Abstract
Objectives
We investigated the relationship between brain lithium levels and the metabolites N-acetyl aspartate (NAA) and myo-inositol (myo-Ino) in the anterior cingulate cortex of a group of older adults with bipolar disorder (BD).
Methods
This cross-sectional assessment included nine subjects (six males and three females) with bipolar I disorder and currently treated with lithium, who were examined at McLean Hospital’s Geriatric Psychiatry Research Program and Brain Imaging Center. The subjects’ ages ranged from 56 to 85 years (66.0 ± 9.7 years) and all subjects had measurements of serum and brain lithium levels. Brain lithium levels were assessed using lithium magnetic resonance spectroscopy. All subjects also had proton magnetic resonance spectroscopy to obtain measurements of NAA and myo-Ino.
Results
Brain lithium levels were associated with higher NAA levels [df = (1, 8), B = 12.53, t = 4.09, p < 0.005] and higher myo-Ino levels [df = (1, 7), F = 16.81, p < 0.006]. There were no significant effects of serum lithium levels on any of the metabolites.
Conclusion
Our findings of a relationship between higher brain lithium levels and elevated NAA levels in older adult subjects with BD may support previous evidence of lithium’s neuroprotective, neurotrophic, and mitochondrial function-enhancing effects. Elevated myo-Ino related to elevated brain lithium levels may reflect increased inositol monophosphatase (IMPase) activity, which would lead to an increase in myo-Ino levels. This is the first study to demonstrate alterations in NAA and myo-Ino in a sample of older adults with BD treated with lithium.
Keywords: brain lithium, geriatric bipolar disorder, myo-inositol, N-acetyl aspartate, proton magnetic resonance spectroscopy
Lithium has been used for over 35 years for the treatment of bipolar disorder (BD), yet the precise neurobiological mechanisms through which lithium exerts its clinical effects are not clear. Concerns regarding neurotoxic side effects of lithium in older adults, including a more prolonged recovery from lithium-induced delirium (1) and the risk of lithium-induced renal insufficiency, have led to both declining rates of lithium use in older adults (2) and questions about the most effective clinical approach to utilizing lithium safely in older adults with BD.
Lithium magnetic resonance spectroscopy (7Li MRS) measures in vivo brain lithium levels. In a recent study at McLean Hospital’s Geriatric Psychiatry Research Program and Brain Imaging Center, Belmont, MA, USA, we demonstrated that elevations in brain lithium levels were associated with frontal lobe dysfunction and higher Hamilton Depression Rating Scale (HDRS) (3) scores in older adults with BD (4). The higher HDRS scores were associated with somatic symptoms of depression, such as fatigue.
Proton magnetic resonance spectroscopy (1H MRS) measures in vivo levels of brain metabolites, including N-acetyl aspartate (NAA), a putative marker of neuronal viability, and myo-inositol containing compounds (myo-Ino). More than half of the studies of adults with BD compared with healthy comparison subjects (HCS) (5–11) report decreased NAA in subjects with BD compared with HCS (5, 6, 10, 11). Lithium has been shown (after four weeks of treatment) to increase brain NAA levels acutely in subjects with BD and in healthy controls (12). Similarly, Silverstone et al. (13) measured increased NAA/Cr levels in the frontal and temporal lobes of BD subjects chronically treated with lithium compared with healthy controls. NAA, which is synthesized in mitochondria, also helps to maintain myelin and is involved with neuron-to-glia signaling (14, 15).
Proton magnetic resonance spectroscopy can also measure myo-Ino levels. The inositol depletion hypothesis suggests that lithium’s efficacy in BD may be related to an inhibition of inositol monophosphatase (IMPase), leading to a reduction in brain myo-Ino and an increase in inositol-1-phosphate (I-1-P) (16).
Given our recent findings of frontal lobe dysfunction associated with higher brain lithium levels, the purpose of this study was to establish whether there was an association between brain lithium levels and NAA levels in the anterior cingulate cortex (ACC) of older adults with BD. Regional neuroanatomical abnormalities in the prefrontal cortex (PFC) and ACC have been consistently implicated in BD (17). We also wished to further explore the effects of lithium on myo-Ino in older adults with BD. We hypothesized that elevations in brain lithium would be associated with an increase in NAA and a decrease in myo-Ino, reflecting alterations in cellular signaling required for the therapeutic efficacy of lithium.
Patients and methods
The Institutional Review Board at McLean Hospital approved this study. Subjects were recruited through referrals from the McLean Hospital Geriatric Psychiatry Inpatient Service, Partial Hospital Program and Outpatient Clinic. Subjects were also recruited through flyers posted around McLean Hospital and advertisements in local newspapers and radio.
Inclusion criteria for this study included current treatment with lithium for DSM-IV-TR bipolar I disorder (18) and a first episode of mania before age 50 (early-onset BD). Subjects aged 50 to 90 years of both sexes were eligible. Exclusion criteria included a serious or unstable medical illness, including cardiovascular, hepatic, renal, respiratory, endocrine, neurologic, or hematologic disease; a first episode of mania after age 50 (late-onset BD); a history of seizure disorder; and a history or current diagnosis of any other DSM-IV-TR Axis I psychiatric illness. One subject had a comorbid diagnosis of alcohol dependence.
All subjects signed a written informed consent form. Subjects meeting inclusion/exclusion criteria continued taking lithium medication without change. All subjects were assessed with a psychiatric interview by a board-certified psychiatrist to establish a diagnosis of BD.
Proton and lithium MRS examinations for all subjects were scheduled to commence between 07:30 and 08:30. Immediately following the 4.0 T MRS examination, subjects had a blood draw to determine trough serum lithium levels. The 7Li MRS examination was scheduled to occur within 8–12 h after the previous lithium dose to determine a trough brain lithium level. All subjects also completed a 1.5 T diagnostic magnetic resonance imaging (MRI) scan to rule out intracranial pathology precluding participation in the study.
MRI and spectroscopy acquisition
Diagnostic imaging was performed on a GE 1.5 T Signa (General Electric Medical Technologies, Milwaukee, WI, USA) with a volume head coil. Brain MRI data were acquired for purposes of ruling out clinically significant central nervous system (CNS) disorders. These MRI scans were reviewed by a board-certified neuroradiologist.
All MRS studies were performed on a 4.0 T Varian Unity/Inova whole body magnetic resonance scanner (Varian NMR Instruments, Palo Alto, CA, USA) equipped with a dual-tuned proton and lithium volumetric head coil (MR Instruments, Minneapolis, MN, USA). Proton spectra were acquired from a 2 cm × 2 cm × 2 cm voxel localized on the ACC using the point resolved spectroscopy sequence [repetition time (TR) = 2 s, echo time (TE) = 30 ms, and averages = 128]. The voxel was placed over the anterior cingulate gyrus, in a predominantly gray-matter area, superior to the orbits and inferior to the genu of the corpus collosum. Lithium spectra were acquired from a 60 mm slice centered on the superior edge of the corpus collosum using the Image Selective In Vivo Spectroscopy (ISIS) spectroscopy sequence (TR = 5 s and averages = 64) (19). The lithium spectrum included signal acquired from the in vivo brain lithium and a LiCl standard attached to the side of the coil. Once the subject had left the magnet, a lithium spectrum from a 6 mM LiCl phantom and the coil standard was acquired under identical conditions to the in vivo study.
MRS analysis
Following data acquisition, the proton spectra were fit using LCModel (Version 6.1-0) (20) and a simulated basis set. The Cramer-Rao spectral inclusion criteria for the spectra were NAA, Cho, Cr, and myo-Ino SD < 15%.
The lithium spectroscopy data were transferred from the 4 T Varian Unity/Inova to a Windows PC (Windows XP Service Pack 2 with a Pentium IV processor) to be processed using FELIX (Accelrys, San Diego, CA, USA). FELIX is a proprietary multidimensional nuclear magnetic resonance processing package capable of peak fitting using a simulated annealing algorithm. Following a direct current offset correction and 10 Hz exponential filtering, the spectra were Fourier transformed to the frequency domain. The frequency spectra were phase corrected and baseline corrected (with a cubic spline) and fit using a Lorentzian fit. The in vivo brain lithium levels were calculated using the following equation:
[Li] = (Sbo)/(Spo) * (Vb/Vp) * [6 mMLiCl] * Q
Sbo = Sb/[1 − EXP(−TR/T1b)]
Spo = Sp/[1 − EXP(−TR/T1p)]
Sb: Signal from brain.
Sp: Signal from phantom.
TR: repetition time of the ISIS sequence = 5 s.
T1b: T1 relaxation time of the lithium brain signal = 4.12 s.
T1p: T1 relaxation time of the lithium phantom signal = 0.52 s.
Sb: integral of lithium signal from the brain measured using FELIX.
Sp: integral of lithium signal from a 6 mM LiCl phantom measured using FELIX.
Vb: sampled volume of the brain measured using NVM (see below). This volume includes only brain and excludes cerebrospinal fluid (CSF) and muscle.
Vp: sampled volume of the phantom measured using NVM.
Q: loading term = Sin[((Standard with phantom/standard with brain)*18) degrees].
Image segmentation and volume calculations
Structural 4.0 T MRI scans were segmented using open-source software, NVM (freely available from Neuromorphometrics, Inc., at http://neuromor-phometrics.org:8080/nvm/), to determine gray matter, white matter, and CSF contributions to the ACC voxel of interest, and to determine the volume of brain tissue (subtracting skull and CSF) contributing to the lithium spectroscopy slab.
Serum lithium
Serum lithium levels were determined by Quest Diagnostics®, Inc.
Statistical analysis
Linear regression with a backward elimination procedure for covariate selection was used as the primary analysis method. For hypothesis-driven tests, statistical significance was defined at an alpha level of 0.05 using two-tailed tests. SPSS 11.0 for Macintosh OSX was used for all computations.
Results
Nine subjects with DSM-IV-TR bipolar I disorder, currently treated with lithium, were examined. The subjects’ ages ranged from 56 to 85 years (66.0 ± 9.7 years); there were six male subjects and three female subjects. Table 1 gives a description of the subjects’ demographics, mean lithium dose, use of extended-release lithium, and the number of other psychotropic medications they were receiving. Serum lithium levels were acquired on two occasions: on the day of their initial assessment [serum lithium level = 0.65 ± 0.07 mEq/L (n = 9)], and on the day of the MRS examination [serum lithium level = 0.71 ± 0.18 mEq/L (n = 7)]. Serum lithium levels were not available for two of the subjects from the day of their MRS examination. For these two subjects, the serum lithium level acquired on the day of their initial assessment was used in the analysis. All lithium levels were obtained as trough levels with subjects on stable lithium dosages, allowing levels across time points to be compared. The Intraclass Correlation Coefficient (ICC) (absolute agreement) for the serum levels between Day 1 and Day 2 was = 0.80 (n = 8).
Table 1.
Age, sex, DSM-IV-TR diagnosis, lithium preparation, lithium dose per day (mg), duration of lithium treatment and other medications for the subjects who participated in this study
| Age | Sex | DSM-IV-TR | Lithium preparation | Lithium dose per day (mg) |
Duration of lithium treatment (years) |
Other medications |
|---|---|---|---|---|---|---|
| 56 | Male | Bipolar I | Lithium carbonate | 1,350 | 35 | None |
| 57 | Male | Bipolar I | Lithium carbonate | 1,200 | 14 | Trazadone Clonazepam |
| 60 | Male | Bipolar I | Lithium carbonate | 1,200 | >20 | Clorazepate Quinapril Atorvastatin Ropinirole |
| 60 | Male | Bipolar I | Lithium carbonate | 600 | >30 | Lamotrigine Lisinopril Atorvastatin Quetiapine Lorazepam |
| 63 | Female | Bipolar I | Lithium carbonate | 600 | >20 | Prednisone Donepezil Metoprolol Nicotine patch Olanzapine Aspirin Azathioprine Nexium Albuterol |
| 67 | Female | Bipolar I | Lithium carbonate | 600 | 22 | Haloperidol Insulin Chlorpromazine Carbamazepine Paroxetine Clonazepam |
| 67 | Female | Bipolar I | Eskalith | 450 | >30 | Venlafaxine Olanzapine Zolpidem Bupropion Ranitidine Lisinopril Alendronate Mirtazapine |
| 79 | Male | Bipolar I | Lithium carbonate | 300 | 9 | Propranolol Allopurinol |
| 85 | Male | Bipolar I | Lithobid | 450 | 33 | Citalopram Pramipexol |
The mean metabolite levels (institutional units) and standard deviations for all the measured proton MRS metabolites are shown in Table 2. For comparison with other studies, we also include metabolite ratios to creatine. Structural 4.0 T MRI scans were segmented using open-source software to determine gray matter, white matter, and CSF contributions to the voxel of interest (see Table 2). The voxel gray matter content was used as a covariate in the statistical analysis. The brain lithium levels, serum lithium levels and the brain-to-serum ratio are also included in Table 2.
Table 2.
Mean and standard deviation for the voxel tissue content, metabolite absolute values, metabolite ratio to creatine, and the lithium measurements
| Voxel tissue content | Mean (%) |
Standard deviation (%) |
| White matter | 14.37 | 2.97 |
| Gray matter | 71.31 | 5.79 |
| Cerebrospinal fluid | 14.31 | 6.72 |
|
Metabolite absolute values |
Mean (IU) |
Standard deviation (IU) |
| N-acetyl aspartate (NAA) | 8.81 | 3.79 |
| myo-Ino (n = 8) | 5.18 | 1.50 |
|
Metabolite ratio to creatine |
Mean |
Standard deviation |
| NAA/Cr | 1.11 | 0.18 |
| myo-Ino/Cr (n = 8) | 0.72 | 0.20 |
| Lithium measurements |
Mean (mEq/L) |
Standard deviation (mEq/L) |
| Serum | 0.67 | 0.19 |
| Brain | 0.22 | 0.25 |
| Mean |
Standard deviation |
|
| Brain-to-serum ratio | 0.33 | 0.38 |
IU = Institutional Units.
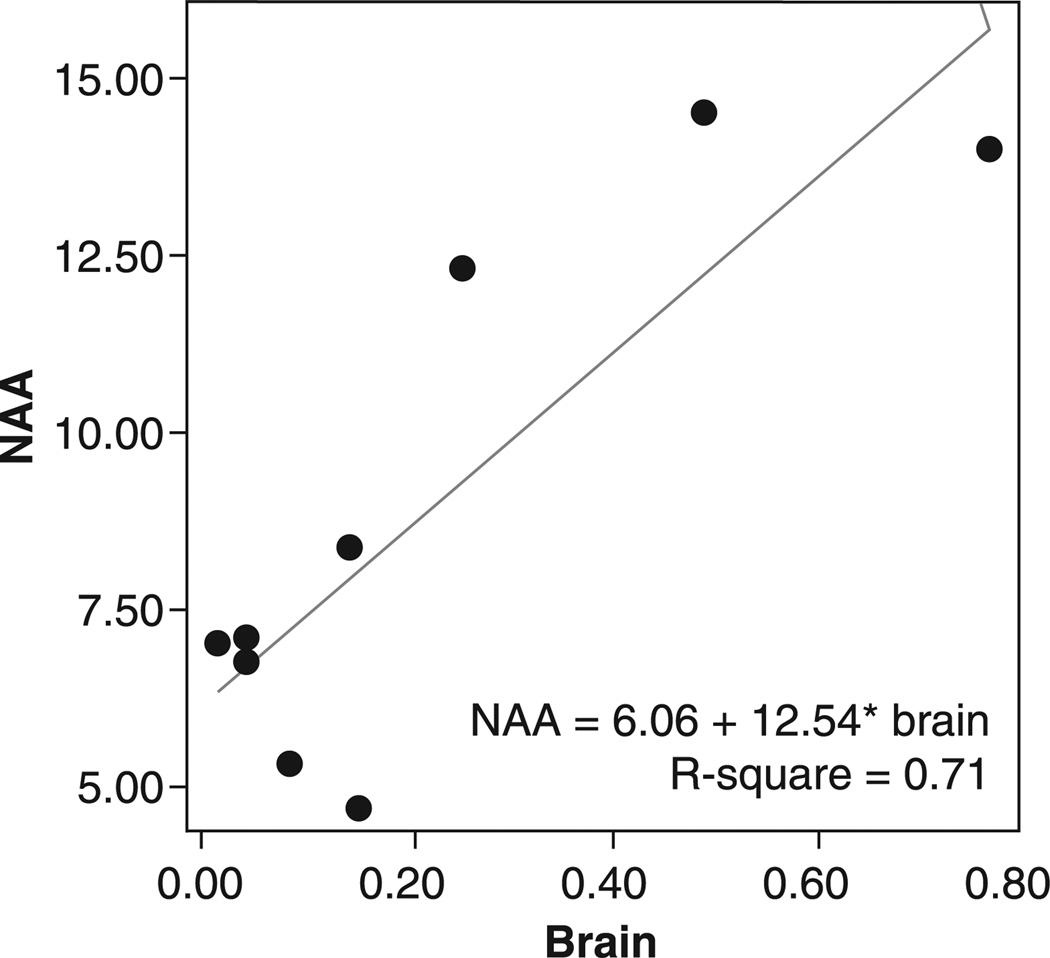
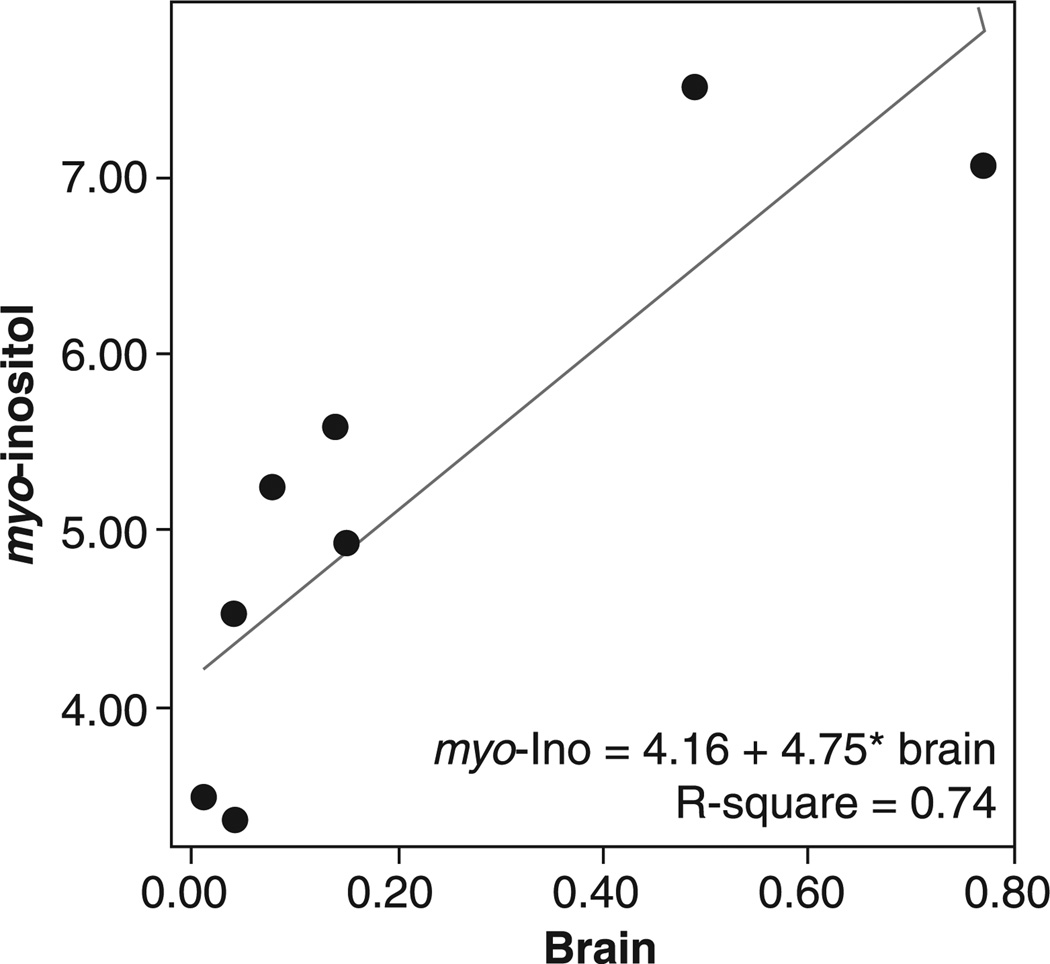
Linear regression with a backward elimination procedure for covariate selection was conducted to look at the effects of age, sex, voxel gray matter content, and brain lithium levels on the ACC NAA and myo-Ino levels. Brain lithium levels were associated with higher NAA levels [df = (1, 8), B = 12.53, t = 4.09, p < 0.005] (see Fig. 1) and higher myo-Ino levels [df = (1, 7), B = 4.75, t = 4.10, p < 0.006] (see Fig. 2).
Fig. 1.
Brain lithium levels (mEq/L) versus anterior cingulate cortex N-acetyl aspartate (NAA) in older adults with bipolar disorder.
Fig. 2.
Brain lithium levels (mEq/L) versus myo-inositol (myo-Ino) in older adults with bipolar disorder.
There was no significant correlation between the other measured metabolites [creatine + phosphocreatine (Cr), choline-containing compounds (Cho), glutamate (Glu), and glutamine (Gln)] and the brain lithium levels. There were no significant effects of serum lithium levels on any of the metabolites.
Discussion
This is the first study to demonstrate alterations in NAA and myo-Ino in a sample of older adults with BD treated with lithium. Previous studies have focused on samples of children, adolescents, and younger adults with BD and found evidence of elevated NAA levels in bipolar patients treated with lithium (21) and mixed reports of lithium’s effects on myo-Ino (22–24). Furthermore, unlike previous studies, we examined the relationship between brain lithium levels and the metabolites NAA and myo-Ino. Consistent with previous studies in adults and with our initial hypothesis, we demonstrated an association between brain lithium levels and higher NAA levels. However, contrary to our original hypothesis, we found an association between brain lithium levels and elevated levels of myo-Ino-containing compounds.
NAA and myo-Ino in proton MRS studies in bipolar disorder
There have been over 20 studies that include a total of over 300 child, adolescent, and adult subjects with BD using proton MRS to study brain metabolites such as NAA, choline, glutamate/glutamine, and myo-Ino (21). NAA levels were generally found to be lower in the frontal lobe structures and hippocampus of euthymic bipolar individuals compared with healthy control subjects. Although lithium treatment in general seemed to be associated with an increase in NAA, some studies found a decrease in NAA with lithium treatment.
In a study by Moore and Galloway (25), lithium (mean levels of 0.8 mEq/L) was shown to increase NAA levels acutely after four weeks of treatment in the brains of 14 subjects with BD and 9 HCS. There was no correlation between serum lithium levels and NAA levels. However, this result is inconsistent with a number of other MRS studies of patients with BD, pre- and post-lithium treatment, including a previous study by the same group (22, 26–28); and a study by Brambilla et al. of HCS pre- and post-lithium treatment (29). None of these studies found significant effects of lithium on NAA.
A number of studies of BD subjects who had been medicated with lithium for at least six months have shown that NAA or NAA/Cr levels were equivalent to or higher than the levels in HCS. In four studies of lithium-treated BD subjects, no significant differences were found in NAA/Cr levels compared with HCS (23, 30–32). In another study, Silverstone et al. (13) found higher NAA/Cr levels in the frontal and temporal lobes among lithium-treated BD subjects compared with HCS. More recently, Brambilla et al. (33) measured increased NAA/Cr in the left dorsolateral prefrontal cortex of lithium-treated subjects compared with subjects who were not treated with lithium, and Frye et al. (34) found decreased NAA in the ACC of 16 BD subjects compared with HCS, but only 5 were treated with lithium. Interestingly, Winsberg et al. (11) and Ohara et al. (31) both report decreased NAA/Cr associated with illness duration.
The findings from proton MRS studies investigating myo-Ino in child, adolescent, and adult BD subjects and healthy controls are less clear. Silverstone et al. (35) failed to find changes in myo-Ino/Cr in the temporal lobes of healthy adults following chronic lithium administration. However, Davanzo et al. (26) looked at the effect of lithium on myo-Ino/Cr in the ACC of youths with BD (manic phase) and found myo-Ino/Cr to be increased at baseline compared with one week of lithium therapy and compared with HCS. These results are similar to those of Moore et al. (28), who observed decreased myo-Ino levels in the frontal lobes of depressed adults with BD following acute lithium treatment. Davanzo et al. (26) also noted that the myo-Ino/Cr decreases were greater in lithium responders than non-responders. These studies lend weight to the inositol depletion hypothesis of lithium’s efficacy in BD.
Support for lithium’s neuroprotective, neurotrophic, and mitochondrial function-enhancing properties
Our findings of a relationship between higher brain lithium levels and elevated NAA levels in older adult subjects with BD support evidence of lithium ’s neuroprotective, neurotrophic and mitochondrial function-enhancing effects. We believe these results fit into a model wherein lithium inhibits GSK-3 (36), increases B-cell lymphoma/leukemia-2 gene (bcl-2) (37), which appears to inhibit necrotic and apoptotic cell death (36), and increases brain-derived neurotrophic factor (BDNF) (38) and hence NAA. Lang et al. (39) found a direct correlation between serum levels of BDNF and ACC NAA in healthy subjects. Therefore, NAA, in addition to being a marker of neuronal viability, may also be a marker for BDNF activity.
Increased gray matter volume has been demonstrated with chronic lithium treatment in patients with BD (23). Elevations in brain NAA levels, therefore, may reflect increased neuronal viability and function in bipolar subjects treated with lithium (23). Elevations in brain NAA associated with brain lithium levels suggest that lithium may also be exerting a neurotrophic effect.
In addition to a neurotrophic and neuroprotective role, lithium may also exert beneficial effects through its ability to enhance mitochondrial function. Recent work has demonstrated that lithium increases mitochondrial mass and adenosine triphosphate (ATP) production (40). NAA is synthesized in mitochondria by the membrane-bound enzyme l-aspartate N-acetyl-transferase, a catalyst that is found only in brain tissue (41). Research suggests that decreased levels of NAA may imply impaired mitochondrial energy production (42, 43) and that NAA levels are closely related to mitochondrial energy metabolism, and thus may serve as a measure of mitochondrial function (41, 44–47).
Lithium has also been shown to increase both bcl-2, an anti-apoptotic protein (37), and NAA (23). Both of these properties suggest that lithium stimulates neurogenesis (the synthesis of proteins, lipoproteins, and enzymes in the brain), which requires significant energy consumption (48). Therefore, although lithium is associated with ATP production, treatment with lithium may also paradoxically lead to decreases in ATP levels as a result of enhancing processes requiring high energy. Finally, although it is difficult to interpret cause and effect in this cross-sectional study, it is possible that the bipolar individuals studied may actually have increased mitochondrial metabolism, and hence greater NAA, and may retain more brain lithium due to enhanced transport into the cell.
Relationship of brain lithium and myo-inositol
Contrary to our original hypothesis, we found a positive association between brain lithium levels and myo-Ino. The proton MRS peak at 3.6 ppm, myo-Ino, represents inositol-containing compounds, which includes myo-Ino and I-1-P (49). However, I-1-P contributes about 1 mM, and myo-Ino contributes about 5 mM to the total peak area (50). This provides support that an increase in this resonance can be primarily attributed to an increase in myo-Ino.
Lithium was first shown to reduce cerebral concentrations of myo-Ino in the rat cerebral cortex in 1977, leading to the myo-inositol depletion hypothesis for lithium’s mechanism of action in BD (51). The inositol depletion hypothesis suggests that lithium’s efficacy in BD may be related to an inhibition of IMPase, leading to a reduction in brain myo-inositol and an increase in I-1-P (52). However, recent studies have shown that chronic lithium treatment increases IMPase activity, which would lead to an increase in myo-Ino levels (52, 53).
Another study, by Patel et al. (54), demonstrated increased myo-Ino at 42 days of lithium treatment compared with seven days of lithium treatment in depressed adolescents with BD. If lithium increased IMPase activity, the downstream consequence would be inhibition of protein kinase C (PKC) (55). Indeed, it has been shown that PKC is inhibited by lithium (56). Furthermore, PKC inhibits GSK-3 (57, 58) which may be the mechanism by which lithium upregulates BDNF and inhibits GSK-3 (38). Therefore, lithium activation of IMPase would lead to increased myo-Ino levels and an increase in BDNF, reflected by the measurement of NAA by proton MRS. Our results of higher myo-Ino levels correlating with elevated brain lithium in subjects receiving chronic lithium therapy are consistent with an increase in IMPase activity and an elevation of NAA.
Complicating the issue of the relationship between lithium and myo-Ino are conflicting findings regarding the relationship of inositol use to treatment response in bipolar adults. Although previous studies have reported a modest effect of inositol therapy for patients with treatment-resistant bipolar depression (59, 60), other studies have paradoxically shown that an inositol-deficient diet may augment the efficacy of lithium in BD (61). Our sample of older bipolar subjects had a mean 28-item HDRS score of 8.5, suggesting a mild degree of depression in this sample. The findings of a positive correlation between myo-Ino and brain lithium levels may be related to a trait marker of disease severity and may differ in euthymic bipolar adults.
Brain lithium levels in older adults with bipolar disorder
Importantly, our findings were limited to a relationship between the metabolites, NAA and myo-Ino, and brain lithium levels. We did not find a significant relationship between any of the brain metabolites measured by proton MRS and serum lithium in the periphery. Although clinicians rely on serum lithium measurements to serve as proxy indicators of brain lithium levels, there is evidence to suggest that a linear relationship between the brain and serum lithium breaks down in adults with BD beyond middle age (4). In the elderly population, the relationship between the brain and serum lithium may become more variable due to age-related changes in metabolism, body composition, and renal function that alter lithium pharmacokinetics. The effects of these age-related changes on serum lithium levels in the elderly include an extended elimination half-life and higher serum lithium levels at lower doses (62).
Furthermore, intracellular neuronal accumulation of lithium may account for lithium-related CNS toxicity even with relatively low serum lithium levels. Lithium, a naturally occurring ion handled similarly to sodium ions (Na+) in the human body (63), crosses the blood-brain barrier with a mean ratio of serum to CSF lithium of 3.6:1. In brain tissue, lithium entry is mainly through the voltage-sensitive Na+ channel (64), while the major route of lithium efflux is through the Na+– Li+ counter exchange system (64). Red blood cells (RBCs), neurons, and glia all transport lithium against an electrochemical gradient by means of Na+–Li+ counter-transport system (65, 66). There appears to be an inhibition of RBC Na+–Li+ counter-transport with long-term lithium treatment (67), leading to an increased intra- to extracellular lithium ratio. Dorus et al. (in an unpublished manuscript) considered that age-related CNS activity in the elderly might be in part related to higher intracellular lithium concentrations in RBCs (68). Thus, lithium neurotoxicity at normally nontoxic serum lithium levels may be due to excessive intracellular lithium accumulation. This effect may be exacerbated by long-term lithium treatment and age.
Limitations to this study include its small sample size, cross-sectional design, and the lack of an age-matched healthy control group or a group with BD treated with other specific therapies, such as divalproex, that would allow for comparisons of treatment-specific mechanisms of action, including the ability to adequately test the hypothesis of lithium’s effects on myo-Ino and NAA. Although likely that there is a brain lithium-level threshold above which lithium treatment is neurotoxic and below which lithium is not clinically effective, the small sample size and lack of a control group of patients with BD taking specific alternative treatments did not allow for an analysis of such a question. In addition, some of our subjects were treated with concomitant psychotropic medications in a non-systematic fashion that may have limited our ability to interpret the relationship between brain metabolite changes and brain lithium levels (See Table 2 for concomitant psychotropic medications used).
Our interpretation of the relationship between brain myo-Ino and brain lithium levels may also be compromised by the clustered distribution of measured brain lithium values (0.00–0.20 and 0.40–0.80 mEq/L; see Fig. 2). Although the absence of brain lithium data between 0.20–0.40 mEq/L limits the interpretation of our findings, we did find that linear and logarithmic curve estimations for myo-Ino versus brain lithium levels significantly fitted the data (F = 16.81, p < 0.006 and F = 34.56, p < 0.001, respectively), with both curves demonstrating myo-Ino increasing with increasing brain lithium levels.
A further limitation to this study is the lack of younger subjects with BD who also received proton MRS to examine age-related effects on brain metabolites that reflect mitochondrial function and membrane synthesis. Previous studies using phosphorus (31P) MRS have detected age-associated effects that may be related to changes in mitochondrial functioning, including elevated levels of phosphocreatine (PCr) and decreased brain pH (69, 70). Age-associated changes in brain chemistry have also been described in studies using 1H MRS (71–74), including specific findings of decreased NAA possibly reflecting impaired mitochondrial energy function (42, 43).
Future prospective controlled studies of lithium compared with alternative treatments, including divalproex or atypical antipsychotics, utilizing MRS in older and younger adults with BD, may help to clarify more specifically age-related changes in brain metabolites and mechanisms of treatment response.
In summary, we found evidence of elevated NAA and increased myo-Ino levels associated with higher brain lithium levels in the ACC of a sample of older adults with BD. This study is the first to examine the association of brain lithium levels with in vivo brain metabolites using proton MRS. It is also the first to exclusively examine an older sample of BD subjects, a group that is likely to expand with the aging of the population and about which there has been little evidence-based treatment intervention research. Furthermore, the fact that older individuals with BD are subject to age-associated cerebral atrophy and mitochondrial dysfunction makes lithium’s potential neuroprotective, neurotrophic, and mitochondrial function-enhancing effects potentially clinically appealing.
Acknowledgements
This study was funded by the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) (CMM), MH58681 (PFR), and the Clinical Investigator Training Program, Harvard/MIT Health Sciences and Technology – Beth Israel Deaconess Medical Center, in collaboration with Pfizer and Merck & Co (BF).
Footnotes
CTF, YAB, MW, and CMM have no reported conflict of interest.
References
- 1.Nambudiri DE, Meyers BS, Young RC. Delayed recovery from lithium neurotoxicity. J Geriatr Psychiatry Neurol. 1991;4:40–43. doi: 10.1177/089198879100400108. [DOI] [PubMed] [Google Scholar]
- 2.Shulman KI, Rochon P, Sykora K, et al. Changing prescription patterns for lithium and valproic acid in old age: shifting practice without evidence. Br Med J. 2003;326:960–961. doi: 10.1136/bmj.326.7396.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forester B, Streeter CC, Berlow YA, et al. Brain lithium levels and effects on cognition and mood in geriatric bipolar disorder: a lithium-7 magnetic resonance spectroscopy study. Am J Geriatr Psychiatry. 2008 doi: 10.1097/JGP.0b013e318172b3d0. [Epub July 2008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolino A, Frye M, Callicott JH, et al. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biol Psychiatry. 2003;53:906–913. doi: 10.1016/s0006-3223(02)01911-x. [DOI] [PubMed] [Google Scholar]
- 6.Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- 7.Dager SR, Friedman SD, Parow A, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 8.Deicken RF, Eliaz Y, Feiwell R, Schuff N. Increased thalamic N-acetylaspartate in male patients with familial bipolar I disorder. Psychiatry Res. 2001;106:35–45. doi: 10.1016/s0925-4927(00)00083-4. [DOI] [PubMed] [Google Scholar]
- 9.Frey BN, Folgierini M, Nicoletti M, et al. A proton magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in acute mania. Hum Psychopharmacol. 2005;20:133–139. doi: 10.1002/hup.671. [DOI] [PubMed] [Google Scholar]
- 10.Hamakawa H, Kato T, Murashita J, Kato N. Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. Eur Arch Psychiatry Clin Neurosci. 1998;248:53–58. doi: 10.1007/s004060050017. [DOI] [PubMed] [Google Scholar]
- 11.Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 12.Moore CM, Demopulos CM, Henry ME, et al. Brain-to-serum lithium ratio and age: an in vivo magnetic resonance spectroscopy study. Am J Psychiatry. 2002;159:1240–1242. doi: 10.1176/appi.ajp.159.7.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverstone PH, Wu RH, O’Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Moore CM, Breeze JL, Gruber SA, et al. Choline, myo-inositol and mood in bipolar disorder: a proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord. 2000;2:207–216. doi: 10.1034/j.1399-5618.2000.20302.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- 16.Atack JR. Inositol monophosphatase, the putative therapeutic target for lithium. Brain Res Brain Res Rev. 1996;22:183–190. [PubMed] [Google Scholar]
- 17.Haldane M, Frangou S. New insights help define the pathophysiology of bipolar affective disorder: neuroimaging and neuropathology findings. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:943–960. doi: 10.1016/j.pnpbp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR) 4th edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 19.Ordidge R, Connelly A, Lohman J. Image-selected in-vivo spectroscopy (ISIS): a new technique for spatially selective NMR spectroscopy. J Magn Reson. 1986;66:283–294. [Google Scholar]
- 20.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: A systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Friedman SD, Dager SR, Parow A, et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 2004;56:340–348. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2′s neurotrophic effects? Biol Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 24.Sharma R, Venkatasubramanian PN, Barany M, Davis JM. Proton magnetic-resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr Res. 1992;8:43–49. doi: 10.1016/0920-9964(92)90059-e. [DOI] [PubMed] [Google Scholar]
- 25.Moore GJ, Galloway MP. Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull. 2002;36:5–23. [PubMed] [Google Scholar]
- 26.Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Fujii K, Shioiri T, Inubushi T, Takahashi S. Lithium side effects in relation to brain lithium concentration measured by lithium-7 magnetic resonance spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:87–97. doi: 10.1016/0278-5846(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 28.Moore GJ, Bebchuk JM, Parrish JK, et al. Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am J Psychiatry. 1999;156:1902–1908. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]
- 29.Brambilla P, Stanley JA, Sassi RB, et al. 1H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology. 2004;29:1918–1924. doi: 10.1038/sj.npp.1300520. [DOI] [PubMed] [Google Scholar]
- 30.Stoll AL, Renshaw PF, Sachs GS, et al. The human brain resonance of choline-containing compounds is similar in patients receiving lithium treatment and controls: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 1992;32:944–949. doi: 10.1016/0006-3223(92)90184-2. [DOI] [PubMed] [Google Scholar]
- 31.Ohara K, Isoda H, Suzuki Y, et al. Proton magnetic resonance spectroscopy of the lenticular nuclei in bipolar I affective disorder. Psychiatry Res. 1998;84:55–60. doi: 10.1016/s0925-4927(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 32.Amaral JA, Tamada RS, Issler CK, et al. A 1HMRS study of the anterior cingulate gyrus in euthymic bipolar patients. Hum Psychopharmacol. 2006;21:215–220. doi: 10.1002/hup.761. [DOI] [PubMed] [Google Scholar]
- 33.Brambilla P, Stanley JA, Nicoletti MA, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86:61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Frye MA, Watzl J, Banakar S, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 35.Silverstone PH, Hanstock CC, Fabian J, Staab R, Allen PS. Chronic lithium does not alter human myo-inositol or phosphomonoester concentrations as measured by 1H and 31P MRS. Biol Psychiatry. 1996;40:235–246. doi: 10.1016/0006-3223(95)00382-7. [DOI] [PubMed] [Google Scholar]
- 36.Manji HK, McNamara R, Chen G, Lenox RH. Signalling pathways in the brain: cellular transduction of mood stabilisation in the treatment of manic-depressive illness. Aust N Z J Psychiatry. 1999;33(Suppl.):S65–S83. doi: 10.1111/j.1440-1614.1999.00670.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Zeng WZ, Yuan PX, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002099. Epub Oct. 9, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry. 2007;62:530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Struewing IT, Barnett CD, Tang T, Mao CD. Lithium increases PGC-1 alpha expression and mitochondrial biogenesis in primary bovine aortic endothelial cells. FEBS J. 2007;274:2749–2765. doi: 10.1111/j.1742-4658.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- 41.Truckenmiller ME, Namboodiri MA, Brownstein MJ, Neale JH. N-acetylation of l-aspartate in the nervous system: differential distribution of a specific enzyme. J Neurochem. 1985;45:1658–1662. doi: 10.1111/j.1471-4159.1985.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 42.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- 43.De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- 44.Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology. 1993;43:2484–2490. doi: 10.1212/wnl.43.12.2484. [DOI] [PubMed] [Google Scholar]
- 45.Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, Vagnozzi R. N-acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J Neurotrauma. 2001;18:977–991. doi: 10.1089/08977150152693683. [DOI] [PubMed] [Google Scholar]
- 46.Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 47.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Li CQ, Shen J. In vivo detection of cortical GABA turnover from intravenously infused [1–13C]D-glucose. Magn Reson Med. 2005;53:1258–1267. doi: 10.1002/mrm.20473. [DOI] [PubMed] [Google Scholar]
- 49.Cerdan S, Hansen CA, Johanson R, Inubushi T, Williamson JR. Nuclear magnetic resonance spectroscopic analysis of myo-inositol phosphates including inositol 1,3,4,5-tetrakisphosphate. J Biol Chem. 1986;261:14676–14680. [PubMed] [Google Scholar]
- 50.Ross BD. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR Biomed. 1991;4:59–63. doi: 10.1002/nbm.1940040205. [DOI] [PubMed] [Google Scholar]
- 51.Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- 52.Kaya N, Resmi H, Ozerdem A, Guner G, Tunca Z. Increased inositol-monophosphatase activity by lithium treatment in bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:521–527. doi: 10.1016/j.pnpbp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Parthasarathy LK, Seelan RS, Wilson MA, Vadnal RE, Parthasarathy RN. Regional changes in rat brain inositol monophosphatase 1 (IMPase 1) activity with chronic lithium treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:55–60. doi: 10.1016/s0278-5846(02)00315-9. [DOI] [PubMed] [Google Scholar]
- 54.Patel NC, DelBello MP, Cecil KM, et al. Lithium treatment effects on myo-inositol in adolescents with bipolar depression. Biol Psychiatry. 2006;60:998–1004. doi: 10.1016/j.biopsych.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manji HK, Lenox RH. Long-term action of lithium: a role for transcriptional and posttranscriptional factors regulated by protein kinase C. Synapse. 1994;16:11–28. doi: 10.1002/syn.890160103. [DOI] [PubMed] [Google Scholar]
- 56.Hahn CG, Umapathy C, Wang HY, Koneru R, Levinson DF, Friedman E. Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39:355–363. doi: 10.1016/j.jpsychires.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- 58.Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59:1160–1171. doi: 10.1016/j.biopsych.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Nierenberg AA, Ostacher MJ, Calabrese JR, et al. Treatment-resistant bipolar depression: A STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163:210–216. doi: 10.1176/appi.ajp.163.2.210. [DOI] [PubMed] [Google Scholar]
- 60.Chengappa KN, Levine J, Gershon S, et al. Inositol as an add-on treatment for bipolar depression. Bipolar Disord. 2000;2:47–55. doi: 10.1034/j.1399-5618.2000.020107.x. [DOI] [PubMed] [Google Scholar]
- 61.Shaldubina A, Stahl Z, Furszpan M, et al. Inositol deficiency diet and lithium effects. Bipolar Disord. 2006;8:152–159. doi: 10.1111/j.1399-5618.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 62.Sajatovic M. Treatment of bipolar disorder in older adults. Int J Geriatr Psychiatry. 2002;17:865–873. doi: 10.1002/gps.719. [DOI] [PubMed] [Google Scholar]
- 63.Sproule BA, Hardy BG, Shulman KI. Differential pharmacokinetics of lithium in elderly patients. Drugs Aging. 2000;16:165–177. doi: 10.2165/00002512-200016030-00002. [DOI] [PubMed] [Google Scholar]
- 64.El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9:23–32. doi: 10.1080/10673220127873. [DOI] [PubMed] [Google Scholar]
- 65.Janka Z, Szentistvanyi I, Juhasz A, Rimanoczy A. Difference in lithium transport between neurones and glia in primary culture. Neuropharmacology. 1980;19:827–830. doi: 10.1016/0028-3908(80)90078-7. [DOI] [PubMed] [Google Scholar]
- 66.Pandey GN, Dorus E, Davis JM, Tosteson DC. Lithium transport in human red blood cells: genetic and clinical aspects. Arch Gen Psychiatry. 1979;36:902–908. doi: 10.1001/archpsyc.1979.01780080076018. [DOI] [PubMed] [Google Scholar]
- 67.Renshaw PF, Yurgelun-Todd DA, Tohen M, Gruber S, Cohen BM. Temporal lobe proton magnetic resonance spectroscopy of patients with first-episode psychosis. Am J Psychiatry. 1995;152:444–446. doi: 10.1176/ajp.152.3.444. [DOI] [PubMed] [Google Scholar]
- 68.Hicks R, Dysken MW, Davis JM, Lesser J, Ripeckyj A, Lazarus L. The pharmacokinetics of psychotropic medication in the elderly: a review. J Clin Psychiatry. 1981;42:374–385. [PubMed] [Google Scholar]
- 69.Rae C, Scott RB, Lee M, et al. Brain bioenergetics and cognitive ability. Dev Neurosci. 2003;25:324–331. doi: 10.1159/000073509. [DOI] [PubMed] [Google Scholar]
- 70.Murashita J, Kato T, Shioiri T, Inubushi T, Kato N. Age-dependent alteration of metabolic response to photic stimulation in the human brain measured by 31P MR-spectroscopy. Brain Res. 1999;818:72–76. doi: 10.1016/s0006-8993(98)01285-2. [DOI] [PubMed] [Google Scholar]
- 71.Moreno-Torres A, Pujol J, Soriano-Mas C, Deus J, Iranzo A, Santamaria J. Age-related metabolic changes in the upper brainstem tegmentum by MR spectroscopy. Neurobiol Aging. 2005;26:1051–1059. doi: 10.1016/j.neurobiolaging.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Scylloinositol in normal aging human brain: 1H magnetic resonance spectroscopy study at 4 Tesla. NMR Biomed. 2005;18:51–55. doi: 10.1002/nbm.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kadota T, Horinouchi T, Kuroda C. Development and aging of the cerebrum: assessment with proton MR spectroscopy. AJNR Am J Neuroradiol. 2001;22:128–135. [PMC free article] [PubMed] [Google Scholar]
- 74.Sijens PE, den Heijer T, Origgi D, et al. Brain changes with aging: MR spectroscopy at supraventricular plane shows differences between women and men. Radiology. 2003;226:889–896. doi: 10.1148/radiol.2263011937. [DOI] [PubMed] [Google Scholar]