Abstract
Gerbillus nanus Blanford, 1875 known as Baluchistan gerbil, is a granivorous solitary naked-footed species. No evidence of its natural infection with the protozoan parasite, Leishmania, has so far been provided. Cutaneous leishmaniasis (CL) is a major public health problem in many parts of the world, including Iran. The annual nationwide incidence of human CL due to Leishmania major (CLM) in endemic rural areas was above 18 000 cases in 2008. The detection of L. major in rodents is of fundamental importance for incriminating them as potential reservoirs of CLM infection. Between April 2007 and April 2008, following detection of 245 clinical cases in Jask region of south-east Iran, wild rodents were captured and checked by the microscopic slide smears for leishmanial infections. Overall, 106 gerbilline rodents were captured from which 17 were identified as Gerbillus nanus. Females of Meriones hurrianae, Tatera indica and G. nanus were found to be naturally infected with L. major. The presence of these parasites in G. nanus has never been reported before. All the amastigote-infected rodents came from the eastern plain of this region, except one T. indica from the western plain which was found to be smear-positive or kinetoplast DNA-positive by PCR. The highest (11.8%) prevalence of infection among rodents confirmed by PCR to be infected with L. major was attributed to Baluchistan gerbil, G. nanus, which is thus incriminated as a potential reservoir host of L. major in Iran.
INTRODUCTION
Rodents are one of the most important small mammalian groups which may carry a wide variety of pathogens responsible for many tropical zoonotic diseases including those caused by the trypanosomatid parasitic protozoa, Leishmania (Ashford, 2000). In the Old World where Leishmania exists, it is transmitted by sand flies of the genus Phlebotomus (Azizi et al., 2010). Those rodents which belong to the subfamily Gerbillinae, though not exclusively, are the principal reservoir hosts of cutaneous leishmaniasis due to Leishmania major (CLM) in endemic parts of Iran (Moemenbellah–Fard et al., 2003).
The genus Gerbillus Desmarest, 1804 is one of the most diversified plurispecific taxa of granivorous rodents inhabiting arid and semi-arid areas. It is widely dispersed in southern, central, and eastern provinces of Iran. Its role in the eco-epidemiology of CLM in Iran has not been clarified. It needs to be reassessed in the light of more recent observations to help in planning control measures. Five species of Gerbillus (G. nanus, G. aquilus, G. mesopotamiae, G. cheesmani and G. henleyi) have so far been described from Iran (Siahsarvie and Darvish, 2007).
Baluchistan gerbil, G. nanus Blanford, 1875 is a polytypic naked-footed species which has previously been recorded from the Konarak Village in the most southern part of the Sistan-Baluchistan Province of Iran. Misonne (1959) recorded Baluchistan gerbil from south-east and south Iran up to Bandar Abbas, the capital city of Hormozgan Province. It is a solitary, nocturnal, desert gerbil. The aim of this study was to identify the role of G. nanus as a potential reservoir host of L. major parasites from a new non-urban focus of CLM in the Jask region on the northern coast of Oman Sea. This is the first report after five decades on detection of G. nanus in a region that lies within its previously defined Iranian boundaries. This is also the first report on microscopic and molecular detection of the parasite, L. major, within naturally-infected G. nanus, thus raising the number of confirmed CLM reservoir hosts to eight species in six different genera of murid rodents in Iran.
ANIMALS AND METHODS
Study Area
The Jask region (≈154 km2) is a wide aeolic plain on the northern coastline of Oman Sea (25°38′N, 57°46′E, at an altitude of 4.8 m above sea level; Fig. 1), situated on the southernmost part of Iranian territories at an ecotone between sand dunes to the south and loess plains leading to the rocky mountains in the north. A roughly 30 km littoral plain belt leads to hilly regions. It is characterized by long dry summers and has a hot humid climate over most of its arid and semi-arid regions with sandy hills. This region is mainly covered with Tamarix tetrandra (Violales: Tamaricaceae) plants which grow in salt marshes near the seasonal drainage ditches. Other halophytic plant communities include: Prosopis juliflora, Salvadora persica, Glycyrrhiza glabra and Ziziphus spinichristi. Its mean annual temperature is 27°C, and its mean annual precipitation is 125 mm. This study was conducted from April 2007 to April 2008, around seven villages (the eastern Gohert, Surak and Lirdaf, the central Gowan, the western Kangan, Negar and Old Jask) in the rural district of Jask.
Fig. 1.
Map of Iran, showing the locations of Hormozgan Province and the district of Jask.
Collection, Identification and Examination of Rodents
Colonies of gerbilline rodents were located on the periphery (1.5 km away) of the selected villages. Sample collections were performed in spring (April–June, 2007), autumn (October–December, 2007) and winter (January–March, 2008) in the Jask district. All colonies of wild gerbils were checked to find the most active burrows characterized by the external activity marks (fresh food remains and faeces, footprints, loose soil on burrow pores). Rodents were captured once every month using 20 standard Sherman live traps (30×15×15 cm wire mesh cage traps) placed next to burrow holes, and baited with a mixture of dates, cucumbers or millet seeds. The traps were set after sunset and checked for gerbils early every morning. Each trap set on one night was considered a ‘trap-night’ and there were 20 ‘trap-nights’ per month.
The trapped gerbils were transferred to the laboratory at Bandar Abbas School of Health (HUMS), Hormozgan, Iran. They were anaesthetized using chloroform, identified to species by morphological features using valid taxonomic keys (Wilson and Reeder, 2005) and tagged after registration. The captures and subsequent handling of rodents were conducted under the permission of the Natural Resources Authority, Hormozgan Environmental Protection Organization. Their internal organs (liver and spleen) were removed and fixed in 70% ethanol for preparing smears.
Duplicate impression smears were prepared from the ear pinnae, tail base, liver, spleen and any patent skin lesions of every gerbil (Edrissian et al., 1982), air-dried, Giemsa-stained and examined under a compound light microscope at ×1000 for detection of Leishmania amastigotes. Furthermore, the kinetoplast DNA (kDNA) of each impression smear was extracted as described elsewhere (Azizi et al., 2008).
PCR Protocol
The variable segment on minicircles of kinetoplast DNA from leishmanial parasites was amplified using nested PCR. The forward primer LINR4 (5′-GGG GTT GGT GTA AAA TAG GG-3′) was used in both stages, while the first stage reverse primer LIN17 (5′-TTT GAA CGG GAT TTC TG-3′) and the second stage reverse primer LIN19 (5′-CAG AAC GCC CCT ACC CG-3′) were involved in a nested PCR method. Reference strains of L. major (MHOM/IR/54/LV39) and L. tropica (MHOM/IR/89/ARD2) were used as standards. The first-round reaction mixture contained 250 μM deoxynucleoside triphosphate (dNTP), 1.5 μM MgCl2, 1 U Taq polymerase, 1 μM LINR4, 1 μM LIN17 and 5 μl DNA extract in ×1PCR buffer in a final volume of 25 μl. This mixture was incubated in a CG1-96 thermocycler set to run for 5 minutes at 94°C, followed by 30 cycles each of 30 seconds at 95°C, 1 minute at 52°C, 1 minute at 72°C and a final extension at 72°C for 10 minutes and kept at 4°C. The first-round product (2 μl of a 4∶1 dilution in ddH2O) was used as template for the second round, in a total volume of 20 μl and under similar conditions to those for the first round but using LINR4 and LIN19 as the primers in 33 cycles.
Agarose Gel Electrophoresis
A 5 μl sample of each second-round PCR product was subjected to electrophoresis in 1.5% agarose gel. The DNA bands were stained with 1% ethidium bromide, visualized on a UV transilluminator, compared with molecular-weight markers and the corresponding second-round products for the L. major and L. tropica standards.
RESULTS
During the study period, 106 individual desert rodent species were caught, 51% of which were male and 49% female (Table 1). Five different gerbilline rodent species or three distinct genera were caught and morphologically identified. Baluchistan gerbil (Gerbillus nanus), a nocturnal desert rodent which solely breeds in salt marshes, ranked as the fourth most abundant species caught (16%). All G. nanus specimens were captured in the eastern plain.
Table 1. The types and numbers of male (♂) and female (♀) rodents caught and found infected with L. major, using light microscopy (LM) or polymerase chain reaction (PCR), in each of the seven study villages.
| Species of rodent | No. of rodents found infected/no. caught in | No. or % of rodents | No. and (%) of infected cases | ||||||
| Eastern plain | Central plain | Western plain | ♂ | ♀ | %Total | LM | PCR | All | |
| M. persicus | 0/1 | 0/0 | 0/28 | 12 | 17 | 27.4 | 0 (0) | 0 (0) | 0/29 |
| T. indica | 0/0 | 0/2 | 1/25 | 13 | 14 | 25.5 | 1♀ (3.7) | 1♀ (3.7) | 1/27 |
| M. hurrianae | 2/24 | 0/0 | 0/2 | 14 | 12 | 24.5 | 1♀ (3.8) | 2♀ (7.7) | 2/26 |
| M. libycus | 0/0 | 0/0 | 0/7 | 5 | 2 | 6.6 | 0 (0) | 0 (0) | 0/7 |
| G. nanus | 2/17 | 0/0 | 0/0 | 10 | 7 | 16 | 1♂ (5.9) | 2♂,♀ (11.8) | 2/17 |
| Any | 4/42 | 0/2 | 1/62 | 54 | 52 | 100 | 3 (2.8) | 5 (4.7) | 5/106 |
A female-biased parasitism with L. major was evident, since infected specimens of Meriones hurrianae, Tatera indica and one G. nanus belonged to female rodents. Those of G. nanus were a mix of male and female and came from two of the three infected study villages. The ratio of male-to-female positivity for all infected rodents was 1–4. The highest prevalence of infection (11.8%) among various rodents confirmed molecularly to be infected with L. major was attributed to Baluchistan gerbil, G. nanus. Most (82%) of the L. major-infected rodents came from eastern plain of the Jask region. Amastigotes were found in each of the duplicate impression smears from each infected gerbilline rodents.
The percentage probabilities of finding any uninfected (i.e. free from L. major) rodents caught in three infected villages, assuming that the prevalence of detectable infection was the same in each rodent species, were also calculated (Table 2). The least probable (3.7%) rodent species being free from leishmanial infections in this region was M. hurrianae, while the most probable (37%) Leishmania-free rodent species was M. libycus. In other words, the most probable main reservoir host of L. major among rodents in the studied area is M. hurrianae.
Table 2. The probabilities (%) of not finding any infected rodents collected from three villages, assuming that the prevalence of detectable infection in each species was the same.
| Probability (%) | ||||
| Species | Surak | Lirdaf | Negar | All villages |
| M. persicus | … | 85 | 27 | 23 |
| T. indica | … | … | 27 | 27 |
| M. hurrianae | 23 | 16 | … | 3.7 |
| M. libycus | … | … | 37 | 37 |
| G. nanus | 37 | 27 | … | 10 |
Overall, 10 smears — six found amastigote-positive and four negative by microscopy — were confirmed positive for leishmanial DNA by PCR. No other smears were PCR-positive. For each PCR-positive sample, the second round products of the nested PCR were identical to those of the L. major reference strain with a main band of 560 bp and distinct from those of the L. tropica standard with a main band of 750 bp (Fig. 2).
Fig. 2.
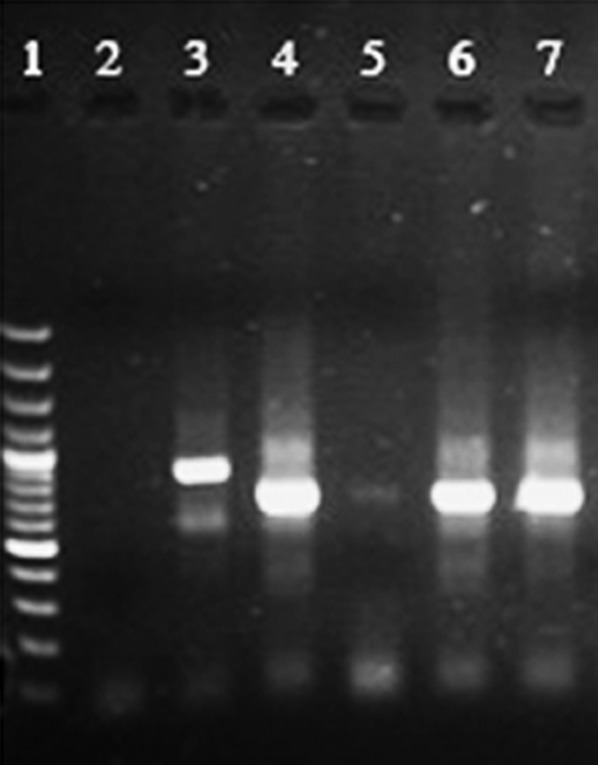
The results of the PCR-based amplification of kinetoplast DNA recovered from liver and spleen smear samples of Gerbillus nanus (lanes 5–7), or from reference samples of Leishmania tropica (lane 3) or L. major (lane 4), a negative control (lane 2) and molecular weight marker (lane1) were run to check for identity.
DISCUSSION
Outbreaks of human cutaneous leishmaniasis due to Leishmania major (CLM) impose particularly serious burden of morbidity on people in rural areas of Iran. The incidences of clinical CLM cases in Bandar Jask were 74.3 and 81.7/10 000 in 2006 and 2007, respectively (unpubl. obs.). The distribution of CLM in the Jask region is unstable. These local outbreaks prompted us to consider the regional pathogenic landscape topography and the patchy dispersion of potential infectious reservoir hosts. The infection could spread to other similar nearby regions. The studies carried out in 2007 and 2008 in Jask region revealed that although the number of different rodent species caught from the western plain was approximately 1.5 times that of the eastern plain, the number of L. major-positive rodents was fourfold in the latter plain; since the latter ecotope lies next to the Sistan-Baluchistan Province bordering Pakistan’s littoral region.
Numerous species of rodents have so far been reported from Iran. Some 70 species were caught by Edrissian et al. (1975), while 65 species of rodents were cited by Darvish (2005). In the present surveillance of the rodents’ fauna, the specimens caught were distributed among five wild (Meriones persicus, M. libycus, M. hurrianae, Tatera indica and Gerbillus nanus) rodent species. No commensal rodent species were captured because samplings were carried out on the outskirts of the study villages. So far, only eight species (Rhombomys opimus, Nesokia indica and Rattus norvegicus as well as all of the five forenamed species presented in this study) of rodents are thus reported by different authors to be infected with L. major in this country (Yaghoobi–Ershadi et al., 1996; Pourmohammadi et al., 2008; Emami et al., 2009; Motazedian et al., 2010; Jask area: the present study). Similar to the zoonotic foci of the disease from Rajasthan region in north-west India (Molyneux and Ashford, 1983), it was found that M. hurrianae was the actual natural reservoir host of CLM in south-east region of Iran (Ashford, 1996). In contrast to our earlier finding in the nearby Fars Province, where M. libycus was the main reservoir of CLM (Moemenbellah–Fard et al., 2003), no Persian or Libyan jirds were found to be infected indicating that these rodents do not have a pivotal role in the epidemiology of CLM in Jask region.
This is the first report of G. nanus being naturally infected with L. major. Previous studies showed that although six different Gerbillus species, including G. nanus, were captured along the western shore of the Dead Sea, none of them were found to be infected with L. major (Schlein et al., 1984). Similarly, no Leishmania were detected in any of six G. campestris, two G. aureus and one G. nanus of Tunisia (Ben–Ismail et al., 1987). No infections were also observed in three G. nanus and three G. dasyurus of Jordan (Saliba et al., 1994). Fichet–Calvet et al. (2003) reported that two species of G. dasyurus and G. pyramidium were infected with L. major by 16% and <1% in Israel and Egypt, respectively. Curiously, two wild G. pyramidium floweri were recently found infected with L. tropica in Egypt which is widely regarded to be the cause of anthroponotic (not zoonotic) disease (Shehata et al., 2009). This finding adds yet another dimension to the already complex eco-epidemiology of CLM in the Old World.
The eastern plain of Jask region included also a relatively high (16%) abundance of wild Baluchistan gerbils, G. nanus, whose role in the eco-epidemiology of CLM in Iran has previously not been clarified. It needs to be reassessed in the wake of more recent observations to help in planning control measures. This is the first report of the presence of G. nanus in a non-urban region which lies within its previously defined geographical limits in Iran after five decades since Misonne’s report (1959). In the present study, the first evidence of natural infection of G. nanus with the protozoan parasite, L. major, sheds some new lights on the incrimination of putative zoonotic reservoirs of CLM infection in this region. The role of G. nanus as potential propagator of the pathogens needs more investigation.
As G. nanus and M. hurrianae were both relatively common and positive for leishmanial amastigotes, it seems likely that they are the main rodent reservoir hosts in the study region, assuming that most of their leishmanial parasites belong to species causing the human CLM disease observed in the region. The recent report of CLM infection from a species of the Indian gerbil, T. indica, and two unspecified genera of Gerbillus s.l. in the south-east region of adjacent Fars Province should thus be reconsidered in the light of the present study (Oryan et al., 2007; Mehrabani et al., 2007).
Although only 10 smears were confirmed positive for leishmanial kDNA by PCR, a female-biased parasitism was evident since most (80%) infected rodent specimens were female. This is in line with observations in other host/parasite systems (Saliba et al., 1994; Krasnov et al., 2005), though it needs to be corroborated by further investigations on the effects of host gender differences in blood protozoan infections of mammals. It is speculated that since females are less mobile, they may thus be expected to be bitten more by infectious sand flies.
Both Phlebotomus papatasi and Ph. salehi sand flies were also captured in the vicinity of rodent burrows in the studied area. They contained L. major parasites confirmed by parasitological and molecular (PCR) methods. It is speculated that Ph. papatasi and Ph. salehi sand flies could be associated with G. nanus and M. hurrianae rodent colonies, respectively, since in unstable zoonotic systems of CLM disease, the L. major parasites are usually associated with M. hurrianae/Ph. salehi in south-east Iran, similar to that in north-western India (Molyneux and Ashford, 1983).
It is concluded that G. nanus is a potential new reservoir host of L. major in this area adding yet another dimension to the pathogenic complexes of CLM disease in southern Iran.
Acknowledgments
The meticulous help provided by the anonymous referees is appreciated. The authors are grateful for the precious logistical support given (to carry out the field and laboratory work) by the Tropical and Infectious Diseases Research Centre, HUMS, Bandar Abbas, Iran. We appreciate help with laboratory work from Mr M. Kalantari, initial rodent identification by Ms M. Adnafi and expert verification of rodent identity by Dr Jamshid Darvish. Finally, the authors are also indebted to the Vice-chancellor for Research and Technology at SUMS for permitting the use of facilities at the university. This paper was the result of research plan (No. M/P/251 dated 1-1-2008) carried out under the auspices of HUMS.
REFERENCES
- Ashford RW.(1996)Leishmaniasis reservoirs and their significance in control. Clinics in Dermatology 14523–532. [DOI] [PubMed] [Google Scholar]
- Ashford RW.(2000)The leishmaniases as emerging and reemerging zoonoses. International Journal for Parasitology 301269–1281. [DOI] [PubMed] [Google Scholar]
- Azizi K, Rassi Y, Javadian E, Motazedian MH, Asgari Q.&Yaghoobi-Ershadi MR.(2008)First detection of Leishmania infantum in Phlebotomus (Larroussius) major (Diptera: Psychodidae) from Iran. Journal of Medical Entomology 45726–731. [DOI] [PubMed] [Google Scholar]
- Azizi K, Rassi Y.&Moemenbellah-Fard MD.(2010)PCR-based detection of Leishmania major kDNA within naturally infected Phlebotomus papatasi in southern Iran. Transactions of the Royal Society of Tropical Medicine and Hygiene 104440–442. [DOI] [PubMed] [Google Scholar]
- Ben-Ismail R, Ben Rashid MS, Gradoni L, Gramiccia M, Helal H.&Bach-Hamba D.(1987)La leishmaniose cutanee zoonotique en Tunisie: Etude du reservoir dans le foyer de douara. Annales de la Société Belge de Médecine Tropicale 67335–343. [PubMed] [Google Scholar]
- Darvish J.(2005)The current status of rodents in Iran [document on the Internet]. Available at: http://iranrodents.com/PersianRodents.aspx (in Persian) [accessed 6 March 2011] [Google Scholar]
- Edrissian GH, Ghorbani M.&Tahvildar-Bidruni G.(1975)Meriones persicus, another probable reservoir of zoonotic cutaneous leishmaniasis in Iran. Transactions of the Royal Society of Tropical Medicine and Hygiene 69517–519. [DOI] [PubMed] [Google Scholar]
- Edrissian G, Zovein Z.&Nadim A.(1982)A simple technique for preparation of smears from the ear of Rhombomys opimus for the detection of leishmanial infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 76706–707. [DOI] [PubMed] [Google Scholar]
- Emami MM, Yazdi M.&Nilforoushzadeh M.(2009)Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of central Iran. Transactions of the Royal Society Tropical Medicine and Hygiene 1031257–1262. [DOI] [PubMed] [Google Scholar]
- Fichet-Calvet E, Jomaa I, Ben Ismail R.&Ashford RW.(2003)Leishmania major infection in the fat sand rat Psammomys obesus in Tunisia: interaction of host and parasite populations. Annals of Tropical Medicine and Parasitology 97593–603. [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Morand S, Hawlena H, Khokholova IS.&Shenbrot GI.(2005)Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 146209–217. [DOI] [PubMed] [Google Scholar]
- Misonne X.(1959)Zoogéographie des mammiféres de l’Iran. Memoires Institute de la Royal Sciences Natural de Belgique 591–157. [Google Scholar]
- Mehrabani D, Motazedian MH, Oryan A, Asgari Q, Hatam GR.&Karamian M.(2007)Microscopical and PCR-based detection and identification of Leishmania major in Tatera indica and rodents of the genus Gerbillus in search of its reservoir in the Larestan region, Fars province, southern Iran. Annals of Tropical Medicine and Parasitology 101315–322. [DOI] [PubMed] [Google Scholar]
- Moemenbellah-Fard MD, Kalantari M, Rassi Y.&Javadian E.(2003)The PCR-based detection of Leishmania major infections in Meriones libycus (Rodentia: Muridae) from southern Iran. Annals of Tropical Medicine and Parasitology 97811–816. [DOI] [PubMed] [Google Scholar]
- Molyneux DH.&Ashford RW.(1983)The Biology of Trypanosoma and Leishmania, Parasites of Man and Domestic Animals London: Taylor & Francis [Google Scholar]
- Motazedian MH, Parhizkari M, Mehrabani D, Hatam G.&Asgari Q.(2010)First detection of Leishmania major in Rattus norvegicus from Fars province, southern Iran. Vector-Borne and Zoonotic Diseases 10969–975. [DOI] [PubMed] [Google Scholar]
- Oryan A, Mehrabani D, Owji SM, Motazedian MH.&Asgari Q.(2007)Histopathologic and electron microscopic characterization of cutaneous leishmaniasis in Tatera indica and Gerbillus spp. infected with Leishmania major. Comparative Clinical Pathology 16275–279. [Google Scholar]
- Pourmohammadi B, Motazedian MH.&Kalantari M.(2008)Rodent infection with Leishmania in a new focus of human cutaneous leishmaniasis in northern Iran. Annals of Tropical Medicine and Parasitology 102127–133. [DOI] [PubMed] [Google Scholar]
- Saliba EK, Disi AM, Ayed RE, Saleh N, Oumeish O.&Al-Ouran R.(1994)Rodents as reservoir hosts of cutaneous leishmaniasis in Jordan. Annals of Tropical Medicine and Parasitology 88617–622. [DOI] [PubMed] [Google Scholar]
- Schlein Y, Warburg A, Schnur LF, Le Blancq SM.&Gunders AE.(1984)Leishmaniasis in Israel: reservoir hosts, sand fly vectors and leishmanial strains in the Negev, Central Arava and along the Dead Sea. Transactions of the Royal Society of Tropical Medicine and Hygiene 78480–484. [DOI] [PubMed] [Google Scholar]
- Shehata MG, Samy AM, Doha SA, Fahmy AR, Kaldas RM, Furman BD.&Villinski JT.(2009)First report of Leishmania tropica from a classical focus of L. major in North-Sinai, Egypt. American Journal of Tropical Medicine and Hygiene 81213–218. [PubMed] [Google Scholar]
- Siahsarvie R.&Darvish J.(2007)New records of naked-footed gerbil Gerbillus nanus and pygmy gerbil Gerbillus cfr. henleyi (Rodentia: Muridae) from Iran. Iranian Journal of Animal Biosystematics 343–48. [Google Scholar]
- Wilson DE.&Reeder DM.(2005)Mammal Species of the World, a Taxonomic and Geographic Reference 3rd Edn. Vol. 21210–1245.Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA.&Mohebali M.(1996)Meriones libycus and Rhombomys opimus (Rodentia: Gerbillidae) are the main reservoir hosts in a new focus of zoonotic cutaneous leishmaniasis in Iran. Transactions of the Royal Society of Tropical Medicine and Hygiene 90503–504. [DOI] [PubMed] [Google Scholar]


