted deception, incapacitation, or honest uncertainty regarding the abused substance hinder determination of accurate and precise information. The variable nature of the SCBs present in different K2 products also presents a hurdle to determining the metabolites that will be formed due to the fact that parent drugs themselves may differ in each case, and the original adulterated plant matter is not always available for analysis. Nonetheless, the availability of easily attainable biological samples such as blood and urine are of great value for initial determination of the metabolic fate of SCBs and the temporal detection of K2 use in humans. One of the first studies elucidating human urinary metabolites of a commonly abused SCB was reported by Sobolevsky et al. (2010). In this study, urine samples were collected from 3 people within 12 hours of smoking an herbal mixture. The investigators confirmed that this mixture contained JWH-018 and a CP-47,497C-8 homologue (cannabicyclohexanol). For the first time, it was reported that (1) the JWH-018 parent compound is not excreted in urine; (2) the two major urinary metabolites of JWH-018 in humans are monohydroxylations of either the indole moiety (Figure 5Bi) or of the alkyl chain (Figure 5Bii); (3) the minor urinary metabolites of JWH-018 include a carboxylated metabolite, dihydroxylated metabolites, reduced trihydroxylated metabolites, reduced dihydroxylated metabolites and N-dealkylated monohydroxylated metabolites; and (4) the major metabolites are almost completely glucuronidated, while some of the minor metabolites are excreted in their free forms (Sobolevsky et al., 2010). Soon after this study, another group validated that JWH-018 monohydroxylated metabolites in urine are sensitive and selective biomarkers of JWH-018 abuse (Moller et al., 2011). The results of these studies prompted investigators at the Arkansas Department of Health, the Arkansas State Crime Lab and Cayman Chemical to develop authentic standards of the major urinary metabolites of JWH-018, and analogous JWH-073 structures, to enable quantification in urine samples. Using these standards, Moran et al. (2011), determined that the indole-monohydroxylated JWH-018 metabolites are oxidized at positions 5 and 6 of the indole ring and excreted in ng/mL concentrations. In this study, two metabolites measured in the greatest abundance were modified at the omega carbon of the alkyl chain by either monohydroxylation or carboxylation. This study also confirmed the original findings that the parent JWH-018 compound is not excreted and that the major JWH-018 urinary metabolites are highly glucuronidated. Interestingly, the carboxylated metabolite of JWH-073 was detected in all samples, despite a reported lack of exposure to JWH-073. This indicates a possible demethylation step before carboxylation resulting in production of JWH-073 from JWH-018, or a prior unreported exposure to JWH-073 (Moran et al., 2011). A later study by the same group demonstrated that monohydroxylation at the omega-1 carbon of the alkyl chain produces the major JWH-018 urinary metabolite (Chimalakonda et al., 2012), followed by monohydroxylation and carboxylation of the omega carbon, as previously shown (Moran et al., 2011). Altogether, studies identifying in vivo urinary metabolites of JWH-018 are generally in close agreement with each other and with in vitro reports obtained using pooled human liver microsomes to generate metabolites (Chimalakonda et al., 2012; Wintermeyer et al., 2010).
Final remarks
SCB abuse has recently emerged as a significant threat to public health and safety, particularly in teenagers and young adults who are unaware of the risks associated with SCB use. Because of the sudden appearance of SCB abuse, little is known about SCB pharmacology or toxicology. However, over the past several years, many research groups have acquired valuable knowledge regarding the variety of multiple SCBs that comprise K2 products, as well as some major and minor SCB urinary metabolites that will serve as useful biomarkers to monitor K2 abuse. The work examined in this review suggests that K2 abuse results in complicated pharmacology involving the multiple SCBs that are present in K2. Possible mediators of K2-induced adverse effects include a resultant entourage of distinct CB1R-active SCB metabolites and/or synergistic drug–drug interactions. As such, the pharmacokinetics of SCBs should be investigated to more fully understand the consequences of exposure to both the parent SCBs and their active metabolites. Direct and indirect pharmacological activity by SCBs on non-CB1/CB2 neurotransmitter and neurochemical signaling, such as in the serotonergic, dopaminergic, glutaminergic and adrenergic systems, should be extensively examined as they likely participate in the effects of SCB use. Monitoring and law enforcement agencies should remain vigilant to identify and mitigate the effects of new drugs of abuse that continue to emerge primarily to circumvent bans on established SCBs as part of the cycle of the K2/Spice phenomenon.
Acknowledgments
This research was supported in part by an award from the University of Arkansas for Medical Sciences Translational Research Institute, which is funded by the National Center for Research Resources [1 UL 1RR029884, Curtis Lowery, PI, PLP, Co-I].
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- AAPCC. [last accessed 17 Dec 2011];Synthetic marijuana data. 2011 [Online] Available from: http://www.aapcc.org/dnn/Portals/0/Synthetic%20Marijuana%20Data%20for%20Website%2012.12.2011.-pdf.
- AAPCC. [last accessed 6 Jun 2013];Synthetic marijuana data [Online] 2013 Available from: https://aapcc.s3.amazonaws.com/files/library/Synthetic_Marijuana_Data_for_Website_5.31.2013.pdf.
- Allsop DJ, Copeland J, Norberg MM, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7:e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Rahman RM, Nair SM, et al. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, et al. CP47,497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwarter V, Dresen S, Weinmann W, et al. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res. 2011;51:26–38. doi: 10.1007/s12026-011-8210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2013;8:523–526. doi: 10.2215/CJN.05690612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C. Teen’s death officically linked to synthetic pot. [last accessed 25 Sep 2012];The Atlanta Journal-Constitution [Online] 2012 Available from: http://www.ajc.com/news/news/local/teens-death-officially-linked-to-synthetic-pot/nQWKk/
- Bosier B, Hermans E, Lambert D. Differential modulation of AP-1- and CRE-driven transcription by cannabinoid agonists emphasizes functional selectivity at the CB1 receptor. Br J Pharmacol. 2008;155:24–33. doi: 10.1038/bjp.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, et al. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, et al. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Zimmerman SM, Saffell AR, et al. Differential drug–drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse and pain therapy. J Pharmacol Exp Therapeutics. 2013;346:350–361. doi: 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton PJ, Marczylo TH, Rana S, et al. Characterization of the endocannabinoid system, CB(1) receptor signalling and desensitization in human myometrium. Br J Pharmacol. 2011;164:1479–1494. doi: 10.1111/j.1476-5381.2011.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR, et al. 2010 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clin Toxicol (Phila) 2011;49:910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- CBP.GOV. Lab results confirm CBP in Ohio Discover Synthetic Narcotics in Incense Packets. [last accessed 7 Jul 2013];U.S. Customs and Border Protection News Release [Online] 2009 Available from: http://www.cbp.gov/archived/xp/cgov/newsroom/news_releases/archives/2009_news_releases/january_2009/01142009_3.xml.html.
- Chimalakonda KC, Bratton SM, Le VH, et al. Conjugation of Synthetic Cannabinoids, JWH-018 [Naphthalen-1-yl-(1-pentylindol-3-yl)methanone] and JWH-073 [naphthalen-1-yl-(1-butylindol-3-yl)methanone], Metabolites by Human UDP-glucuronosyltransferases. Drug Metab Dispos. 2011;39:1967–1976. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, Seely KA, Bratton SM, et al. Cytochrome p450-mediated oxidative metabolism of abused synthetic cannabinoids found in k2/spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Morrison S, Greenberg J, Saidinejad M. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. 2012;129:e1064–e1067. doi: 10.1542/peds.2011-1797. [DOI] [PubMed] [Google Scholar]
- Condie R, Herring A, Koh WS, et al. Cannabinoid inhibition of adenylate cyclase-mediated signal transduction and interleukin 2 (IL-2) expression in the murine T-cell line, EL4.IL-2. J Biol Chem. 1996;271:13175–13183. doi: 10.1074/jbc.271.22.13175. [DOI] [PubMed] [Google Scholar]
- Couch RA, Madhavaram H. Phenazepam and cannabinomimetics sold as herbal highs in New Zealand. Drug Test Anal. 2012;4:409–414. doi: 10.1002/dta.349. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Hallak JE, Machado-de-Sousa JP, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2012;38:162–164. doi: 10.1111/jcpt.12018. [DOI] [PubMed] [Google Scholar]
- Department of Justice. [last accessed 07 Dec 2012];Schedules of controlled substances: temporary placement of five synthetic cannabinoids into schedule I. 2011 [Online] Available from: http://www.gpo.gov:80/fdsys/pkg/FR-2011-03-01/pdf/2011-4428.pdf.
- Dresen S, Ferreiros N, Putz M, et al. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psycho-active compounds. J Mass Spectrom. 2010;45:1186–1194. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- Drugs-Forum. [last accessed 26 Sep 2012];Snorting JWH-018. Substance information network (S.I.N.) foundation. 2009 [Online] Available from: http://www.drugs-forum.com/forum/showthread.php?t=101492.
- Drugs-Forum. JWH-018 for a non-smoker and a person with new home/insufflation. [last accessed 26 Sep 2012];Substance Information Network (S.I.N.) Foundation. 2010a [Online] Available from: http://www.drugs-forum.com/forum/showthread.php?t=127290.
- Drugs-Forum. Experiencing the addictive properties of synthetic cannabinoids. [last accessed 26 Sep 2012];Substance Information Network (S.I.N.) Foundation. 2010b [Online] Available from: http://www.drugs-forum.com/forum/showthread.php?t=124787.
- Drugs-Forum. Am-2201 addiction…Seeking advice. [last accessed 26 Sep 2012];Substance Information Network (S.I.N.) Foundation. 2011 [Online] Available from: http://www.drugs-forum.com/forum/showthread.php?t=168986.
- Durand D, Delgado LL, de la Parra-Pellot DM, Nichols-Vinueza D. Psychosis and severe rhabdomyolysis associated with synthetic cannabinoid use. Clin Schizophr Relat Psychoses. 2013 [PubMed] [Google Scholar]
- ElSohly MA, Gul W, ElSohly KM, et al. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35:487–495. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- EMCDDA. EMCDDA-Europol 2008 Annual Report on the implementation of Council Decision 2005/387/JHA. [last accessed 7 Dec 2012];Lisbon. 2009 [Online] Available from: http://www.emcdda.europa.eu/attachements.cfm/att_132902_EN_2008_Implementation%20report.pdf.
- Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2012;27:19–27. doi: 10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- Ernst L, Kruger K, Lindigkeit R, et al. Synthetic cannabinoids in “spice-like” herbal blends: first appearance of JWH-307 and recurrence of JWH-018 on the German market. Forensic Sci Int. 2012;222:216–222. doi: 10.1016/j.forsciint.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Ernst L, Schiebel HM, Theuring C, et al. Identification and characterization of JWH-122 used as new ingredient in “Spice-like” herbal incenses. Forensic Sci Int. 2011;208:e31–e35. doi: 10.1016/j.forsciint.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117:152–157. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, et al. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Farkas I, Kallo I, Deli L, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Franklin JM, Carrasco GA. Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5-HT(2A)) receptor activity via ERK1/2 signaling. Synapse. 2013;67:145–159. doi: 10.1002/syn.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JM, Mathew M, Carrasco GA. Cannabinoid-induced upregulation of serotonin 2A receptors in the hypothalamic paraventricular nucleus and anxiety-like behaviors in rats. Neurosci Lett. 2013;548:165–169. doi: 10.1016/j.neulet.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp A, Bortoff K, Zhang Y, et al. Diphenyl purine derivatives as peripherally selective cannabinoid receptor 1 antagonists. J Med Chem. 2012;55:10022–10032. doi: 10.1021/jm301181r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Mcmahon LR, Sanchez JJ, Javors MA. Purity of synthetic cannabinoids sold online for recreational use. J Anal Toxicol. 2012;36:66–68. doi: 10.1093/jat/bkr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford JA, et al. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol. 2012;34:161–167. doi: 10.1016/j.ntt.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, et al. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34:962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo R, Chiarini A, Dal Pra I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. J Mass Spectrom. 2012;47:141–146. doi: 10.1002/jms.2036. [DOI] [PubMed] [Google Scholar]
- Goupil E, Laporte SA, Hebert TE. Functional selectivity in GPCR signaling: understanding the full spectrum of receptor conformations. Mini Rev Med Chem. 2012;12:817–830. doi: 10.2174/138955712800959143. [DOI] [PubMed] [Google Scholar]
- Grabenauer M, Krol WL, Wiley JL, Thomas BF. Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: implications for nontargeted screening of designer drugs. Anal Chem. 2012;84:5574–5581. doi: 10.1021/ac300509h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug Alcohol Depend. 2012;121:159–162. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105:951–953. doi: 10.1111/j.1360-0443.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int. 2012;109:495–501. doi: 10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GW Pharmaceuticals. [last accessed 06 Dec 2012];Third phase III Sativex cancer pain trial commences [Online] 2012a Available from: http://www.gwpharm.com/Third%20phase%20III%20Sativex%20cancer%20pain%20trial%20commences.aspx.
- GW Pharmaceuticals. [last accessed 06 Dec 2012];Package leaflet: Information for the patient, Sativex Oromucosal Spray [Online] 2012b Available from: www.medicines.org.uk/EMC/pdfviewer.aspx?isAttachment=true&documentid=23228.
- Habayeb OM, Taylor AH, Bell SC, et al. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- Harris CR, Brown A. Synthetic cannabinoid intoxication: a case series and review. J Emerg Med. 2012;44:360–366. doi: 10.1016/j.jemermed.2012.07.061. [DOI] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kneisel S, Szabo B, Auwarter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: Clinical and laboratory findings. Addiction. 2012;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Weston SE, Jones NA, et al. Delta-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia. 2010;51:1522–1532. doi: 10.1111/j.1528-1167.2010.02523.x. [DOI] [PubMed] [Google Scholar]
- Horne AW, Phillips 3rd JA, Kane N, et al. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS One. 2008;3:e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Primack BA, Barnett TE, Cook RL. College students and use of K2: an emerging drug of abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16–20. doi: 10.1186/1747-597X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S, Ramsey J, King L, et al. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol. 2010;34:252–260. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- Jin MJ, Lee J, In MK, Yoo HH. Characterization of in vitro metabolites of cp 47,497, a synthetic cannabinoid, in human liver microsomes by LC-MS/MS. J Forensic Sci. 2012;58:195–199. doi: 10.1111/j.1556-4029.2012.02261.x. [DOI] [PubMed] [Google Scholar]
- Jinwala FN, Gupta M. Synthetic cannabis and respiratory depression. J Child Adolesc Psychopharmacol. 2012;22:459–462. doi: 10.1089/cap.2011.0122. [DOI] [PubMed] [Google Scholar]
- Johnston LD, Malley PM, Bachman JG, Schulenberg JE. [last accessed 16 Dec 2011];Marijuana use continues to rise among U.S. teens, while alcohol use hits historic lows. 2011 [Online] Available from: http://www.monitoringthefuture.org.
- Kneisel S, Westphal F, Bisel P, et al. Identification and structural characterization of the synthetic cannabinoid 3-(1-adamantoyl)-1-pentylindole as an additive in ‘herbal incense’. J Mass Spectrom. 2012;47:195–200. doi: 10.1002/jms.2059. [DOI] [PubMed] [Google Scholar]
- Kovacs FE, Knop T, Urbanski MJ, et al. Exogenous and endogenous cannabinoids suppress inhibitory neurotransmission in the human neocortex. Neuropsychopharmacology. 2012;37:1104–1114. doi: 10.1038/npp.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G, Tam J. The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163:1423–1431. doi: 10.1111/j.1476-5381.2011.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci U S A. 2005;102:19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SE, Paro R, Borriello L, et al. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. Int J Androl. 2012;35:731–740. doi: 10.1111/j.1365-2605.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Moriello AS, Starowicz K, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, et al. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Liu X, Jutooru I, Lei P, et al. Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a:ZBTB10 in breast cancer. Mol Cancer Ther. 2012;11:1421–1431. doi: 10.1158/1535-7163.MCT-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H, Berk A, Kaiser CA, et al. Molecular cell biology. 7th ed. New York: W.H. Freeman; 2012. [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou ZY, Chen C, He Q, et al. Targeting CB(2) receptor as a neuroinflammatory modulator in experimental autoimmune encephalomyelitis. Mol Immunol. 2011;49:453–461. doi: 10.1016/j.molimm.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci. 2005;77:1667–1673. doi: 10.1016/j.lfs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM. Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br J Pharmacol. 1997;120:1397–1398. doi: 10.1038/sj.bjp.0701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moreno AM, Brera B, Spuch C, et al. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J Neuroinflammation. 2012;9:8. doi: 10.1186/1742-2094-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer HH, Sauer C, Theobald DS. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28:447–453. doi: 10.1097/01.ftd.0000211812.27558.6e. [DOI] [PubMed] [Google Scholar]
- Mayo N. Coroner: Lamar Jack ingested chemical found in fake marijuana before he died. [last accessed 6 Dec 2012];Anderson Independent Mail [Online] 2011 Available from: http://www.independentmail.com/news/2011/oct/15/coroner-lamar-jack-ingested-chemical-found-fake-ma/
- Mcallister SD, Griffin G, Satin LS, Abood ME. Cannabinoid receptors can activate and inhibit G protein-coupled inwardly rectifying potassium channels in a xenopus oocyte expression system. J Pharmacol Exp Ther. 1999;291:618–626. [PubMed] [Google Scholar]
- Mcintosh BT, Hudson B, Yegorova S, et al. Agonist-dependent cannabinoid receptor signalling in human trabecular meshwork cells. Br J Pharmacol. 2007;152:1111–1120. doi: 10.1038/sj.bjp.0707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcquade D, Hudson S, Dargan PI, Wood DM. First European case of convulsions related to analytically confirmed use of the synthetic cannabinoid receptor agonist AM-2201. Eur J Clin Pharmacol. 2012;69:373–376. doi: 10.1007/s00228-012-1379-2. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2012;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola G, Aturki Z, D’orazio G, et al. Analysis of synthetic cannabinoids in herbal blends by means of nano-liquid chromatography. J Pharm Biomed Anal. 2012;71:45–53. doi: 10.1016/j.jpba.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622–e1627. doi: 10.1542/peds.2010-3823. [DOI] [PubMed] [Google Scholar]
- Moller I, Wintermeyer A, Bender K, et al. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. 2011;3:609–620. doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Seddon AL, Fisk JE, et al. Cannabis-related deficits in real-world memory. Hum Psychopharmacol. 2012;27:217–225. doi: 10.1002/hup.1273. [DOI] [PubMed] [Google Scholar]
- Moran CL, Le VH, Chimalakonda KC, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- Musah RA, Domin MA, Cody RB, et al. Direct analysis in real time mass spectrometry with collision-induced dissociation for structural analysis of synthetic cannabinoids. Rapid Commun Mass Spectrom. 2012;26:2335–2342. doi: 10.1002/rcm.6354. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Madea B, Kernbach-Wighton G, et al. Driving under the influence of synthetic cannabinoids (“Spice”): a case series. Int J Legal Med. 2013 doi: 10.1007/s00414-013-0864-1. [DOI] [PubMed] [Google Scholar]
- National Conference of State Legislatures. [last accessed 11 Oct 2012];Synthetic CAnnabinoids (a.k.a. “K2/”Spice) enactments [Online] 2012 Available from: http://www.ncsl.org/issues-research/justice/synthetic-cannabin-oids-enactments.aspx.
- Office of Diversion Control. National Forensic Laboratory Information System: Year 2008 annual report. Washington, DC: U.S. Drug Enforcement Administration; 2009. [last accessed 7 Dec 2012]. [Online] Available from: www.deadiversion.usdoj.gov/nflis/2008annual_rpt.pdf. [Google Scholar]
- Office of Diversion Control. Special report: synthetic cannabinoids and synthetic cathinones reported in National Forensic Laboratory Information System, 2009–2010. Springfield (VA): U.S. Drug Enforcement Administration; 2011. [last accessed 7 Dec 2012]. [Online] Available from: www.deadiver-sion.usdoj.gov/nflis/2010rx_synth.pdf. [Google Scholar]
- Office of Diversion Control. National Forensic Laboratory Information System: Midyear report 2011. Springfield (VA): U.S. Drug Enforcement Administration; 2012. [last accessed 7 Dec 2012]. [Online] Available from: http://www.deadiversion.usdoj.gov/nflis/2011midyear.pdf. [Google Scholar]
- Patton AC, Moran C, McCain K, et al. K2 toxicity: Fatal case of psychiatric complications following AM2201 exposure. J Forensic Sci. 2013 doi: 10.1111/1556-4029.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB and CB. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Martinez AA, Seillier A, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:2177–2186. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos D, Vinod KY, Cao J, et al. Cannabinoid receptor 1 signaling in embryo neurodevelopment. Birth Defects Res B Dev Reprod Toxicol. 2012;95:137–150. doi: 10.1002/bdrb.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychonaut Web Mapping Research Group. [last accessed 07 Dec 2012];Spice report. 2009 [Online] Available from: http://www.psychonautproject.eu/documents/reports/Spice.pdf.
- Psychoyos D, Vinod KY. Marijuana, spice ‘herbal high’, and early neural development: implications for rescheduling and legalization. Drug Test Anal. 2012;5:27–45. doi: 10.1002/dta.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran M, Brents LK, Franks LN, et al. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol. 2013;269:100–108. doi: 10.1016/j.taap.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard JBS, Coughlin SR. [last accessed 18 Jul 2013];Anatomical profiling of CB1R expression [Online] 2008a Available from: http://pdsp.med.unc.edu/ShaunCell/webfiles/Cnr1%20graph%201.jpg.800.jpg.
- Regard JBS, Coughlin SR. [last accessed 18 Jul 2013];Antomical profiling of CB2R expression [Online] 2008b Available from: http://pdsp.med.unc.edu/ShaunCell/webfiles/Cnr2%20graph%201.jpg.800.jpg.
- Riedel G, Fadda P, McKillop-Smith S, et al. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera P, Luque-Rojas MJ, Pastor A, et al. Diet-dependent modulation of hippocampal expression of endocannabinoid signaling-related proteins in cannabinoid antagonist-treated obese rats. Eur J Neurosci. 2012;37:105–117. doi: 10.1111/ejn.12012. [DOI] [PubMed] [Google Scholar]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Robles EM, Bagues Arias A, Martin Fontelles MI. Cannabinoids and muscular pain: Effectiveness of the local administration in rat. Eur J Pain. 2012;16:1116–1127. doi: 10.1002/j.1532-2149.2012.00115.x. [DOI] [PubMed] [Google Scholar]
- Savini D. 2 Investigators: Synthetic marijuana product blamed in teen’s deadly crash. [last accessed 06 Dec 2012];CBS Chicago [Online] 2011 Available from: http://chicago.cbslocal.com/2011/07/07/2-investigators-synthetic-marijuana-product-blamed-in-teens-deadly-crash/
- Schecter A, Brian R. Retailers selling legal high “Time Bombs” to kids, doctor says. [last accessed 25 Sep 2012];ABC News [Online] 2011 Available from: http://abcnews.go.com/Blotter/retailers-selling-legal-high-time-bombs-kids-doctor/story?id=13753898.
- Schubart CD, Sommer IE, van Gastel WA, et al. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130:216–221. doi: 10.1016/j.schres.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekula K, Zuba D, Stanaszek R. Identification of naphthoylindoles acting on cannabinoid receptors based on their fragmentation patterns under ESI-QTOFMS. J Mass Spectrom. 2012;47:632–643. doi: 10.1002/jms.3004. [DOI] [PubMed] [Google Scholar]
- Shim JY, Bertalovitz AC, Kendall DA. Probing the Interaction of SR141716A with the CB1 Receptor. J Biol Chem. 2012;287:38741–38754. doi: 10.1074/jbc.M112.390955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Seely KA, Reed RL, et al. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem. 2007;101:87–98. doi: 10.1111/j.1471-4159.2006.04346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, et al. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(−/−) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol. 2012;258:256–267. doi: 10.1016/j.taap.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. 2012 doi: 10.1002/dta.1418. [DOI] [PubMed] [Google Scholar]
- Steup C. Untersuchung des handelsproduktes “Spice” (Investigation of the commercial product “Spice”) Frankfurt, Germany: THC Pharm GmbH; 2008. [last accessed 07 Dec 2012]. [Online] Available from: http://usualredant.de/downloads/analyse-thc-pharm-spice-jwh-018.pdf. [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chem Neurosci. 2012;3:349–355. doi: 10.1021/cn300014e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surugiu ME, Minca DG. European dimension of cannabinoid-like products use. J Med Life. 2012;5:79–81. [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Delta(9) tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol. 2012;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalji J. Use of synthetic marijuana linked to drowning death of Clearwater teen. [last accessed 25 Sep 2012];Tampa Bay Times [Online] 2012 Available from: http://www.tampabay.com/news/publicsafety/use-of-synthetic-marijuana-linked-to-drowning-death-of-clearwater-teen/1223770.
- Tofighi B, Lee JD. Internet highs - Seizures after consumption of synthetic cannabinoids purchased online. J Addict Med. 2012;6:240–241. doi: 10.1097/ADM.0b013e3182619004. [DOI] [PubMed] [Google Scholar]
- Tung CK, Chiang TP, Lam M. Acute mental disturbance caused by synthetic cannabinoid: a potential emerging substance of abuse in Hong Kong. East Asian Arch Psychiatry. 2012;22:31–33. [PubMed] [Google Scholar]
- Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198:31–38. doi: 10.1016/j.forsciint.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Unimed Pharmaceuticals. Marinol (Dronabinol) new drug application. FDA; 2004. [last accessed 02 Jan 2013]. [Online] Available from: http://www.fda.gov/ohrms/dockets/dockets/05n0479/05N-0479-emc0004-04.pdf. [Google Scholar]
- United States 21 U.S.C. x 813. Treatment of controlled substance analogues. U.S. Government Printing Office; 1986. [last accessed 01 Jan 2013]. p. 452. [Online]. Available from: http://www.gpo.gov:80/fdsys/pkg/USCODE-2008-title21/pdf/USCODE-2008-title21-chap13-subchapI-partB-sec813.pdf. [Google Scholar]
- United States. Synthetic drug abuse prevention act of 2012. U.S. Government Printing Office; 2012. [last accessed 7 Dec 2013]. pp. 138–140. [Online] Available from: http://www.gpo.gov/fdsys/pkg/BILLS-112s3187enr/pdf/BILLS-112s3187enr.pdf. [Google Scholar]
- Valdeolivas S, Satta V, Pertwee RG, et al. Sativex-like combination of phytocannabinoids is neuroprotective in malonatelesioned rats, an inflammatory model of Huntington’s disease: Role of CB(1) and CB(2) receptors. ACS Chem Neurosci. 2012;3:400–406. doi: 10.1021/cn200114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valoti E, Casagni E, Dell’Acqua L, et al. Identification of 1-butyl-3-(1-(4-methyl)naphtoyl)indole detected for the first time in “herbal high” products on the Italian market. Forensic Sci Int. 2012;223:e42–e46. doi: 10.1016/j.forsciint.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of Spice products (synthetic cannabinoids) Drug Alcohol Depend. 2011;120:238–241. doi: 10.1016/j.drugalcdep.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197:157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Vidinsky B, Gal P, Pilatova M, Vidova Z, et al. Anti-proliferative and anti-angiogenic effects of CB2R agonist (JWH-133) in non-small lung cancer cells (A549) and human umbilical vein endothelial cells: an in vitro investigation. Folia Biol (Praha) 2012;58:75–80. doi: 10.14712/fb2012058020075. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal F, Sonnichsen FD, Thiemt S. Identification of 1-butyl-3-(1-(4-methyl)naphthoyl)indole in a herbal mixture. Forensic Sci Int. 2012;215:8–13. doi: 10.1016/j.forsciint.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Wintermeyer A, Moller I, Thevis M, et al. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem. 2010;398:2141–2153. doi: 10.1007/s00216-010-4171-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [last accessed 02 Jan 2013];Management of substance abuse [Online] 2012 Available: http://www.who.int/substance_abuse/facts/cannabis/en/
- Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma P, Cole RB, Wang G. Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS. Anal Bioanal Chem. 2006;386:1345–1355. doi: 10.1007/s00216-006-0717-6. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma P, Iszard M, et al. In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist. Drug Metab Dispos. 2002;30:1077–1086. doi: 10.1124/dmd.30.10.1077. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, et al. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW. History of cannabis as a medicine: a review. Rev Bras Psiquiatr. 2006;28:153–157. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- Zuba D, Byrska B, Maciow M. Comparison of “herbal highs” composition. Anal Bioanal Chem. 2011;400:119–126. doi: 10.1007/s00216-011-4743-7. [DOI] [PubMed] [Google Scholar]