Abstract
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominate cancer syndrome that leads to an increased risk of developing invasive diffuse type (signet ring cell) gastric carcinoma. Approximately 30% of HDGC cases are caused by a germline mutation involving the E-cadherin (CDH1) gene. Those with the CDH1 mutation have an 80% and 60% cumulative lifetime risk of developing diffuse type gastric carcinoma and lobular breast carcinoma respectively. Due to the focal nature of early diffuse type gastric carcinoma, identifying early lesions with surveillance endoscopy is limited. As a result, elective risk-reducing total gastrectomy is currently recommended. In this report, the clinical, intraoperative, and pathologic work-up is reviewed regarding a patient with known CDH1 germline mutation.
Introduction
Gastric cancer is the second leading cause of cancer death world-wide with nearly one million new diagnoses per year. More than three-fourths of those individuals die from their disease.1 In Hawai‘i, gastric cancer is the fifth leading cause of cancer-related death as reported by the Hawai‘i Tumor Registry. The majority of gastric cancers are intestinal type adenocarcinomas that have been linked to Helicobacter pylori colonization and diet.2 However, 1%–3% of all gastric cancers are attributed to hereditary diffuse gastric cancer (HDGC).3 HDGC is an uncommon autosomal dominant form of diffuse type gastric carcinoma that generally presents at an early age with advanced stage and poor prognosis.4
The first indication of a genetic link to certain diffuse-type gastric cancers was reported in 1998 when Guilford and colleagues discovered 3 germline truncating mutations in the E-cadherin (CDH-1) gene within a large New Zealand family of Maori ethnicity.4 Following this discovery, other germline CDH1 mutations have been identified in patients with HDGC from a wide range of ethnic backgrounds, including but not limited to Filipino, African American, Korean, Japanese, and European.5–8 Mutations in the CDH1 gene result in its loss of expression that leads to defective intercellular adhesion (Figure 1). The Gastric Cancer Linkage Consortium currently specifies two criteria for the diagnosis of HDGC (Table 1).9,10,16 Of those individuals meeting either of these two criteria, approximately 25%–30% will demonstrate the CDH1 germline mutation.11 The mechanism of disease in the remaining HDGC cases is largely unknown.
Figure 1.
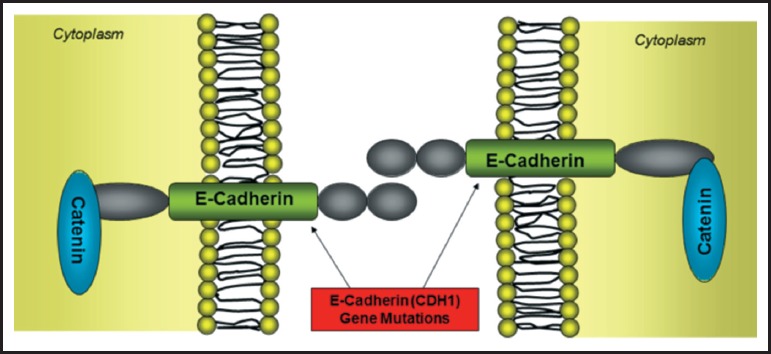
Germline mutations within the epithelial cadherin (CDH1) gene results in loss of expression and ultimately defective intercellular adhesions between cells.
Table 1.
The Gastric Linkage Consortium Criteria for Hereditary Diffuse Gastric Cancer requires Criteria 1 or Criteria 2 to be met before the diagnosis of HDGC.
| Gastric Cancer Linkage Consortium Criteria for Hereditary Diffuse Gastric Cancer9,10,16 | |
| Criteria 1 | Two or more documented cases of diffuse gastric cancer in first or second degree relatives, with at least one diagnosed before the age of 50 |
| Criteria 2 | Three or more cases of documented diffuse gastric cancer in first/second degree relatives, independent of age of onset |
Once an individual meets the criteria for HDGC, it is recommended that the patient and at-risk family members be tested for the CDH1 germline mutation. Those with the mutation will carry an 80% risk of developing diffuse type gastric carcinoma by 80 years of age.11 In addition, female patients carry an additional 60% lifetime risk of developing lobular breast carcinoma by 80 years of age.12 Many of these individuals undergo annual endoscopic evaluation. However, the sensitivity of endoscopic biopsies has been called into question owing to the focal nature of early invasive/in-situ gastric carcinoma.3 In addition, there is conflicting data as to the predominant site of involvement, proximal versus distal, which may further limit the sensitivity of screening endoscopic biopsies.13
Pathologic handling of the total gastrectomy specimen consists of submitting the entire specimen for routine histologic examination. Traditionally only hematoxylin and eosin staining has been used. However, periodic acid-schiff (PAS) staining has demonstrated improved detection of invasive and in situ gastric carcinoma.11,12 Few studies have demonstrated the complete mapping of the total gastrectomy specimen using PAS staining. Therefore, this institution's experience using this technique is described.
Case Report
A 22-year-old woman presented for genetic work-up due to strong family history of early onset diffuse gastric carcinoma. The patient's mother was diagnosed with stage IV diffuse type gastric cancer at the age of 41 despite having negative endoscopic gastric biopsies the year prior (Figure 2). In addition, the patient's aunt died of gastric cancer in her early 40's and maternal grandfather died of gastric cancer in his 50's (Figure 2). As a result of her strong family history, the patient underwent CDH1 germline testing that demonstrated a deleterious trp20stop CDH1 germline mutation. She subsequently underwent three screening endoscopic gastric biopsy evaluations that were negative for malignancy or dysplasia. It was the patient's decision then to undergo a prophylactic total gastrectomy.
Figure 2.
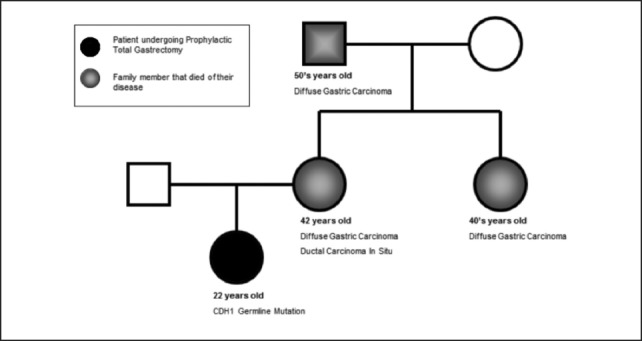
Patient's pedigree showing strong family history of diffuse gastric carcinoma.
Pathology
Intraoperative consult was obtained to ensure that margins were free of gastric mucosa. This was done by taking sections of the entire proximal and distal margins and embedding en face. Once negative margins for gastric mucosa were obtained, the specimen was opened along the greater curvature, pinned to a cork board, and allowed to fix in formalin overnight. No lesions were identified grossly (Figure 3). The entire specimen was mapped using a photograph with superimposed graph (Figure 4) and submitted for histologic examination in 225 cassettes. Each section was stained with PAS as recommended by current guidelines.11,12
Figure 3.
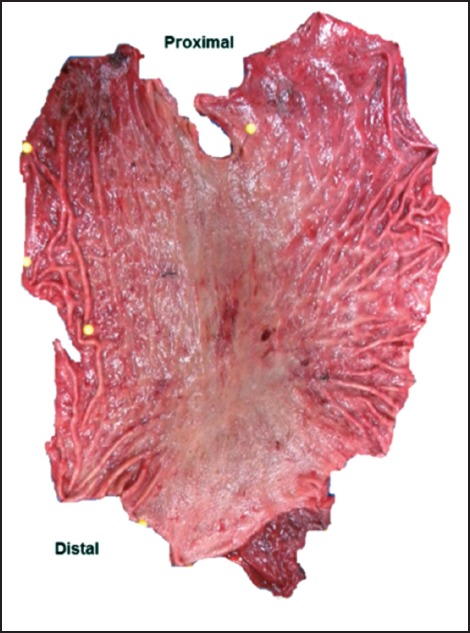
Gross total gastrectomy specimen opened along the greater curvature with no identifiable lesions.
Figure 4.
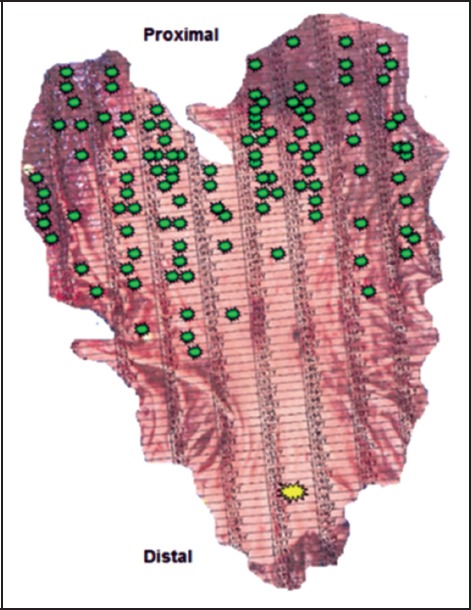
Total gastrectomy specimen with grid used for mapping. Green indicates foci of invasive or in-situ diffuse-type gastric carcinoma. Yellow indicates a single focus of invasive diffuse-type gastric carcinoma in the distal half of the stomach.
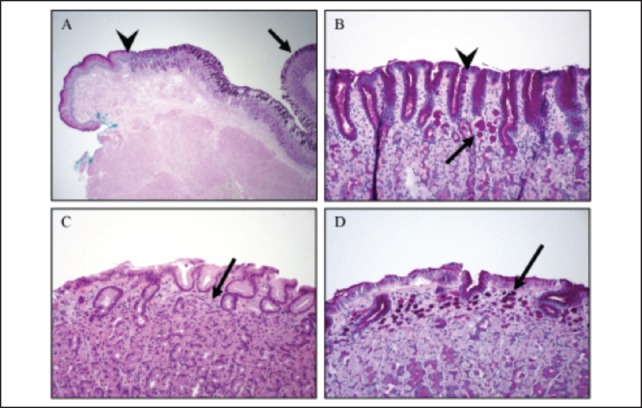
Histologic examination revealed 109 foci of invasive diffuse type (signet ring cell) gastric carcinoma and 6 foci of in situ (including pagetoid spread) diffuse type gastric carcinoma. The size of invasive foci ranged from single cells to 1.5 mm in greatest dimension (Figure 5). They were predominantly seen within the proximal two-thirds of the stomach with a single focus in the distal one-third (Figure 4). The invasive component was limited to the superficial lamina propria and no perineural or lymph-vascular invasion was identified. Fifteen regional lymph nodes were also examined that were negative for metastasis.
Figure 5.
Discussion
The world-wide incidence of sporadic gastric cancers has been decreasing over recent years.1 One hypothesis for the decrease in sporadic carcinoma is increased recognition and treatment of Helicobacter pylori infection. While the incidence of sporadic gastric carcinoma appears to be decreasing there has been increased awareness of non-sporadic gastric carcinomas such as hereditary diffuse gastric carcinoma.
This institution received the risk-reducing total gastrectomy specimen from a patient known to harbor the CDH1 germline mutation. As has been reported by Rogers and colleagues, when dealing with prophylactic total gastrectomy specimens there is commonly no gross evidence of disease.13 It is therefore necessary to sample the entire specimen in order to detect microscopic foci of invasive and/or in situ tumor.
It was found that carefully graphing a photograph of the pinned out gastrectomy was the most efficient means of correlating the gross with microscopic findings. Two hundred and twenty-five slides were examined that revealed 109 and 6 foci of invasive and in situ diffuse-type gastric (signet ring cell) carcinoma, respectively. The 6 small foci of in-situ diffuse-type carcinoma (Figure 5) are characteristic of HDGC as described by Oliveira and colleagues.14 Moreover, it is this institution's experience that the use of PAS staining was helpful in identifying these small tumor foci. This technique as outlined by Lee and colleagues allows for the detection of invasive and in situ components with increased sensitivity as compared to routine hematoxylin and eosin sections.12 The average number of foci identified in gastrectomy specimens as seen in prior reports was 10.9 using H&E alone, compared to the 115 foci found in this case using PAS.13
In addition, this case showed tumor burden was concentrated within the proximal stomach. A review of the literature shows this is in concordance with Rogers and colleagues.13 However, Charlton and colleagues reported two out of six cases with a distal stomach predominance.15 This suggests variability may exist in regards to the predominant location of early CDH1 diffuse gastric carcinoma. In addition to this variability, multiple foci of tumor may be identified throughout the entire specimen as highlighted in this case by a single focus of invasive diffuse gastric carcinoma within the distal stomach (Figure 4). Such variability reiterates the importance of submitting the entire gastrectomy specimen for histologic examination.
Conclusion
Recognition of a strong family history of diffuse type gastric carcinoma and/or lobular breast carcinoma is critical for identifying those patients at risk for HDGC. Individuals at risk can be tested for the germline mutation of the epithelial cadherin (CDH1) gene and those with a positive test should be offered prophylactic/risk-reducing total gastrectomy.11 Lastly, it is this institution's experience that surveillance endoscopy has limited sensitivity in identifying occult diffuse gastric carcinoma in patients with CDH1 germline mutations.
Disclaimer
The findings and conclusions of this study do not necessarily represent the views of the Queen's Medical Center.
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Epplein M, Nomura A, et al. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control. 2008;19:869–877. doi: 10.1007/s10552-008-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira C, Seruca R, et al. Hereditary gastric cancer. Best Pract Res Clin Gastroenterol. 2009;23(2):147–157. doi: 10.1016/j.bpg.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Guilford P, Hopkins J, Harraway J, MeLeod M, McLeod N, Harwira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 5.Shinmura K, Kohno T, Takahashi M, et al. Familial gastric cancer: clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20:1127–1131. doi: 10.1093/carcin/20.6.1127. [DOI] [PubMed] [Google Scholar]
- 6.Yoon KA, Ku JL, Yang HK, et al. Germline mutations of E-cadherin gene in Korean familial gastric cancer patients. J Hum Genet. 1999;44:177–180. doi: 10.1007/s100380050137. [DOI] [PubMed] [Google Scholar]
- 7.Gayther SA, Gorringe KL, Ramus SJ, et al. Identification of germline E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998;58:4086–4089. [PubMed] [Google Scholar]
- 8.Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–2372. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 9.Caldas C, Carneiro F, Lynch HT, Yokota J, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet. 1999;36:873e80. [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira C, Bordin MC, Grehan N, Huntsman D, et al. Screening E-cadherin in gastric cancer families reveals germ-line mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19:510e17. doi: 10.1002/humu.10068. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AF, Rees H, Owen DA, et al. Periodic acid-schiff is superior to hematoxylin and eosin for screening prophylactic gastrectomies from CDH1 mutation carriers. AM J Surg Pathol. 2012;34:1007–1013. doi: 10.1097/PAS.0b013e3181e28985. [DOI] [PubMed] [Google Scholar]
- 13.Rogers WM, Dobo E, Norton JA, et al. Risk-reducing total gastrectomy for germline mutations in E-cadherin (CDH1): pathologic findings with clinical implications. Am J Surg Pathol. 2008;32:799–809. doi: 10.1097/PAS.0b013e31815e7f1a. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira C, Moreira H, Seruca R, et al. Role of pathology in the identification of hereditary diffuse gastric cancer: report of a Portuguese family. Virchows Arch. 2005;446:181–184. doi: 10.1007/s00428-004-1156-4. [DOI] [PubMed] [Google Scholar]
- 15.Charlton A, Blair V, Shaw D, et al. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53:814–820. doi: 10.1136/gut.2002.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JG, Yang HK, Kim WH, et al. Report on the first meeting of the International Collaborative Group on Hereditary Gastric Cancer. J Natl Cancer Inst. 2000;92:1781. doi: 10.1093/jnci/92.21.1781. [DOI] [PubMed] [Google Scholar]


