Abstract
Aseptic loosening of cemented tibial components in total knee arthroplasty (TKA) has been related to inadequate cement penetration into the trabecular bone bed during implantation. Recent postmortem retrieval work has also shown there is loss of interlock between cement and bone by resorption of trabeculae at the interface. The goal of this study was to determine if TKAs with more initial interlock between cement and bone would maintain more interlock with in vivo service (in the face of resorbing trabeculae) and have less micro-motion at the cement–bone interface. The initial (created at surgery) and current (after in vivo service) cement–bone interlock morphologies of sagittal implant sections from postmortem retrieved tibial tray constructs were measured. The implant sections were then functionally loaded in compression and the micro-motion across the cement–bone interface was quantified. Implant sections with less initial interdigitation between cement and bone and more time in service had less current cement–bone interdigitation (r2 = 0.86, p = 0.0002). Implant sections with greater initial interdigitation also had less micro-motion after in vivo service (r2 = 0.36, p = 0.0062). This work provides direct evidence that greater initial interlock between cement and bone in tibial components of TKA results in more stable constructs with less micro-motion with in vivo service.
Keywords: Knee arthroplasty, Loosening, Postmortem, Micro-motion, Radiolucent lines
1. Introduction
Over 600,000 total knee arthroplasties (TKAs) are performed in the US each year and the probability of needing a revision during the lifetime of the patient has been estimated to be 15% for males and 17.5% for females (Losina et al., 2012). Loosening of the tibial component is the most common cause of knee arthroplasty failure, but details about how loosening occurs is not fully understood. Aseptic loosening of cemented implants is often seen as a progression of radiolucent lines on x-ray at the interface between the cement and bone (Schneider and Soudry, 1986). At this point, gaps can form between the cement and bone and are often filled with fibrocartilage tissue (Hori and Lewis, 1982). Poor penetration of cement into the bone bed at the time of surgery increases the propensity for radiolucency formation (Ritter et al., 1994).
Pressurization of cement into trabecular bone results in a mechanical interlock between the cement and bone and this provides the initial fixation for the implant. However, with in vivo service, the trabeculae that interlock with the cement layer can resorb. A recent morphological study of postmortem retrieved tibial trays showed that 75% of the cement–bone interlock was lost within 10 years of in vivo service (Miller et al., 2014). These findings suggest that the fixation that exists at the completion of surgery does not remain over the functional lifetime of the joint arthroplasty. Excessive trabecular bone resorption at the cement–bone interface could contribute to increased micro-motion and eventual implant loosening.
The goal of this study was to determine the influence of the initial state of cement–bone interlock on the loss of trabecular interlock and interface micro-motion following in vivo service. This was accomplished using en bloc postmortem-retrieved tibial components from TKAs; these implants were not revisions obtained for a loose implant. We hypothesized that tibial components with more initial interlock between cement and bone would maintain more interlock with in vivo service and have less micro-motion at the cement–bone interface.
2. Methods
2.1. Specimen preparation
Ten fresh-frozen cemented total knee arthroplasties (TKAs) were obtained en bloc from the Anatomical Gift Program at SUNY Upstate Medical University (Table 1). All components had metal tibial trays with stems/keels and polyethylene inserts. All tibial components were determined to be radiographically well fixed (radiolucent lines less than 2 mm in 1 or fewer zones).
Table 1.
Donor information for two laboratory-prepared (implants A and B) 10 postmortem-retrieved (implants C–L) total knee arthroplasties (TKA). Implants C & D were from the same donor.
| Implant | Time in service (years) |
Sex | Age | BMI (kg/m2) |
Cause of death | Implant; Manufacturer | CR or PS | PE insert wear score |
|---|---|---|---|---|---|---|---|---|
| A | 0 | M | 70 | 34.3 | Cardiac arrest | Triathlon; Stryker | CR | 0 |
| B | 0 | F | 67 | 36.4 | Acute MI | Scorpio; Stryker | PS | 0 |
| C | 1 | F | 73 | 22.7 | Cardiac arrest | Vanguard; Biomet | CR | 17 |
| D | 2 | F | 73 | 22.7 | Cardiac arrest | Vanguard; Biomet | CR | 14 |
| E | 2.5 | F | 82 | 36.5 | Acute congestive heart failure | PFC Sigma; Depuy | PS | 17 |
| F | 3 | F | 87 | 23.9 | Pulmonary infection | Nexgen; Zimmer | PS | 7 |
| G | 5 | M | 61 | 32.9 | Cardiac arrest | Triathlon; Stryker | CR | 13 |
| H | 6.5 | F | 83 | 23.7 | Carcinoma | Scorpio; Stryker | PS | 25 |
| I | 9 | F | 74 | 31.9 | Cardiac arrest, renal failure | Nexgen LPS; Zimmer | PS | 9 |
| J | 11 | F | 69 | 22.5 | Rectal cancer | Advanced PS: Wright Medical Technology | PS | 52 |
| K | 16 | F | 75 | 31.4 | Heart disease, cardiac arrest | Duracon; Howmedica | CR | 45 |
| L | 3* | M | 86 | 25.5 | Cardiac arrest | Nexgen; Zimmer | CR | 9 |
Time in service for implant L was estimated from the polyethylene (PE) insert wear score versus time in service linear relationship (*).
CR indicates cruciate retaining and PS indicates posterior stabilized implant type.
Articulating surface wear was quantified using the grading scheme of Hood et al. (1983) for abrasion, burnishing, cement debris, delamination, pitting, scratching, and surface deformation. Each wear type was given a score of 0–3 based on severity over 8 regions of the articulating surface. The total wear score was calculated as the sum of the component scores.
Two additional fresh-frozen cadaver knees (without implants) were cemented with metal tibial trays to represent cases with zero years in service. Each proximal tibia was warmed in a calcium buffered bacteriostatic agent (Race et al., 2005) at 37 °C; transverse and keel cuts were made, and the surface was cleaned with pulsed lavage. PMMA cement (Simplex, Stryker Orthopedics, Mahwah, NJ) was applied to the cut surface in a doughy state, pressurized using a mixing spatula, and the metal tray was pressed onto the tibial surface.
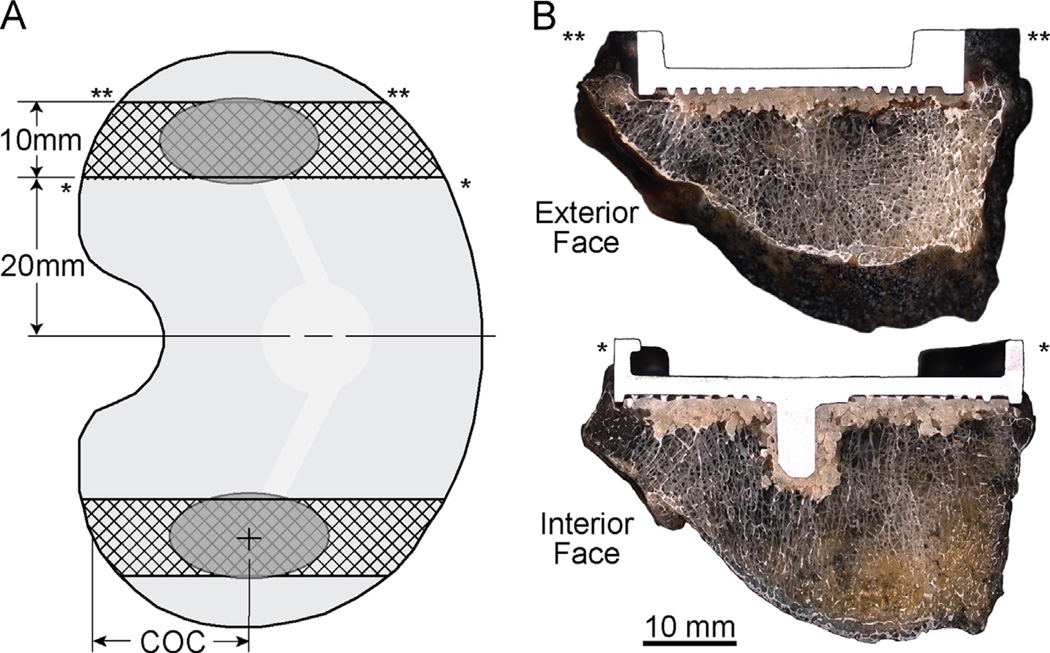
The constructs were sectioned in the sagittal plane with a water irrigated, silicon carbide blade (Isomet® 2000; Buehler Inc, Lake Bluff, Il). Sagittal cuts were made 20 and 30 mm from the midline of the tibial tray in medial and lateral directions (Fig. 1A) creating two specimens per TKA with a width of ~9–10 mm (Fig. 1B). The specimen faces were polished to 1200 grit using a grinder/polisher (Ecomet® 6, Buehler Inc, Lake Bluff, Il, USA). A total of 24 specimens were created (medial and lateral sections for 10 postmortem retrievals and 2 laboratory prepared tibial trays).
Fig. 1.
Tibial tray constructs were sectioned in the sagittal plane to create medial and lateral test specimens (A). The center of the contact patch (COC) on the polyethylene insert was used to define the load application point on the test specimens. Specimens were imaged at high resolution on interior and exterior faces to document interface morphology (B).
2.2. Interface morphology
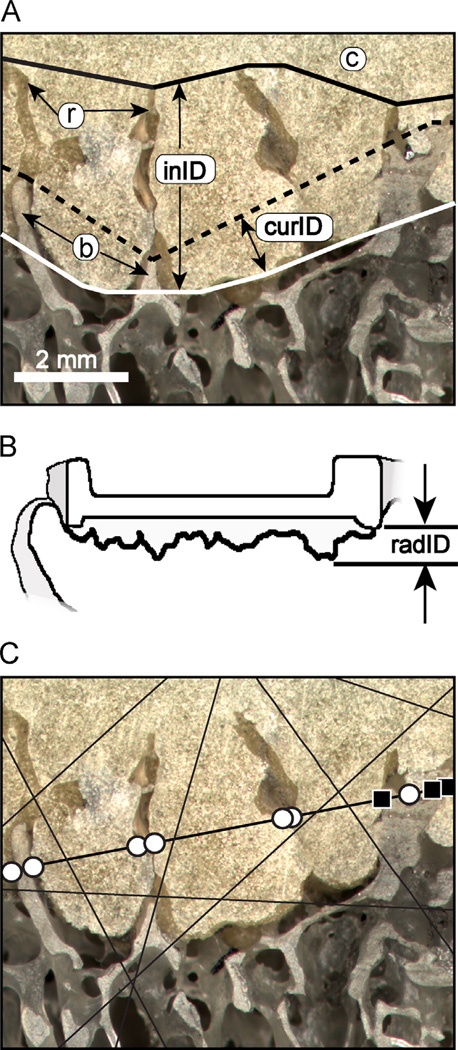
The quantity of fixation from the cement–bone interface was measured in terms of the interdigitation depth of trabeculae into the cement layer and the contact fraction between cement and bone (Mann et al., 2012; Miller et al., 2014). The initial cement–bone interlock morphology was documented using the mold shape of the cement that flows and cures around the trabeculae during the cementing process, as was first recognized by Charnley (1970). With in vivo service, the interlocked bone may resorb, leaving an empty cavity in the cement with a form of the original interlocking trabecular structure (Fig. 2A). We recently described this as a being analogous to a trace fossil from the field of paleontology, where an initial imprint is created in a substrate, and is left to fossilize with time (Miller et al., 2014). With this approach, the initial state of fixation can be estimated for each specimen face using the ‘trace fossil’ approach.
Fig. 2.
The cement–bone interface morphology of a postmortem retrieved tibial component shows trabecular bone (b) interdigitated with cement (c) and areas of resorbed (r) bone (A). The initial interdigitation depth (inID) and current interdigitation depth (curID) were determined. Radiographic interdigitation depth (radID) was calculated as the distance from the rim on the tibial tray to the maximum depth of cement into the bone (B). Cement–bone contact fraction (CF) was determined using a random ray stereology method to document contact (black squares) or non-contact (white circles) at the cement–bone interface (C).
A high-resolution camera imaging system (0.0057 mm/pixel) was used to create detailed reflected white light images of the cement–bone interface for each specimen face. Piece-wise linear traces of the original extent of interdigitation of bone into the cement layer (solid black line), current interdigitation of bone in the cement layer (dashed line), and cement layer into the bone (white line, Fig. 2A) were constructed. The initial interdigitation depth (inID) was calculated using a moving average, local minimum point-to-point distance measurement (ImagePro, Media Cybernetics, Rockville, MD) from the original extent of bone in the cement layer (solid black line) to the extent of cement into the bone (solid white line). The current interdigitation depth (curID) was measured between the current extent of bone in the cement layer (dashed black line) and the extent of cement into the bone (white line). An analog of the radiographic A–P projected cement interdigitation (radID) (Fig. 2B) was calculated using the perpendicular distance from the base of the tray to the distal extent of the cement layer.
The contact fraction (CF) between cement and bone at the interface was calculated using a random ray stereology method. One hundred random rays were projected across the cement–bone interface (Fig. 2C) and visual inspection was used to determine if there was bone in contact with cement for the ray crossing the interface. To calculate the contact fraction (CF) the sum of cement–bone points in contact was divided by the total number of intersections. Morphology measures were made on internal and external faces and averaged for each specimen.
2.3. Mechanical testing
An axial compressive loading regime was chosen for this study because recent instrumented TKR studies (D’Lima et al., 2007) (Kutzner et al., 2010) have shown that the axial component of loading predominates during most activities of daily living. Axial compressive loads were limited to the equivalent to one body weight (1 BW) to prevent permanent damage to the specimens during loading and this was scaled according to the cross sectional area of the test specimen as follows:
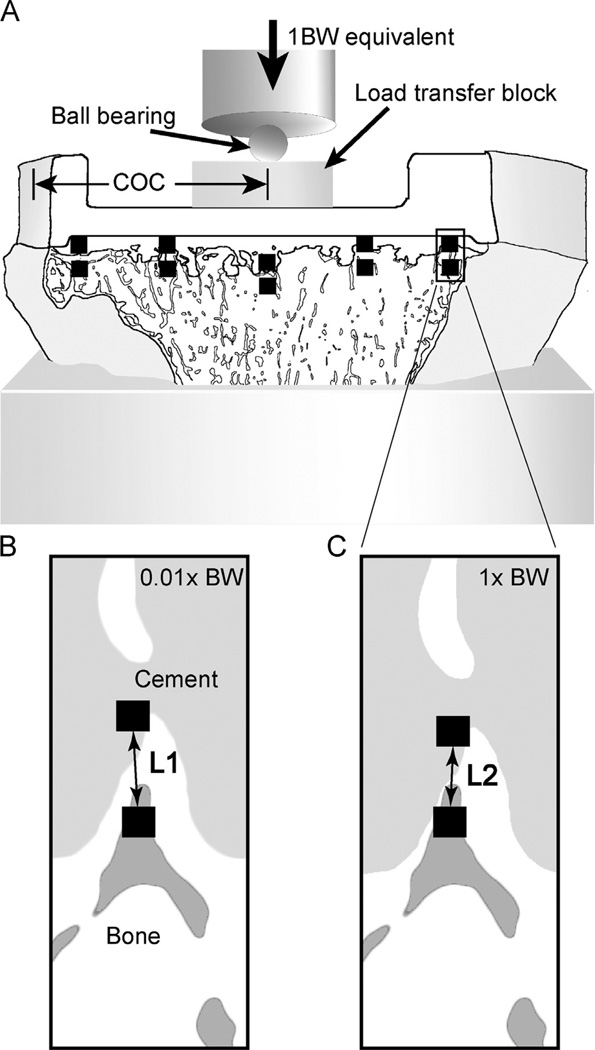
Specimens were potted in an acrylic cement mold, 15 mm distal from the cement–bone interface, and fixed (Fig. 3A) to the base of a mechanical testing frame (Q-Test, MTS, Eden Prairie, MN). Because loads were not applied through the polyethylene insert, a loading block was used to distribute load over an area consistent with previously reported pressure measurements (Villa et al., 2004) made across the polyethylene insert–tray interface (28% of A–P dimension). The loading block was positioned in the A–P direction based on the center of contact (COC) (Fig. 1A) of the wear patch from the polyethylene insert.
Fig. 3.
Tibial tray sections (A) were loaded axially in compression to one body weight (BW) equivalent loading through a load transfer block that was positioned coincident with the polyethylene insert center of contact (COC). Digital image correlation imaging was used to measure the relative cement–bone micro-motion at five locations along the interface per side of the specimen (black squares). The distance between cement and bone measurement points with 0.01 BW loading (B) and with 1.0 BW loading (C) was used to determine micro-motion (L1–L2).
The micro-motion at the cement–bone interface was documented using a digital image correlation (DIC) system consisting of a digital camera with a telecentric lens and DIC software (Rapid Correlator 1.0, X-Stream Software, Ottawa, ON). The camera was positioned at 10 sampling locations along the interface, with five locations per face (Fig. 3A). The relative micro-motion of the cement–bone interface was measured between pairs of DIC sampling squares (40 × 40 pixels, 0.1 mm2) located on the cement and adjacent trabecular bone (Fig. 3B) for negligible load (0.01 BW) and one body weight (1 BW) equivalent load (Fig. 3C). The difference in measured lengths (L1 – L2) was used to define the micro-motion at the sampling location. Specimens were loaded for 4 cycles at 1 mm/min, with image capture during the fourth cycle. The camera was moved to the next sampling location, and the loading sequence was repeated. The median cement–bone micro-motion for the 10 sampling locations was used as primary outcome measure to describe mechanical stability for each specimen. The RMS error of the DIC system for measurement between two sampling squares was 0.784 µm (Mann et al., 2008).
2.4. Statistical method
To test the hypothesis that tibial trays with more initial cement–bone interlock maintain more interlock with in vivo service, a two-parameter linear regression model was used with current interdigitation depth (curID) as the dependent variable, and initial interdigitation depth (inID) and time in service as the independent variables. Square root transformations were performed on the variables, prior to performing the linear regression analysis. To test the hypothesis that tibial trays with more initial and current interlock have less interface micro-motion, regression analysis was performed with micro-motion as the dependent variable and inID, curID or CF as the independent variables. Log transformations were performed for the CF-micro-motion and ID-micro-motion regression analyses resulting in a power-law response. Finally, linear regression analysis was performed to determine if there was a correlation between polyethylene wear score and interface micro-motion.
3. Results
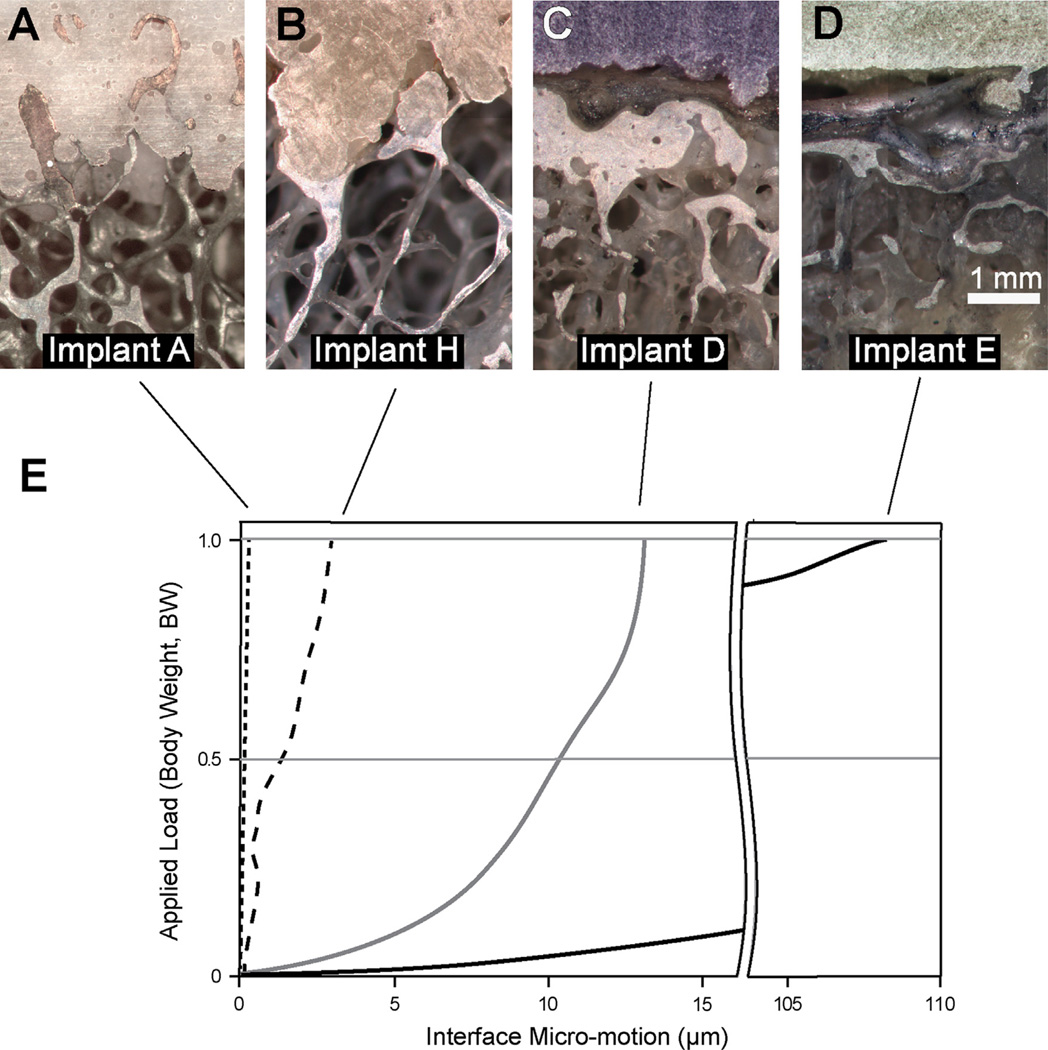
The lateral specimen from Implant C failed during preparation due to a very weak cement–bone interface leaving 23 test specimens from 12 TKAs for testing and analysis. Specimens had a wide range of cement–bone interlock, from laboratory prepared specimens where there was no resorption of the trabecular bone (Fig. 4A), to postmortem retrievals with some remaining interlock (Fig. 4B), to specimens with little or no bone in the interdigitated region (Fig. 4C and D). The mean initial interdigitation depth (inID = 1.16 mm) was about twice the current interdigitation depth (curID = 0.65 mm) (p < 0.0001, paired t-test) (Table 2). The radiographic interdigitation depth (radID) was much larger (3.63 mm) than either inID (p < 0.0001, paired t-test) or curID (p < 0.0001, paired t-test). The cement–bone contact fraction (CF) ranged from 0% to 53.6% with a mean of 16.8%.
Fig. 4.
A lab-prepared specimen (A) with substantial interdigitation and contact between cement and bone had virtually no interface micro-motion (<1 µm). A postmortem retrieval with some remaining interdigitation (B) had interface micro-motions of 4 µm. A postmortem retrieval with no interdigitation along the specimen surface (C) had 12 µm of micro-motion with a non-linear response to the applied load. A postmortem retrieval with extensive soft tissue (D) between the cement and the bone had 110 µm of micro-motion. The micro-motion response of these four representative sections of the tibial tray constructs is shown (E).
Table 2.
Interface morphology and micro-motion measures for postmortem-retrieved and laboratory-prepared total knee arthroplasties (TKA). There were 23 specimens obtained from 12 TKAs. Mean, (standard deviation), and [range] are shown.
| Test parameter | Postmortem retrieved TKA (n = 19) | Laboratory prepared TKA (n = 4) | Combined groups (n = 23) |
|---|---|---|---|
| Initial interdigitation depth, inID (mm) | 1.07 (0.58) [0.19–2.05] | 1.57 (0.04) [1.51–1.61] | 1.16 (0.56) [0.19–2.05] |
| Current interdigitation depth, curID (mm) | 0.46 (0.47) [0–1.51] | 1.55 (0.04) [1.49–1.59] | 0.65 (0.60) [0–1.60] |
| Radiographic interdigitation depth, radID (mm) | 3.37 (1.57) [1.22–6.56] | 4.87 (1.41) [3.29–6.27] | 3.63 (1.62) [1.22–6.56] |
| Contact fraction, CF (%) | 10.2 (10.2) [0–36] | 48 (6) [42–54] | 17 (18) [0–53.6] |
| Interface micro-motion (µm) | 20.9 (42.7) [0.75–178] | 1.97 (0.79) [0.82–2.54] | 17.6 (39.3) [0.75–178] |
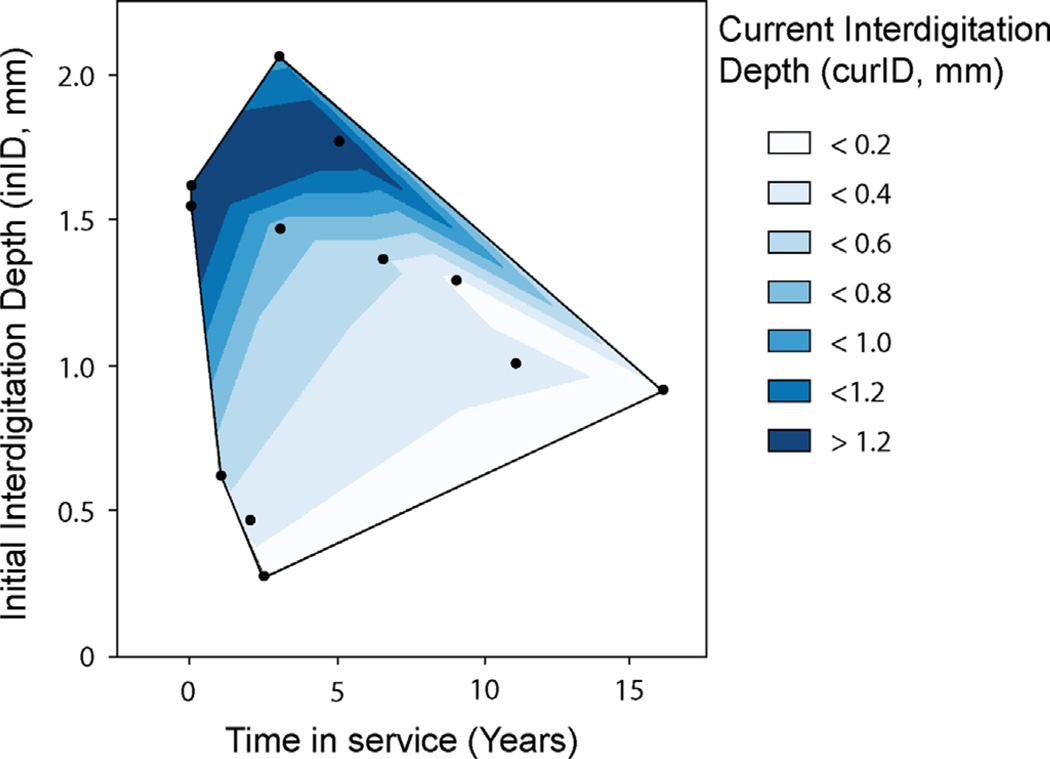
Using the two-parameter regression model with curID as the dependent variable, the initial interdigitation depth (p = 0.0004) and time in service (p = 0.0015) had significant contributions in the estimate of curID (r2 = 0.86, p = 0.0002). Implants with less initial interdigitation and more time in service had less current cement–bone interdigitation (Fig. 5). For the 10 postmortem retrievals, the loss of interdigitation depth per year was quite variable (median: 0.11 mm/year, range: 0.05–0.38 mm/year). Normalizing to initial interdigitation depth, the loss of interdigitation depth ((inID−curID)/inID = 4.6%/year) was proportional to time in service (r2 = 0.48, p = 0.013).
Fig. 5.
Contour plot of current cement–bone interdigitation depth (curID) as a function of time in service and initial interdigitation depth (inID) for 12 tibial tray constructs. Implants with more time in service and less initial interdigitation had the least amount of current interdigitation.
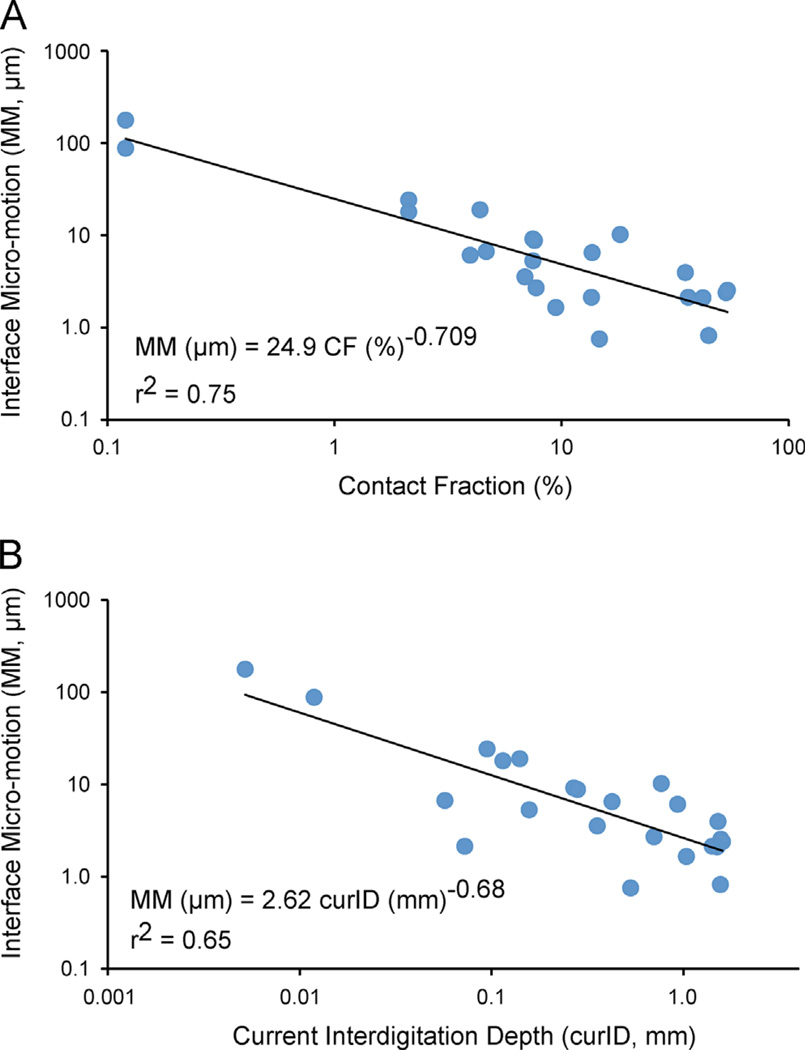
The cement–bone micro-motion with 1 BW equivalent loading ranged from 0.75 to 178 µm (mean: 17.6 ± 39.3 µm). Specimens with small micro-motions had a linear response between applied load and micro-motion (Fig. 4E). In contrast, specimens with large micro-motions often had a non-linear load/micro-motion response with an increase in stiffness (slope) with increasing load. There was an inverse power-law relationship (r2 = 0.75, p = 0.0001) between the amount of current fixation and micro-motion; specimens with greater contact fraction (CF) had less micro-motion (Fig. 6A). Specimens with greater current interdigitation depth (curID) also had less micro-motion (r2 = 0.65, p = 0.0001) (Fig. 6B). Initial interdigitation depth (inID) was also inversely correlated with micro-motion (r2 = 0.36, p < 0.0062).
Fig. 6.
There was a power-law response between current contact fraction (CF) and cement–bone interface micro-motion (MM) (A) where interfaces with greater CF had less micro-motion. A similar relationship between current interdigitation depth (curID) and micro-motion (MM) was also found (B).
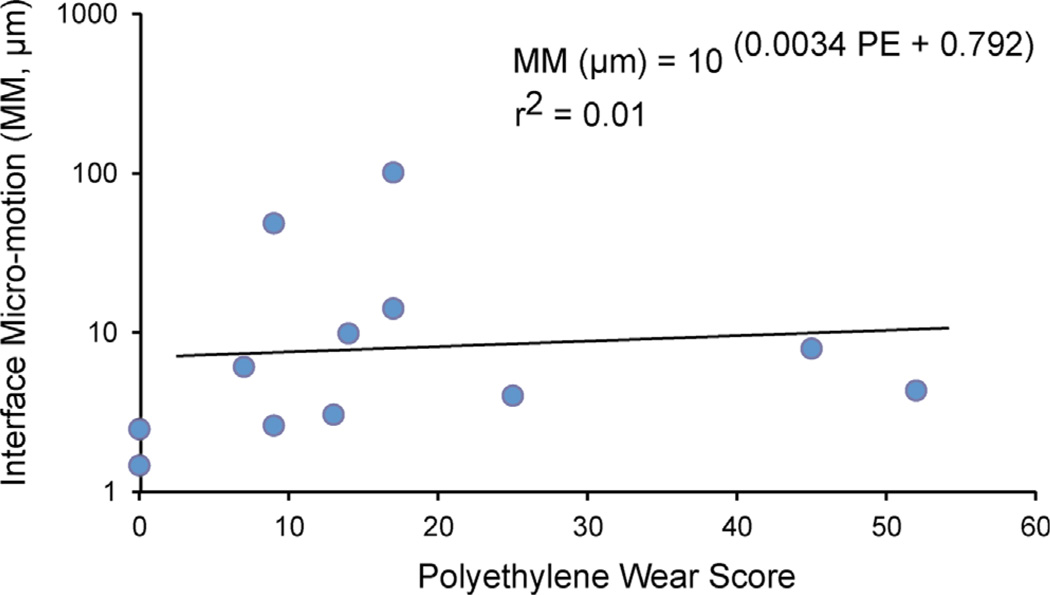
The amount of polyethylene insert wear, quantified using the Hood method, was not correlated (r2 = 0.01, p = 0.67) with interface micro-motion (Fig. 7). There was a positive correlation between PE wear and time in service (r2 = 0.66, p = 0.0024).
Fig. 7.
There was no correlation between polyethylene insert wear score (PE) and micro-motion (MM) at the cement–bone interface.
4. Discussion
Postmortem retrieved tibial components from cemented total knee arthroplasties (TKA) were used to determine if implants that started with better initial interlock to bone maintained interlock with in vivo service, and also had greater mechanical stability. There was a loss of cement–bone interlock with in vivo service, and specimens with greater initial interlock maintained more interlock with time in service. With functional loads applied to the sagittal sections, there was less interface micro-motion when there was greater initial and current interlock between the cement and bone. To our knowledge, this is the first biomechanical study of TKA fixation to quantify the relationship between the initial state of fixation and the functional micro-motion at the cement–bone interface.
There are several limitations to this study. First, the retrievals were from our Anatomical Gift Program and may not represent the entire clinical population. However, our donor population is similar to that receiving TKAs in the US; 75% were female (62% in US population) (Kurtz et al., 2011) and 33% were less than 65 year old at time of surgery (44% for US population) (Kurtz et al., 2011). There were different implant designs, but all had cemented metal trays with stems. Details of the cement type, cement technique, and viscosity of cement during cementation were not known; therefore the effectiveness of these clinical variables could not be assessed. However, detailed measures of the resulting cement mantle and quantity of interdigitation were made. The initial interdigitation depth was based on the assumption that the mold shape of the cement layer represented the initial interlock of trabeculae into cement. We previously reported that the root mean square (RMS) error in the estimate of initial interdigitation depth was 0.06 mm (Miller et al., 2014).
To measure micro-motion along the trabecular bone–cement interface and also quantify the morphology, sagittal sections of the cemented tibial tray were created. As such, the mechanical behavior of the whole construct was not assessed and it is possible that load transfer would be different from the transverse sections studied here. However, the micro-motion magnitudes were similar to those measured for full postmortem retrieved TKA constructs by Rao et al. (2010) (mean: 18.2 µm, range: 2.7–34.5 µm) and our group (mean: 32 µm, range: 1.5–105 µm) (Mann et al., 2014). Only axial compressive loads were applied, and shear forces were neglected. However, axial compressive loading is the predominant loading direction during ambulation (Kutzner et al., 2010).
A morphological assessment of the entire cement–bone interface of postmortem-retrieved tibial components (Miller et al., 2014), including tray and keel regions, showed that contact fraction of TKAs was limited (10.2%, range: 4.2–32.2%). Medial and lateral test sections used in the present study had similar contact fractions (10.1%, range: 0–36%). This suggests that the morphology for the specimens used in this study may be representative of the TKA as a whole. Interestingly, the cement–bone contact fraction for postmortem total hip femoral components was also reported to be in a similar range (10.4%, range: 0.4–32.5%) (Mann et al., 2010). Overall, it appears that cemented component fixation may begin with substantial interlock or apposition between cement and bone at the completion of surgery, but there is a loss of fixation with in vivo service. The quantity of interlock required for stable clinical fixation is not known but interfaces that have micro-motion in the range of 150 µm (Jasty et al., 1997) are known to result in fibrous tissue formation. The two specimens with micro-motions in this range (88–178 µm) had zero contact between cement and bone.
Inducible displacements (a measure of micro-motion) and overall migration of tibial components have been documented clinically using Radio-Stereometeric Analysis (RSA). Tibial trays with larger initial inducible displacement have greater migration (Toksvig-Larsen et al., 1998; Uvehammer and Karrholm, 2001; Wilson et al., 2010) and excessive early migration has been correlated with an increased risk of tray loosening (Pijls et al., 2012). Loss of cement–bone interlock that occurs during in vivo service, and the relationship with increased cement–bone micro-motion, as found in the present study, could be responsible for the inducible displacements measured with RSA. If there were no loss of cement–bone interlock, it would be unlikely that inducible displacements could be detected with RSA, as micro-motion would be very small (<3 µm for lab-prepared specimens in Table 2). Of course, if there was no interlock between cement and bone at the time of surgery, then there would likely be substantial micro-motion at the cement–bone interface and conditions would not be favorable for maintaining stable fixation. This work focused on the relationship between interface micro-motion and the local cement–bone interface morphology. Component migration, as measured by RSA, would be influenced by architectural changes to bone supporting the cement–bone interface in addition to changes in interface morphology.
The mechanics of the cement–bone interface has been studied extensively, particularly with regard to the relationship between cementing technique, penetration of cement into the bone bed, and the resulting mechanical strength of the interface. Laboratory studies have shown that the mechanical strength of the cement–bone interface increases with greater interdigitation depth of cement into trabecular bone (Krause et al., 1982; Walker et al., 1984). Increased cement penetration into bone also has been shown to decrease micro-motion at the cement–bone interface (Bert and McShane, 1998). These studies provide a sound biomechanical basis to achieve good interlock between cement and bone at the time of surgery, but they do not address the biomechanics in the presence of the biological changes that occur at the interface with in vivo use.
The effect of in vivo service on fixation strength has been studied using postmortem retrieved cemented tibial components (Gebert de Uhlenbrock et al., 2012), where the force required to pull the tibial tray from the bone was used as a measure of fixation. The pull-off force was greater for cases where there was more cement–bone interdigitation. In addition, the cement–bone interface was weaker for TKAs with greater time in service. This latter finding is consistent with the current observation of loss of interlock with in vivo service due to trabecular resorption, as this would affect the stability and strength of the interface.
The amount of polyethylene (PE) wear on the tibial insert articulating surface did not correlate with the micro-motion found at the cement–bone interface. The TKA with the highest wear (52 Hood score with 11 years service) had small micro-motions (4 µm), while the component with the most micro-motion (178 µm with 2.5 years service) had much less wear (17 Hood score). It is possible that the amount of initial interlock plays a more important role in the resulting micro-motion in the short term. In the longer term, PE wear may be a more important factor, given that there was a positive correlation between PE wear and time in service. An assessment of the presence of PE debris at interfaces in our retrievals is planned to further explore this issue.
Clinically, the results from the present study further support the concept of achieving good cement penetration into the trabecular bone bed of the proximal tibia during surgical implantation. Because there is loss of trabecular interlock in the cement layer with in vivo service, starting with more initial interlock can result in more current interlock and less micro-motion. Clinical studies have shown that increasing cement penetration, as documented radiographically, can reduce the incidence of radiolucent lines and failures (Guha et al., 2008; Ritter et al., 1994; Walker et al., 1984). An ideal cement penetration of 3–4 mm has been proposed based on the concept that a minimum of 2 mm is needed to interlock with transverse trabeculae (Cawley et al., 2013;Walker et al., 1984). Additional penetration (>5 mm) can increase the risk of thermal necrosis (Sih et al., 1980). It is interesting to note that for specimens that had radID >3 mm, micro-motions were uniformly small (mean (sd): 4.7 ± 2.9 µm, range: 0.8–10.2 µm). For those with radID <3 mm, micro-motions were much more variable (mean (sd): 47 ± 65 µm, range: 0.8–178 µm). These findings suggest that obtaining 3 mm of cement layer thickness may result in interfaces with consistently small micro-motions during in vivo service, while thinner cement layers may not always result in stable fixation.
Acknowledgments
The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award number AR42017. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge the assistance of Dan Jaeger for providing the postmortem retrievals and tissue from the SUNY Upstate Anatomical Gift Program. Timothy Izant serves as a paid consultant for Stryker Orthopaedics for clinical total joint replacement studies unrelated to the content of this manuscript.
Footnotes
Conflict of interest statement
Timothy Izant serves as a paid consultant for Stryker Orthopaedics for clinical total joint replacement studies unrelated to the content of this manuscript. All other authors have no conflicts of interest to disclose.
References
- Bert JM, McShane M. Is it necessary to cement the tibial stem in cemented total knee arthroplasty? Clin. Orthop. Relat. Res. 1998;356:73–78. doi: 10.1097/00003086-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Cawley DT, Kelly N, McGarry JP, Shannon FJ. Cementing techniques for the tibial component in primary total knee replacement. Bone Joint J. 2013;95-B:295–300. doi: 10.1302/0301-620X.95B3.29586. [DOI] [PubMed] [Google Scholar]
- Charnley J. The reaction of bone to self-curing acrylic cement. A long-term histological study in man. J. Bone Joint Surg. Br. 1970;52:340–353. [PubMed] [Google Scholar]
- D’Lima DD, Patil S, Steklov N, Chien S, Colwell CW., Jr in vivo knee moments and shear after total knee arthroplasty. J. Biomech. 2007;40(Suppl. 1):S11–S17. doi: 10.1016/j.jbiomech.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Gebert de Uhlenbrock A, Puschel V, Puschel K, Morlock MM, Bishop NE. Influence of time in-situ and implant type on fixation strength of cemented tibial trays—a post mortem retrieval analysis. Clin. Biomech. (Bristol, Avon) 2012;27:929–935. doi: 10.1016/j.clinbiomech.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Guha AR, Debnath UK, Graham NM. Radiolucent lines below the tibial component of a total knee replacement (TKR)—a comparison between single-and two-stage cementation techniques. Int. Orthop. 2008;32:453–457. doi: 10.1007/s00264-007-0345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. J. Biomed. Mater. Res. 1983;17:829–842. doi: 10.1002/jbm.820170510. [DOI] [PubMed] [Google Scholar]
- Hori RY, Lewis JL. Mechanical properties of the fibrous tissue found at the bone–cement interface following total joint replacement. J. Biomed. Mater. Res. 1982;16:911–927. doi: 10.1002/jbm.820160615. [DOI] [PubMed] [Google Scholar]
- Jasty M, Bragdon C, Burke D, O’Connor D, Lowenstein J, Harris WH. in vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J. Bone Joint Surg. Am. 1997;79:707–714. doi: 10.2106/00004623-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Krause WR, Krug W, Miller J. Strength of the cement–bone interface. Clin. Orthop. 1982;163:290–299. [PubMed] [Google Scholar]
- Kurtz SM, Ong KL, Lau E, Widmer M, Maravic M, Gomez-Barrena E, de Fatima de Pina M, Manno V, Torre M, Walter WL, de Steiger R, Geesink RG, Peltola M, Roder C. International survey of primary and revision total knee replacement. Int. Orthop. 2011;35:1783–1789. doi: 10.1007/s00264-011-1235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010;43:2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J. Bone Joint Surg. Am. 2012;94:201–207. doi: 10.2106/JBJS.J.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Cleary RJ, Janssen D, Verdonschot N. Experimental micromechanics of the cement–bone interface. J. Orthop. Res. 2008;26:872–879. doi: 10.1002/jor.20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Goodheart JR, Izant TH, Cleary RJ. Peri-implant bone strains and micro-motion following in vivo service: a postmortem retrieval study of 22 tibial components from total knee replacements. J. Orthop. Res. 2014;32:355–361. doi: 10.1002/jor.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Pray CL, Verdonschot N, Janssen D. A new approach to quantify trabecular resorption adjacent to cemented knee arthroplasty. J. Biomech. 2012;45:711–715. doi: 10.1016/j.jbiomech.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Verdonschot N, Izant TH, Race A. Functional interface micromechanics of 11 en-bloc retrieved cemented femoral hip replacements. Acta Orthop. 2010;81:308–317. doi: 10.3109/17453674.2010.480938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Goodheart JR, Izant TH, Rimnac CM, Cleary RJ, Mann KA. Loss of cement–bone interlock in retrieved tibial components from total knee arthroplasties. Clin. Orthop. Relat. Res. 2014;472:304–313. doi: 10.1007/s11999-013-3248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijls BG, Valstar ER, Nouta KA, Plevier JW, Fiocco M, Middeldorp S, Nelissen RG. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop. 2012;83:614–624. doi: 10.3109/17453674.2012.747052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race A, Miller MA, Clarke MT, Mann KA. Cement/implant interface gaps explain the poor results of CMW3 for femoral stem fixation: a cadaver study of migration, fatigue and mantle morphology. Acta Orthop. 2005;5:679–687. doi: 10.1080/17453670510041763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AS, Engh JA, Engh GA, Parks NL. Mechanical stability of well-functioning tibial baseplates from postmortem-retrieved total knee arthroplasties. J. Arthroplast. 2010;25:481–485. doi: 10.1016/j.arth.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Ritter MA, Herbst SA, Keating EM, Faris PM. Radiolucency at the bone–cement interface in total knee replacement. The effects of bone-surface preparation and cement technique. J. Bone Joint Surg. Am. 1994;76:60–65. doi: 10.2106/00004623-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Schneider R, Soudry M. Radiographic and scintigraphic evaluation of total knee arthroplasty. Clin. Orthop. Relat. Res. 1986:108–120. [PubMed] [Google Scholar]
- Sih GC, Connelly GM, Berman AT. The effect of thickness and pressure on the curing of PMMA bone cement for the total hip joint replacement. J. Biomech. 1980;13:347–352. doi: 10.1016/0021-9290(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Toksvig-Larsen S, Ryd L, Lindstrand A. Early inducible displacement of tibial components in total knee prostheses inserted with and without cement: a randomized study with roentgen stereophotogrammetric analysis. J. Bone Joint Surg. Am. 1998;80:83–89. [PubMed] [Google Scholar]
- Uvehammer J, Karrholm J. Inducible displacements of cemented tibial components during weight-bearing and knee extension observations during dynamic radiostereometry related to joint positions and 2 years history of migration in 16 TKR. J. Orthop. Res. 2001;19:1168–1177. doi: 10.1016/S0736-0266(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Villa T, Migliavacca F, Gastaldi D, Colombo M, Pietrabissa R. Contact stresses and fatigue life in a knee prosthesis: comparison between in vitro measurements and computational simulations. J. Biomech. 2004;37:45–53. doi: 10.1016/s0021-9290(03)00255-0. [DOI] [PubMed] [Google Scholar]
- Walker PS, Soudry M, Ewald FC, McVickar H. Control of cement penetration in total knee arthroplasty. Clin. Orthop. Relat. Res. 1984:155–164. [PubMed] [Google Scholar]
- Wilson DA, Astephen JL, Hennigar AW, Dunbar MJ. Inducible displacement of a trabecular metal tibial monoblock component. J. Arthroplast. 2010;25:893–900. doi: 10.1016/j.arth.2009.06.015. [DOI] [PubMed] [Google Scholar]