Cysticercosis (Cysticercus cellulosae) is a zonotic disease caused by the larval form of the nematode Taenia solium or pork tapeworm. Humans harbour the tapeworm in the intestine and are the definitive hosts in the parasite’s life cycle. Infection occurs after ingestion of undercooked pork or by ingestion of its eggs in contaminated food or water. Cysticercosis is a public health problem and is endemic in several developing countries of Asia, Central Africa and South America (Rajshekhar et al., 2003; Prasad et al., 2008). However, due to frequent migration and changes in travel patterns, it is now increasingly seen in developed nations also (Prasad et al., 2008). The commonest site for cysticercosis is the central nervous system. Involvement of the breast is extremely rare (Chi and Chi, 1978) and only a few cases are reported. In the breast, this parasite presents as a lump. Due to the rarity of the condition, these lumps are often mistaken for other common pathologies, such as cyst, fibroadenoma, or even carcinoma posing serious concern. We report here a case of an isolated cysticercosis of the breast masquerading as breast tumour, along with a brief review of the reported cases in the literature.
PATIENTS AND METHODS
The patient was a 54-year-old female, a strict vegetarian from a middle class family of New Delhi, India. She presented with a breast lump in the lower inner quadrant of her left breast for 3 months. The lump was 2×2 cm in size, hard in consistency, had an irregular surface and was free from the overlying skin and the underlying muscle. It was non-tender and the bilateral axillae were normal. Her eosinophil count and erythrocyte sedimentation rate were normal. There was a small dot-like calcification with a surrounding halo in the mammogram (Fig. 1). The ultra-sonography of the breast showed a well-defined round cyst within a collection and a brightly echogenic protrusion from the wall. Fine needle aspiration showed clear fluid, the cytology of which revealed macrophages and eosinophils. Due to the suggestive radiological and cytological findings and with the patient being from an endemic region, a possible diagnosis of parasitic infestation was made. The computed tomographic scan of her brain and the ophthalmic examination were normal. Routine microscopy of her stool revealed no parasites. She was subjected to wide local excision of the breast lump. The cut section showed a fluid-filled bladder-like structure in the centre of the lump (Figs. 2 and 3). The histo-pathological examination confirmed the diagnosis of cysticercosis (Fig. 4). She was advised albendazole tablet 400 mg daily for 2 weeks.
Fig. 1.
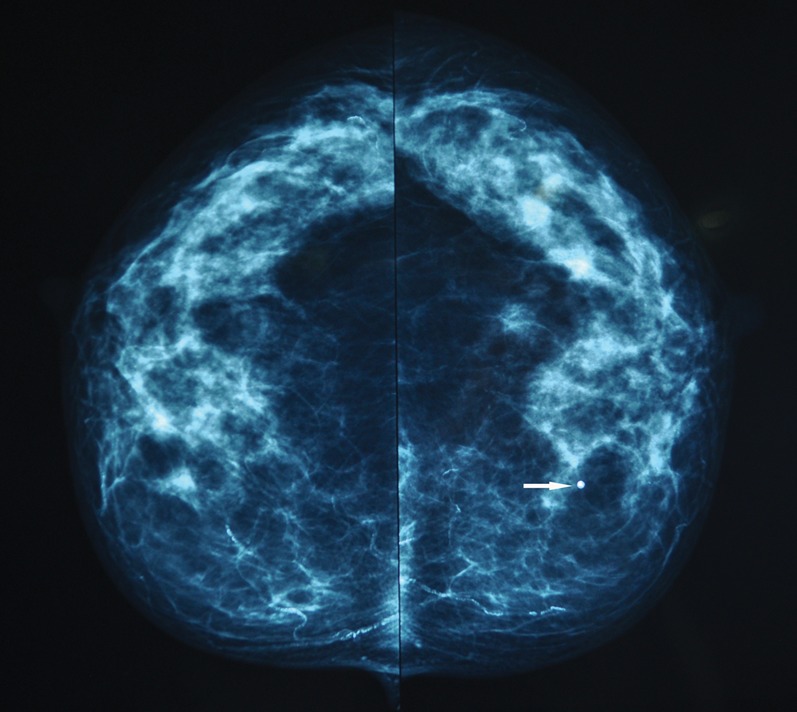
The mammogram of the left breast showed a dot-like calcification (arrow) with a surrounding halo. On comparing the surgical specimen with the mammogram, this calcified dot corresponded to the scolex and the halo to the cyst wall.
Fig. 2.
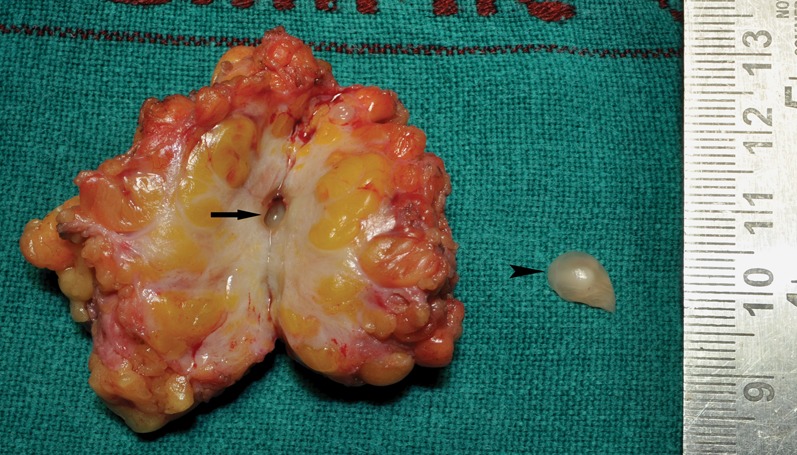
The cut section of the breast lump revealed the larva in the centre of the lump with surrounding fibrosis. The empty cavity (arrow) is seen in the centre of the lump after the larva (arrow head) had been removed from it.
Fig. 3.
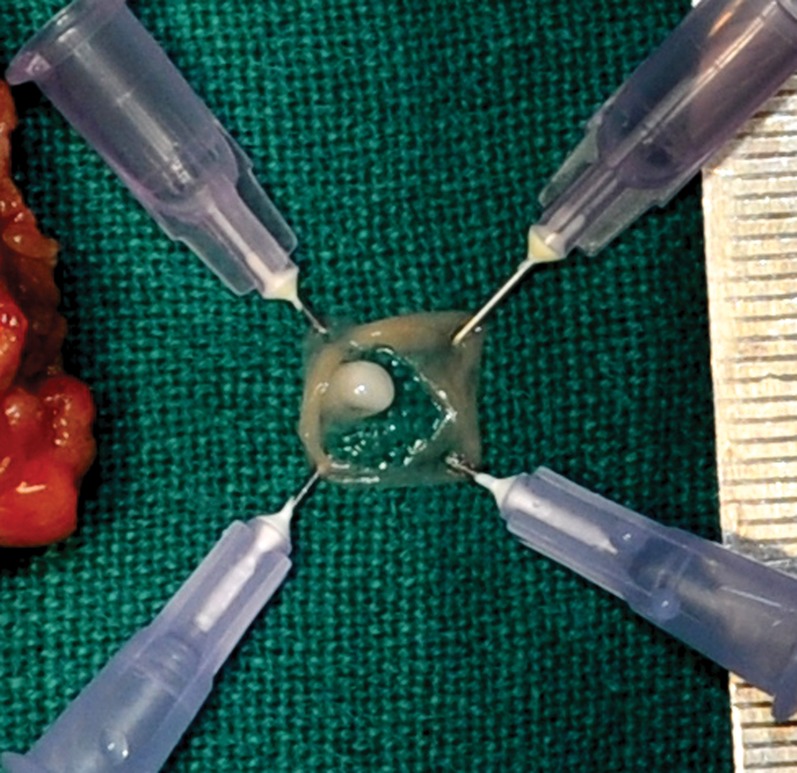
The larva is split open. The pearly white structure is seen protruding from the wall of the larva to its lumen, It was 2×3 mm in size, firm in consistency and had smooth glistening surface.
Fig. 4.
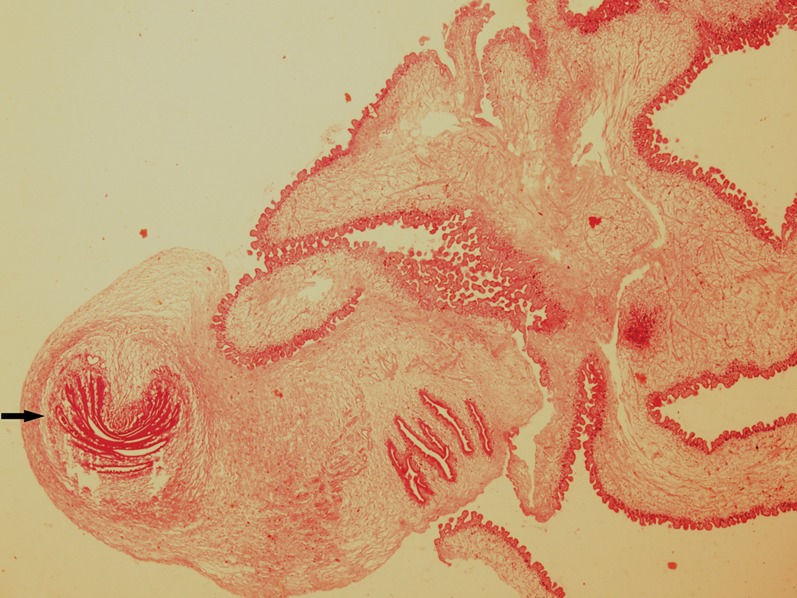
The microscopic appearance of the parasite. The characteristic scolex (arrow) is seen at the cephalic end of the parasite (haematoxylin and eosin stains, ×40 magnification).
DISCUSSION
Here we have presented a case of isolated cysticercosis of the breast. Although cysticercosis is not a rarity in our region, its presentation as a breast lump is exceptional. Only a few cases are reported in the literature. An overview of 13 published reports and the present case is presented in the Table. In the present case, it was clinically mimicking a breast tumour. However, being in an endemic area, our radiologist and the pathologist had a high index of suspicion for a parasitic lesion. This high index of suspicion and awareness were the key to make the probable pre-operative diagnosis of a parasitic breast disease.
An overview of 13 published reports and the present case.
| Authors | Age (years) | Presentation | FNAC | Imaging | Treatment | Histopathology |
| Lucarelli et al., 2008 | 63 | Breast-WNL | … | CT scan brain: two well-defined lesions in the brain similar to T. solium larvae Mammogram: tubular, worm-like structure suggestive of calcified larvae in the intermuscular area of the pectoral major of left breast | Excision followed by albendazole | Characteristic scolex, with four suckers and a double row of hooks |
| Sinha et al., 2008 | 65 | Painless, mobile lump(1×1 cm) in the left breast | … | Mammogram: a small high-density lesion with round, well-defined margins in the upper outer quadrant Ultrasound: an anechoic lesion with a central hyperechoic focus, consistent with a scolex | Excision biopsy | … |
| Agnihotri et al., 2006 | 22 | Non-tender lump on right breast | … | … | Excision biopsy | Three-layered wall of the cysticercus. Multinucleated giant cells and foreign body granulomas in the wall of the cyst |
| Conde et al., 2006 | 26 | Painless, mobile mass (2.0 cm) in the right breast | … | Ultrasonography: an oval nodule with circumscribed margins, hypoechoic homogeneous content and posterior enhancement | Resection of the mass | Cystic cavity containing larvae, consistent with cysticerci |
| Sah et al., 2001 | 25 | Painless, mobile, firm lump (3.5×2.5 cm) on right breast | … | … | Excision biopsy | Main mass consistent with fibroadenoma, inside which there was a nodule containing scolex with feature of cysticercous cellulose |
| Sahai et al., 2002 | 22 | Breast lump, 2×1 cm | Clear, acellular fluid | … | … | Cysticercus |
| 43 | Breast lump, 1.5×1 cm | Eo, AIE, palisading histiocytes, cysticercus | … | … | … | |
| 35 | Breast lump, 1×1 cm | Cysticercus | … | … | … | |
| 30 | Breast lump, 1×1 cm | Eo, AIE, palisading histiocytes, cysticercus | … | … | … | |
| 30 | Breast lump, 15×2 cm | Few polys, Eo, cysticercus | … | … | … | |
| 32 | Breast lump, 2×2 cm | Eo, AIE, palisading histiocytes, cysticercus | … | … | … | |
| 42 | Breast lump, 1×1.5 cm | Eo, AIE, palisading histiocytes, degenerating cysticercus infiltrated by polys, giant cells, BDC | … | … | … | |
| 16 | Brest lump, 2×2 cm | Few polys, cysticercus | … | … | … | |
| 49 | Brest lump, 3×3 cm | Few polys, Eo, palisading histiocytes, cysticercus | … | … | … | |
| 45 | Breast lump, 2×3 cm | Eo, AIE, palisading histiocytes, cysticercus, BDC | … | … | … | |
| 35 | Breast lump, 2×2 cm | Eo, AIE, palisading histiocytes, FBGC, cysticercus | … | … | … | |
| 30 | Breast lump, 4×3 cm | Palisading histiocytes, cysticercus | … | … | … | |
| 30 | Breast lump, 2×3 cm | Eo, AIE, palisading histiocytes, cysticercus | … | … | … | |
| 49 | Breast lump, 2×3 cm | Eo, AIE, palisading histiocytes, degenerating cysticercus infiltrated by polys, calcareous corpuscles | … | … | … | |
| 15 | Breast lump, 2×3 cm | Few polys, palisading histiocytes, cysticercus | … | … | … | |
| 14 | Breast lump, 3×3 cm | AIE, degenerating cysticercus infiltrated by polys | … | … | … | |
| Amatya and Kimula, 1999* | ||||||
| Kapila and Verma,1996† | ||||||
| Vuong, 1989 | 30 | Breast mass, 2 cm | Inflammatory cells mixed with spiked spherules | Excision biopsy | Scolex with four large suckers | |
| Alagaratnam et al.,1988 | 43 | Breast lump, 0.5 cm | No cellular elements | … | Excision biopsy | Rostellum bearing hooklets |
| Kunkel and Hawksley, 1987 | 25 | Well-demarcated breast mass, 1 cm | … | … | Excision biopsy | Parasite with an invaginated scolex |
| Leggett, 1983 | 29 | Breast lump, 1.5 cm | … | … | Excision biopsy | Scolex with hooklets and suckers |
| Chi and Chi, 1978‡ | ||||||
| Bhattacharjee et al., 2011 | 54 | Painless, hard, breast lump, 2×2 cm | Clear fluid, the cytology of which revealed macrophages and eosinophils | Mammogram: small dot-like calcification with a surrounding halo | Wide local excision followed by albendazole | Scolex at the cephalic end of the parasite |
| Ultrasonography- well-defined round cyst within a collection and a brightly echogenic protrusion from the wall |
WNL, within normal limit; AIE, acute inflammatory exudate; BDC, benign ductal cells; Eo, eosinophils; FBGC, foreign body giant cells; polys, polymorphonuclear leucocytes.
The paper document five cases of cysticersosis of the breast among 23 402 biopsy specimens examined over 5 years; no detailed data of these particular cases were presented.
The paper represents the first eight cases of Sahai et al. (2002).
This paper reported 191 cases of cysticercosis, of which seven cases were seen in the breast. No details of the individual cases were presented.
Due to the rarity of the disease, there is no detailed description of the mammographic or ultra sonographic appearances of breast cysticercosis. Sinha et al. reported a case with mammographic appearance of a small high-density lesion with round well-defined margins. There was no specific feature to suggest parasitic infestation in their case (Sinha et al., 2008). Lucarelli et al. reported a tubular worm-like appearance suggestive of calcified larvae in the intermuscular area of the pectoral major (Lucarelli et al., 2008). In our case, the architecture of the breast parenchyma was well preserved. There was a small dot-like calcification with a surrounding halo in the mammogram. On comparing the surgical specimen with the mammogram, this calcified dot corresponded to the scolex and the halo to the cyst wall.
Vijayaraghavan described four different types of sonographic appearances for soft tissue cysticercosis (Vijayaraghavan 2004). These are: (1) well-defined round cyst within a collection, with a brightly echogenic protrusion from the wall; (2) loculated collection of fluid with internal echoes with a well-defined round cyst within, with an eccentric echogenic protrusion from the wall, representing the scolex; (3) irregular cyst with minimal fluid collection on one side with an extruded scolex in it; and (4) elliptical calcified cysticercus cysts. Our case had the imaging appearance as described in type 1 cyst.
Although the radiological findings were suggestive of the benign nature of the disease, we went ahead with wide local excision of the lump. This was carried out to obtain a definitive diagnosis as well as to remove the fear of malignancy from the patient’s mind.
The definitive diagnosis of cysticercosis is based on the characteristic features of the cephalic extremity of the parasite. In our case, the fine needle aspiration showed clear fluid and the cytology showed only eosinophils and macrophages. The presence of inflammatory cells mixed with spiked spherules may suggest the presence of encysted flat worms, but is not specific of cysticercosis (Vuong, 1989). Fine needle aspiration cytology could be diagnostic when a fragment of cysticercus is seen on the aspirate (Kapila and Verma, 1996; Sahai et al., 2002). In our case, the final histo-pathological examination demonstrated the scolex at the cephalic extremity of the parasite with characteristic suckers and the hooks, which were diagnostic.
Another interesting aspect of our case was that she was a pure vegetarian. She had probably ingested the eggs of T. solium through the contaminated food or water. The apparent implication of this is the inadequacy of public health measures.
In conclusion, cysticercosis of the breast is rare. However, it should be kept in the differential diagnosis of breast lumps, especially in endemic areas. Clinically, it may mimic a breast tumour. Imaging features are helpful in pre-operative diagnosis. Definitive diagnosis is possible after the histological examination of the parasite. Due to widespread travel of people between countries and continents, the clinician should be aware of this rare but potentially serious breast disease.
Acknowledgments
The authors thank Dr Chandan J. Das, consultant radiologist of Diwan Chand Satyapal Aggarwal Imaging and Research Centre, New Delhi, for his expert opinion on the mammogram.
REFERENCES
- Agnihotri S, Talwar OP, Pudasaini S.&Baral R.(2006)Polish Journal of Pathology 5753–54. [PubMed] [Google Scholar]
- Amatya BM.&Kimula Y.(1999)American Journal of Surgical Pathology 231276–1279. [DOI] [PubMed] [Google Scholar]
- Alagaratnam TT, Wing YK.&Tuen H.(1988)American Journal of Tropical Medicine and Hygiene 38601–602. [DOI] [PubMed] [Google Scholar]
- Chi HS.&Chi JG.(1978)Korean Journal of Parasitology 16123–133. [DOI] [PubMed] [Google Scholar]
- Conde DM, Kashimoto E, Carvalho LE, Hidalgo SR.&Torresan RZ.(2006)Breast Journal 12179. [DOI] [PubMed] [Google Scholar]
- Kapila K.&Verma K.(1996)Acta Cytologica 40653–656. [DOI] [PubMed] [Google Scholar]
- Kunkel JM.&Hawksley CA.(1987)Human Pathology 181190–1191. [DOI] [PubMed] [Google Scholar]
- Leggett CA.(1983)Australian and New Zealand Journal of Surgery 53281. [DOI] [PubMed] [Google Scholar]
- Lucarelli AP, Martins MM, de Oliveira VM, Rinaldi JF, Roveda D, Jr, Galvão MA, Aoki T.&Piato S.(2008)American Journal of Tropical Medicine and Hygiene 79864–865. [PubMed] [Google Scholar]
- Prasad KN, Prasad A, Verma A.&Singh AK.(2008)Journal of Biosciences 33571–582. [DOI] [PubMed] [Google Scholar]
- Rajshekhar V, Joshi DD, Doanh NQ, van De N.&Zhou XN.(2003)Acta Tropica 8753–60. [DOI] [PubMed] [Google Scholar]
- Sah SP, Jha PC, Gupta AK.&Raj GA.(2001)Indian Journal of Pathology and Microbiology 4459–61. [PubMed] [Google Scholar]
- Sahai K, Kapila K.&Verma K.(2002)Postgraduate Medical Journal 78165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Sharma R, Kandpal H, Seenu V.&Sharma M.(2008)Acta Radiologica 49506–509. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan SB.(2004)Journal of Ultrasound in Medicine 23423–427. [DOI] [PubMed] [Google Scholar]
- Vuong PN.(1989)Acta Cytologica 33659–662. [PubMed] [Google Scholar]


