Abstract
Faecal samples were collected from the rectum of 540 domestic dogs from four districts (Lusaka, Katete, Petauke and Luangwa) in Zambia between 2005 and 2006 and prevalences of canine alimentary tract parasites were determined by coprological examination. Thirteen different ova and parasites including strongyle (43.3%), Spirocerca lupi (18.7%), taeniid (13.1%), Toxocara canis (7.6%), Sarcocystis sp.* (7.5%), Isospora sp.* (5.7%), Physaloptera sp.* (4.6%), Capillaria sp.* (2.8%), Dipylidium caninum (2.2%), Mesocestoides sp.* (2.0%), Ascaris sp.* (1.7%), Trichuris vulpis* (0.4%) and Schistosoma mansoni* (0.4%) were detected, Ascaris and Schistosoma probably originating from coprophagy. The species with asterisks and later-described Taenia multiceps are for the first time reported from dogs in Zambia. A coproantigen enzyme-linked immunosorbent assay (CoproAg-ELISA) developed for Echinococcus spp. revealed 43 positive dogs and 37 of these harboured taeniid eggs. From 63 of the 71 taeniid egg-positive samples, eggs and DNA thereof were isolated and subjected to a multiplex polymerase chain reaction for differentiating E. granulosus sensu lato, E. multilocularis and Taenia spp. Amplicons indicative for Taenia spp. were obtained from 60 samples. Sequencing of amplicons spanning part of the mitochondrial cytochrome c oxidase subunit 1 gene, which was possible with 38 samples, revealed 35 infections with T. hydatigena and 3 with T. multiceps. Therefore, the CoproAg-ELISA showed some positives, but concrete evidence for the existence of canine E. granulosus infection could not be established. Comparison of the results of the CoproAg-ELISA and Taenia species identification indicated that the CoproAg-ELISA cross-reacts with patent infections of T. hydatigena (57%) and T. multiceps (33%).
INTRODUCTION
It is very common in most African countries to see free-ranging dogs under poor hygiene condition and in close contact with people, especially in rural settings. Although the potential role of dogs as source of zoonotic parasites to humans has been recognized as a significant public health problem, investigation on the prevalence of such parasite infections has hardly been conducted in most African countries. The conditions combined with poor veterinary services and a lack of awareness of zoonotic diseases exacerbate the risks of disease transmission from dogs to humans.
Echinococcus spp. belonging to such canine zoonotic parasites can cause serious diseases in humans. Therefore, the precise diagnosis of definitive hosts that are shedding infective eggs is of primary importance for evaluating the endemicity and the risk posed to humans in a region, and for assessing control programs. For detecting Echinococcus adult infections, we developed a monoclonal antibody-based coproantigen detection assay (Morishima et al., 1999), which in turn has been used for epidemiological studies in foxes, dogs and cats mainly in Hokkaido, Japan (Tsukada et al., 2000; Nonaka et al., 2009a). The assay showed high specificity against antigens of common canine parasites including Toxocara canis, Dipylidium caninum, Spirometra erinaceieuropaei and some Taenia species (Sakashita et al., 1995); however, cross-reactivity of the assay was recognized against patent T. hydatigena infections (Malgor et al., 1997). Nevertheless, there have been few practical problem in using this assay in Hokkaido because the prevalences of most Taenia species are very low there. Only T. taeniaeformis is commonly found in cats, but the assay showed no cross-reactivity with this parasite.
When considering using the assay in other regions of the world, its specificity should be further evaluated with samples from regions where common canine parasites, including Echinococcus and Taenia species, are prevalent. In Zambia, it has been estimated that E. granulosus G1 strain (sheep strain), which has a broad range of intermediate hosts including sheep, cattle, goats and pigs, is prevalent (Macpherson and Wachira, 1997); thus, the parasite might be present in most of the typical villages in Zambia where animal husbandry of cattle, goats, and pigs is common. Indeed, it has been reported that metacestodes of E. granulosus were routinely found in lungs and livers of cattle at abattoirs in Lusaka (Pandey, 1987; Pandey and Sharma, 1987). Unfortunately, no information is available on the prevalence of canine parasites from that region except for one report in which a parasitic helminth necropsy survey was conducted with 85 dogs in Lusaka, revealing the existence of various parasites such as D. caninum (25% in prevalence), T. hydatigena (18%), Toxocara canis (14%), Ancylostoma caninum (8%), Toxascaris leonina (7%), Ancylostoma braziliensis (2%), Spirocerca lupi (2%) and E. granulosus (1%) (Islam and Chizyuka, 1983).
In this survey, at first, in order to clarify the prevalence of alimentary tract parasites in dogs in Zambia, fresh faecal samples from dogs raised in rural villages and in Lusaka were examined for faecal helminth eggs and coccidian oocysts/sporocysts. Samples containing taeniid eggs were further characterized for identifying the species of taeniid cestodes by multiplex polymerase chain reaction (PCR) and nucleotide sequence analysis of part of the mitochondrial cytochrome c oxidase subunit 1 (CO1) gene. To evaluate its specificity, the developed coproantigen detection assay for canine echinococcosis was performed on all faecal samples.
SUBJECTS AND METHODS
Collection of Faecal Samples and Questionnaire
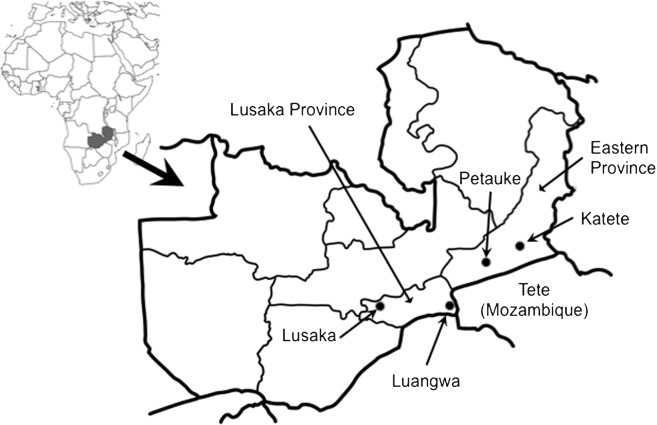
Since we obtained local information (unpublished) that E. granulosus cysts were found in cattle from the Tete Province in Mozambique, we selected for this study two provinces of Zambia that are located next to this province. Fresh faecal samples were collected per rectum from 540 dogs raised in rural villages in Katete and Petauke (eastern province) and Luangwa and Lusaka (Lusaka province) between 2005 and 2006 (Fig.). In order to facilitate the capture and holding of these free-ranging dogs, sample collections were announced to village residents 1 day before. The purpose of the survey was explained and a simple questionnaire was administered to dog owners at the day of sample collection. The questionnaire included the dog’s age and sex.
Study area in Zambia.
Examination for Faecal Helminth Eggs and Coccidian Oocysts/Sporocysts, Coproantigen ELISA
Coproscopic examination (ova-examination) and a coproantigen sandwich enzyme-linked immunosorbent assay (CoproAg-ELISA) were performed as described by Morishima et al. (1999). In order to kill Echinococcus eggs, all faecal samples were stored at −80°C for more than 7 days before examination. Briefly, 0.5 g of faecal samples were weighed and put into plastic tubes. Then 1% formalin containing 0.3% Tween 20 were added to make a total volume of 15 ml. After a centrifugation step (1000g for 10 minutes), the centrifugal sucrose (specific gravity = 1.27) flotation method (Ito, 1980) was applied to the resultant sediments for ova-examination. The supernatants were used for CoproAg-ELISA developed for detecting Echinococcus coproantigen using the monoclonal antibody EmA9. Two cutoff values were used in CoproAg-ELISA to discriminate between negative and suspicious samples [mean (μ)+3 standard deviations (SD) of negative controls; OD = 0.206] and between suspicious and positive samples (μ+5SD; OD = 0.289). The negative controls used were faecal samples from 605 companion dogs raised and kept only on the main island of Japan that is free of Echinococcus infections.
Preparation of Egg DNA
From all samples in which taeniid eggs were detected, 1–10 eggs per sample were collected manually from the cover glass used for ova-examination under a stereomicroscope. Egg DNA was then extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instruction.
Multiplex PCR for Discriminating E. multilocularis, E. granulosus sensu lato and Taenia species
The multiplex PCR for discriminating taeniid eggs was done as described by Trachsel et al. (2007) using egg DNA as template. This method can distinguish E. multilocularis, E. granulosus s.l. and Taenia species. The amplified products [395 base pairs (bp) for E. multilocularis, 117 bp for E. granulosus s.l. and 267 bp for Taenia spp.] were examined by agarose gel electrophoresis. Positive controls of DNA extracted from metacestodes of E. granulosus genotype G1, E. multilocularis and T. hydatigena using QIAamp DNA Mini Kit (Qiagen) and a negative control without DNA were included in all the tests.
Nucleotide Sequencing
Egg DNA identified as Taenia sp. by the multiplex PCR was subjected to another PCR and sequence analysis. Part (491 bp) of the mitochondrial cytochrome CO1 gene was amplified using the primers PR-A (5′-TGG TTT TTT GTG CAT CCT GAG GTT TA-3′) and PR-B (5′-AGA AAG AAC GTA ATG AAA ATG AGC AAC-3′) according to Okamoto et al. (1995). Nucleotide sequences of the products were determined by a Beckman CEQ 8000 DNA analyser using a GenomeLab DTCS Quick Start kit (Beckman Coulter, Fullerton, CA, USA) following the manufacturer’s instruction. The sequences obtained were subjected to BLAST sequence similarity search (National Center for Biotechnology Information, Bethesda, MD, USA) to identify the species of Taenia.
Statistical Analysis
Ninety-five per cent confidence intervals of prevalence were calculated on the basis of binomial distributions using the software program R (version 2.8.1, R Development Core Team, 2008). For the ova and parasites showing more than 5% in overall prevalence, logistic regression analysis was performed to evaluate the effect of site (district), sex and age of dogs on the prevalence using the software program JMP 8 (SAS Institute, Tokyo, Japan). Since sex was not recorded from the dogs from Lusaka, the data of these dogs were excluded in the above analysis. Dogs were grouped as younger or older than 6 months (⩽6 months old versus >6 months old) for comparing T. canis prevalences, and younger or older than one year (⩽1 year old versus >1 year old) for the other parasites. If the effects of interaction between sites and other factors were significant (P<0.05), the same analysis was further performed on the effect of sexes and ages at each site separately. For dogs from Lusaka, the difference in prevalence between age groups was analysed by Fisher’s exact test using the software program R.
RESULTS
Faecal Examination for Helminth Eggs and Coccidian Oocysts/Sporocysts
From 540 canine faecal samples examined, 13 kinds of helminth eggs and coccidian oocysts/sporocysts were detected (Table 1). Among all the ova and parasites found, strongyle eggs were most abundant and were found in 43.3% of the dogs. The prevalence was, however, significantly lower in Luangwa district (17.0%) as compared to the other districts. Taeniid eggs were discovered in 71 (13.1%) dogs in total, and a significantly higher prevalence (33.9%) was observed in Luangwa district where goat husbandry is more prominent than in other districts. The number of taeniid eggs detected ranged from 1 to more than 1000 with a median of 20. Less than 10 eggs were found in 24 dogs. Eggs of the other potential zoonotic parasites such as T. canis, D. caninum and Schistosoma mansoni were also found in less than 10% of the dogs. The prevalence of T. canis was significantly higher in Lusaka (25.8%) than in the other districts. Interestingly, Ascaris sp. eggs were found in nine dogs (four in Katete and five in Lusaka), thus in 1.7% of the samples.
Table 1. Prevalences based on detecting helminth eggs and coccidian oocysts/sporocysts in rectal faeces of free-ranging dogs from four districts in Zambia.
| Parasite | Prevalence (%) (95% confidence interval) | ||||
| Katete | Petauke | Luangwa | Lusaka | Total | |
| (n = 224) | (n = 89) | (n = 165) | (n = 62) | (n = 540) | |
| Strongyle* | 60.7 (54.2–66.9) | 48.3 (38.2–58.5) | 17.0 (12.0–23.4) | 43.5 (31.9–55.9) | 43.3 (39.2–47.5) |
| Spirocerca lupi | 7.1 (4.4–11.3) | 41.6 (31.9–52.0) | 26.1 (20.0–33.2) | 8.1 (3.5–17.5) | 18.7 (15.6–22.2) |
| Taeniid† | 4.5 (2.2–8.1) | 4.5 (1.2–11.1) | 33.9 (27.2–41.5) | 1.6 (0.0–8.7) | 13.1 (10.6–16.3) |
| Toxocara canis | 9.8 (6.6–14.4) | 2.2 (0.6–7.8) | 0.6 (0.0–3.3) | 25.8 (16.6–37.9) | 7.6 (5.6–10.1) |
| Sarcocystis sp.‡ | ND‡ | 9.0 (4.6–16.7) | 6.7 (3.8–11.5) | ND‡ | 7.5 (4.8–11.4) |
| Isospora sp. | 6.3 (3.8–10.2) | 10.1 (5.4–18.1) | 4.2 (2.1–8.5) | 1.6 (0.0–8.7) | 5.7 (4.1–8.0) |
| Physaloptera sp. | 4.0 (2.1–7.5) | 4.5 (1.2–11.0) | 7.3 (4.2–12.3) | 0 (0.0–4.7) | 4.6 (3.2–6.7) |
| Capillaria sp. | 2.2 (1.0–5.1) | 11.2 (6.2–19.5) | 0 (0.0–1.8) | 0 (0.0–4.7) | 2.8 (1.7–4.5) |
| Dipylidium caninum | 2.2 (1.0–5.1) | 4.5 (1.2–11.0) | 1.2 (0.1–4.3) | 1.6 (0.0–8.7) | 2.2 (1.3–3.8) |
| Mesocestoides sp. | 2.7 (1.2–5.7) | 4.5 (1.2–11.0) | 0.6 (0.0–3.3) | 0 (0.0–4.7) | 2.0 (1.1–3.6) |
| Ascaris sp. | 1.8 (0.5–4.5) | 0 (0.0–3.3) | 0 (0.0–1.8) | 8.1 (3.5–17.5) | 1.7 (0.9–3.1) |
| Trichuris vulpis | 0 (0.0–1.3) | 1.1 (0.0–6.1) | 0 (0.0–1.8) | 1.6 (0.0–8.7) | 0.4 (0.0–1.3) |
| Schistosoma mansoni | 0.4 (0.0–2.5) | 0 (0.0–3.3) | 0.6 (0.0–1.8) | 0 (0.0–4.7) | 0.4 (0.0–1.3) |
*Strongyle possibly includes the genera Ancylostoma, Uncinaria and others whose eggs cannot be distinguished morphologically.
†Taeniid possibly includes the genera Taenia and Echinococcus whose eggs cannot be distinguished morphologically.
‡The samples of Katete and Lusaka were not carefully examined for the small sporocysts of Sarcocystis sp.
In the logistic regression analysis (n = 339), significant differences in prevalences among districts were obtained for taeniids, Spirocerca lupi and strongyle eggs. Differences in prevalences between age groups were significant for S. lupi in Luangwa, and Sarcocystis sp. in Petauke. However, in Lusaka (n = 51), no parasites showed significant differences between age groups. Moreover, differences in prevalences between sex groups were not significant for any parasite.
Among the 540 samples, 160 (29.6%) samples showed neither parasite, a single parasite kind was diagnosed in 244 (45.2%) samples and multiple different ova and parasites were found in 136 (25.2%) samples (two kinds in 100, three kinds in 25, four kinds in 10 and five kinds in 1).
Multiplex PCR and Nucleotide Sequencing
From 63 of the 71 samples containing taeniid eggs, DNA was isolated from the eggs. PCR products were obtained from 60 samples in the multiplex PCR, and all were of the size specific for Taenia spp. There were 18 samples with less than 10 eggs detected, and three of them showed no products in the multiplex PCR.
PCR targeting part of the mitochondrial CO1 gene was successful with 38 samples out of the available 63 DNA samples. Sequencing and BLAST sequence similarity searches showed that 35 sequences had 98.4–100% identity with those of T. hydatigena registered in GenBank (accession no. DQ995656, AM503318 or EU544552). The sequences of three samples revealed T. multiceps with identities of 94.4–100% (GenBank accession no. EF393620). For the remaining 25 samples, no amplicons (n = 11) were obtained in the PCR with primers PR-A and PR-B or no clear sequences (n = 14) were obtained by direct sequencing. T. hydatigena was found in three districts (Katete, Petauke and Luangwa), whereas T. multiceps was identified only in the Luangwa district.
CoproAg-ELISA
The CoproAg-ELISA for Echinococcus spp. was positive in 43 dogs and suspicious in 6 dogs, while 37 and 5 of those had taeniid eggs, respectively, after coproscopic examination (Table 2). The multiplex PCR performed on taeniid egg DNA of the 32 CoproAg-ELISA-positive and the 4 CoproAg-ELISA-suspicious samples, however, showed that the eggs of all samples were those of Taenia spp.
Table 2. Comparison of the results of coproantigen ELISA and faecal examination for taeniid eggs.
| Results of coproantigen examination | Results of faecal examination for taeniid eggs | ||
| Positive | Negative | Total | |
| Positive | 37 | 6 | 43 |
| Suspicious | 5 | 1 | 6 |
| Negative | 29 | 462 | 491 |
| Total | 71 | 469 | 540 |
Among the 38 samples identified as harbouring taeniid species by nucleotide sequence analysis, 20 of 35 samples (57%) identified as T. hydatigena and 1 of 3 samples (33%) as T. multiceps showed positive reactions in the CoproAg-ELISA.
DISCUSSION
The coproscopic examination revealed that the dogs in the study area of Zambia were infected with a variety of zoonotic parasites. In the previous study conducted in Lusaka, Islam and Chizyuka (1983) found nine helminth species, of which all but two (T. leonina and E. granulosus) also were identified in the present study. In addition, Sarcocystis sp., Isospora sp., Physaloptera sp., Capillaria sp., Mesocestoides sp., T. vulpis, S. mansoni, Ascaris sp. and T. multiceps were observed, which are thus the first reports from dogs of Zambia.
The highest prevalence was observed for strongyles (43.3%). From the study of Islam and Chizyuka (1983), it can be speculated that A. caninum and A. braziliense are the dominant species among strongyles. Since A. braziliense is more frequently involved in cutaneous larva migrans than A. caninum (Bowman, 2009), species identification of Ancylostoma by molecular methods should be considered in future studies for public health risk assessment. In this study, we detected lower prevalence of strongyles in the Luangwa district than in other districts. Luangwa is a valley with a very hot and humid climate throughout the year. Therefore, taking into account that climatic conditions of Luangwa are suitable for parasite transmission, results obtained in this study need further clarification.
For infections with T. canis, an age resistance limit of 6 months has been indicated (Webster, 1956) and therefore, prevalences for this parasite of age groups younger and older than 6 months were compared. However, no significant difference was observed in this study which is in agreement with recent findings (Fahrion et al., 2008) demonstrating that a low infective dose of 100 embryonated eggs consistently induced patency in adult dogs with and without previous exposure to the parasite. Since an overall prevalence of 5.1% (19/371) was observed in dogs older than 6 months, infection with T. canis in older dogs may constantly be occurring in Zambia. It is noteworthy that a higher prevalence of T. canis was observed in Lusaka, but the reason for this observation was not further elucidated in this study. Nevertheless, T. canis is one of the most important zoonotic parasites, and thus, the high prevalence in Lusaka would be of significance in public health because this district is the most populated city in Zambia and holds many high-density residential areas.
Eggs of S. mansoni were found in two dogs, but without intact miracidium inside the eggs. In general, trematode eggs are not isolated by the flotation techniques. It is known that dogs are not an appropriate host for S. mansoni, and combined with the absence of an intact miracidium in our results, it is most probable that the observation could be a result of coprophagy of human faeces by the dogs. Coprophagy of either human or pig faeces by dogs is also suspected by the finding of Ascaris sp. eggs in this study. A similar observation was reported from India (Traub et al., 2005). Recently, infection of A. lumbricoides in dogs was reported in an endemic region for this human parasite (Shalaby et al., 2010), suggesting that active infection with A. lumbricoides may occur in dogs in the study area. The finding of S. mansoni and Ascaris sp. eggs in dog faeces could also indicate their endemicity in the region.
It should be also noted that we used frozen samples for the faecal egg examination because of the biohazard concern of Echinococcus eggs. As it was observed that freezing of faeces reduced significantly the egg count of ovine gastro-intestinal strongyles in flotation techniques (van Wyk and van Wyk, 2002; Rinaldi et al., 2011), the result obtained in this study may be an underestimation.
Taeniid eggs were found in 71 samples. Canine taeniid species include both zoonotic and non-zoonotic species. Since taeniid eggs cannot be distinguished by their morphology, we used immunological and molecular techniques that were recently developed for distinguishing the genus and species of taeniid cestodes, and the result was used for evaluating potential cross-reactivity of the CoproAg-ELISA.
Various coproantigen detection methods for adult Echinococcus spp. infection have been developed (Deplazes and Eckert, 1996; Benito and Carmena, 2005; Benito et al., 2006; Huang et al., 2007). The CoproAg-ELISA used was developed to detect adult infections of E. multilocularis (Sakashita et al., 1995) and E. granulosus (Malgor et al., 1997). The sensitivity and specificity of the test had been evaluated for E. multilocularis infection using fox samples in Hokkaido, measuring 92.2% in sensitivity and 96.6% in specificity (Morishima et al., 1999; Yimam et al., 2002). Cross-reactivity with patent T. hydatigena (Malgor et al., 1997) and T. pisiformis infections (unpublished) has been observed, but not with T. taeniaeformis or T. crassiceps (Sakashita et al., 1995). In this study, positive reactions in the CoproAg-ELISA were observed in 43 samples, of which taeniid eggs were detected in 37 samples. The multiplex PCR revealed that all of the egg DNAs extracted were those of Taenia spp. These results indicate that the prevalence of E. granulosus in the study area was low or negligible. On the other hand, among 35 samples containing T. hydatigena eggs, 20 (57%) showed positive reactions in the CoproAg-ELISA. In addition, one of three samples containing T. multiceps eggs was positive. These results suggest a cross-reactivity of the test in patent infections with T. hydatigena and T. multiceps. Higher specificities for the detection of E. granulosus coproantigen were reported for a test system using polyclonal antibodies (Deplazes et al., 1992; Deplazes et al., 1994).
The multiplex PCR on egg DNA yielded products in 60 out of 63 samples. It is known that PCR on copro-DNA is often unsuccessful because of the presence of a variety of PCR inhibitors in faeces (Monteiro et al., 1997). In this study, individual eggs were picked up under a stereomicroscope, and this method for isolating taeniid eggs effectively excludes PCR inhibitors.
Sequence analysis of the partial CO1 gene revealed the occurrence of T. hydatigena and T. multiceps. Especially, in Luangwa where a significantly higher prevalence of taeniid eggs was observed than in other districts, both Taenia species were found with T. hydatigena being more prevalent. The observed higher prevalence of Taenia spp. in Luangwa could be related to the difference in the local animal husbandry system. In typical local villages in Zambia, it is common to raise cattle, goats and pigs. However, in Luangwa, goats, which can serve as an intermediate host of both T. hydatigena and T. multiceps, are the main animal in the local husbandry system, and self-consuming of goats is more popular. In contrast, pigs are the main animal in Katete and Petauke, whereas goat husbandry is less popular there. Although pigs can also serve as an intermediate host of T. hydatigena, self-consuming of pigs, or even goats and cattle are limited in those districts. In Lusaka, the capital urban city, animal husbandry is not popular.
We tried to detect E. granulosus infection in dogs in the study area in Zambia using recently developed diagnostic techniques for Echinococcus spp.; however, concrete evidence of the infection could not be obtained. Nevertheless, the study revealed the potential cross-reactivity of the CoproAg-ELISA with patent T. hydatigena and T. multiceps infections. The previous experimental infection study showed that the cross-reactivity of the CoproAg-ELISA with T. hydatigena was only observed in its patency period (Malgor et al., 1997). Therefore, the relationship of the cross-reactivity with T. multiceps and the patency should also be clarified. Moreover, the evaluation of the antigens playing a role in this cross-reactivity is also of future interest.
Since the CoproAg-ELISA showed cross-reactivity with patent infection with Taenia spp., similar surveys in future should be conducted in combination with molecular techniques that enable genus and species identification of taeniid eggs. A technique using PCR-based restriction fragment length polymorphism has been developed for this purpose (Trachsel et al., 2007). With the PCR-based restriction fragment length polymorphism, mixed infection with multiple species of Taenia can be identified simultaneously.
For conducting a survey for gastrointestinal parasite infection, faeces would provide valuable information about the animal itself and the aetiological agents that it harbours (Nonaka et al., 2009b). Moreover, a survey upon faeces is a non-invasive method, thus causing minimal disturbance to the animal condition, ecology and life. Classical approaches such as conducting faecal egg examination alone did not provide adequate information for parasite identification especially for the parasites producing morphologically similar eggs. However, as shown in this and in earlier studies (Bruzinskaite et al., 2009; Davidson et al., 2009; Ziadinov et al., 2008), faecal egg examination in combination with the recently developed molecular techniques would provide results with more accuracy, and being a useful tool in surveys of zoonotic parasite infections in dogs.
Acknowledgments
We thank Professor Yoshimitsu Maede for his valuable support for this survey. We are grateful to the staff of the Laboratory of Parasitology, Graduate School of Veterinary Medicine, Hokkaido University, and the staff of Laboratory of Helminthology, Samora Machel School of Veterinary Medicine, University of Zambia, for their valuable support. We also thank local veterinary officers and veterinary assistants in the surveyed area in Zambia for their support in the survey. This work was supported by Twenty-first Century COE Program ‘Program of Excellence for Zoonosis Control’, MEXT, by the Japan Society for the Promotion of Science (grant no. 15380205) and by the Ministry of Health, Labor and Welfare, Japan (grant for ‘The control of emerging and reemerging diseases in Japan’).
REFERENCES
- Benito A.&Carmena D.(2005)Double-antibody sandwich ELISA using biotinylated antibodies for the detection of Echinococcus granulosus coproantgens in dogs. Acta Tropica 959–15. [DOI] [PubMed] [Google Scholar]
- Benito A, Carmena D, Joseph L, Matinez J, Guisantes JA.(2006)Dog echinococcosis in northern Spain: comparison of coproantigen and serum antibody assays with coprological exam. Veterinary Parasitology 142102–111. [DOI] [PubMed] [Google Scholar]
- Bowman D D.(Ed).(2009)Georgis’ Parasitology for Veterinarians 9th EdnSt Louis, MO: Saunders Elsevier [Google Scholar]
- Bruzinskaite R, Sarkunas M, Torgerson PR, Mathis A, Deplazes P.(2009)Echinococcosis in pigs and intestinal infection with Echinococcus spp. in dogs in southwestern Lithuania. Veterinary Parasitology 160237–241. [DOI] [PubMed] [Google Scholar]
- Davidson RK, Oines O, Madslien K, Mathis A.(2009)Echinococcus multilocularis — adaptation of a worm egg isolation procedure coupled with a multiplex PCR assay to carry out large-scale screening of red foxes (Vulpes vulpes) in Norway. Parasitology Research 104509–514. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Eckert J.(1996)Diagnosis of the Echinococcus multilocularis infection in final hosts. Applied Parasitology 37245–252. [PubMed] [Google Scholar]
- Deplazes P, Gottstein B, Eckert J, Jenkins DJ, Ewald D, Jimenez-Palacios S.(1992)Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitology Research 78303–308. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Jimenez-Palacios S, Gottstein B, Skaggs J, Eckert J.(1994)Detection of Echinococcus coproantigen in stray dogs of northern Spain. Applied Parasitology 35297–301. [PubMed] [Google Scholar]
- Fahrion AS, Staebler S, Deplazes P.(2008)Patent Toxocara canis infections in previously exposed and in helminth-free dogs after infection with low numbers of embryonated eggs. Veterinary Parasitology 152108–115. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yang W, Qiu J, Chen X, Yang Y, Qiu D, Xiao N, Xiao Y, Heath D.(2007)A modified coproantigen test used for surveillance of Echinococcus spp. in Tibetan dogs. Veterinary Parasitology 149229–238. [DOI] [PubMed] [Google Scholar]
- Islam AWMS, Chizyuka HGB.(1983)Prevalence of helminth parasites of dogs in Lusaka, Zambia. Tropical Animal Health Production 15234–326. [DOI] [PubMed] [Google Scholar]
- Ito S.(1980)Modified Wisconsin sugar centrifugal-flotation technique for nematode eggs in bovine feces. Journal of Japan Veterinary Medical Association 33424–429. [Google Scholar]
- Macpherson CNL, Wachira TWM.(1997)Cystic echinococcosis in Africa south of the Sahara In Compendium on Cystic Echinococcosis in Africa and in Middle Eastern Countries with Special Reference to MoroccoAndersen F L, Ouhelli H, Kachani M.245–277.Provo, UT: Brigham Young University Print Services [Google Scholar]
- Malgor R, Nonaka N, Basmadjian I, Sakai H, Carambula B, Oku Y, Carmona C, Kamiya M.(1997)Coproantigen detection in dogs experimentally and naturally infected with Echinococcus granulosus by a monoclonal antibody-based enzyme-linked immunosorbent assay. International Journal for Parasitology 271605–1612. [DOI] [PubMed] [Google Scholar]
- Monteiro L, Bonnemaison D, Vekris A, Petry KG, Bonnet J, Vidal R, Cabrita J, Mégraud F.(1997)Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. Journal of Clinical Microbiology 35995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Tsukada H, Nonaka N, Oku Y, Kamiya M.(1999)Evaluation of coproantigen diagnosis for natural Echinococcus multilocularis infection in red foxes. Japanese Journal of Veterinary Research 46185–189. [PubMed] [Google Scholar]
- Nonaka N, Kamiya M, Kobayashi F, Ganzorig S, Ando S, Yagi K, Iwaki T, Inoue T, Oku Y.(2009a)Echinococcus multilocularis infection in pet dogs in Japan. Vector-borne Zoonotic Diseases 9201–205. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Sano T, Inoue T, Arumua MT, Fukui D, Katakura K, Oku Y.(2009b)Multiplex PCR system for identifying the carnivore origins of faeces for an epidemiological study on Echinococcus multilocularis in Hokkaido, Japan. Parasitology Research 10675–83. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Bessho Y, Kamiya M, Kurosawa T, Horii T.(1995)Phylogenetic relationships within Taenia taeniaeformis variants and other taeniid cestodes inferred from the nucleotide sequence of the cytochrome c oxidase subunit I gene. Parasitology Research 81451–458. [DOI] [PubMed] [Google Scholar]
- Pandey GS.(1987)A study of condemned bovine liver at Lusaka abattoir. Indian Journal of Veterinary Pathology 1118–22. [Google Scholar]
- Pandey GS, Sharma RN.(1987)A survey of bovine pulmonary diseases at Lusaka abattoir in Zambia. Bulletin of Animal Healt and Production in Africa 35336–338. [Google Scholar]
- R Development Core Team(2008)R: A Language and Environment for Statistical Computing [document on the Internet]. Vienna: R Foundation for Statistical Computing. Available at: http://www.R-project.org (accessed March 2009) [Google Scholar]
- Rinaldi L, Coles GC, Maurelli MP, Musella V, Cringoli G.(2011)Calibration and diagnostic accuracy of simple flotation, McMaster and LOTAC for parasite egg counts in sheep. Veterinary Parasitology 177345–352. [DOI] [PubMed] [Google Scholar]
- Sakashita M, Sakai H, Kohno H, Ooi H.-K, Oku Y, Yagi K, Ito M, Kamiya M.(1995)Detection of Echinococcus multilocularis coproantigens in experimentally infected dogs using murine monoclonal antibody against adult worms. Japanese Journal of Parasitology 44413–420. [Google Scholar]
- Shalaby HA, Abdel-Shafy S, Derbala AA.(2010)The role of dogs in transmission of Ascaris lumbricoides for humans. Parasitology Research 1061021–1026. [DOI] [PubMed] [Google Scholar]
- Trachsel D, Deplazes P, Mathis A.(2007)Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 134911–920. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Robertson ID, Irwin PJ, Mencke N, Thompson RCA.(2005)Canine gastrointestinal parasitic zonooses in India. Trends in Parasitology 2142–48. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Morishima Y, Nonaka N, Oku Y, Kamiya M.(2000)Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology 120423–428. [DOI] [PubMed] [Google Scholar]
- van Wyk JA, van Wyk L.(2002)Freezing of sheep faeces invalidates Haemonchus contortus faecal egg counts by the McMaster technique. Onderstepoort Journal of Veterinary Research 69299–304. [PubMed] [Google Scholar]
- Webster G.(1956)A preliminary report on the biology of Toxocara canis (Werner 1782). Canadian Journal of Zoology 34725–726. [Google Scholar]
- Yimam AW, Nonaka N, Oku Y, Kamiya M.(2002)Prevalence and intensity of Echinococcus multilocularis in red foxes (Vulpes vulpes schrencki) and raccoon dogs (Nyctereutes procyonoides albus) in Otaru city, Hokkaido, Japan. Japanese Journal of Veterinary Research 49287–296. [PubMed] [Google Scholar]
- Ziadinov I, Mathis A, Trachsel D, Rysmukhambetova A, Abdyjaparov TA, Kuttubaev OT, Deplazes P, Torgerson PR.(2008)Canine echinococcosis in Kyrgyzstan: using prevalence data adjusted for measurement error to develop transmission dynamics models. International Journal for Parasitology 381179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]