Abstract
A total of 554 fleas were collected in the Moroccan Casablanca and Tiznit regions from domesticated animals and ruminants between August 2007 and October 2008 and were tested for the presence of Rickettsia spp. and Bartonella spp. using molecular methods. For the first time in Morocco, we found Rickettsia felis, the agent of flea-borne spotted fever in Ctenocephalides felis; B. henselae, an agent of cat scratch disease; and Bartonella clarridgeiae, a cat pathogen and potentially a human pathogen.
INTRODUCTION
Fleas are insects in the order Siphonaptera and include ∼2500 species and subspecies (Bitam et al., 2010). Fleas are medically and economically important because they are vectors of several diseases relevant to human health, including plague (Bitam et al., 2006a), murine typhus and bartonelloses (Bitam et al., 2010). Flea-borne disease organisms are widely distributed throughout the world in endemic disease foci that contain components of the enzootic cycle (Bitam et al., 2010).
In Morocco, studies of flea and human flea-borne infections have been limited, even though murine typhus, or ‘endemic typhus’, is prevalent and plague has been reported (Blanc et al., 1936). During the French protectorate of Morocco in the 1930s, the Pasteur Institute of Morocco isolated the agent of the murine typhus, R. typhi (formerly R. mooseri) from rats of the city and port of Casablanca. Two years later, the first case of murine typhus in Morocco was confirmed (Blanc et al., 1936). However, current data are unavailable regarding flea-borne rickettsioses in Morocco.
In this study, we analysed fleas collected in Morocco for evidence of Rickettsia spp. and Bartonella spp. infection to identify the possible aetiological agents and vectors for flea-borne agents that affect humans.
MATERIALS AND METHODS
Collection and Identification of Fleas
From August 2007 to October 2008, fleas were collected from sheep, cats and dogs in Casablanca as well as from sheep and goats in the Tiznit region in southern Morocco and were stored in 70% alcohol and transported to France. Using taxonomic keys (Beaucournu and Launay, 1990), one researcher (NB) identified the fleas morphologically, and a second researcher (JCB) reexamined 5% of each batch.
Polymerase Chain Reaction and Sequencing
All arthropod specimens were surface disinfected by 5-minute immersion in iodinated alcohol before they were rinsed with sterile distilled water for 10 minutes and dried. They were crushed individually in a sterile Eppendorf tube. DNA was extracted from each specimen using the QIAamp Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Each sample was tested for the presence of Rickettsia spp. DNA by real-time semi-quantitative PCR (qPCR) in a Smartcycler, using primers and Taqman probes that targeted a partial sequence of the citrate synthase (gltA) gene (Berrelha et al., 2009). Positive samples were further tested by real-time semi-quantitative PCR using primers and probes that targeted the R. felis bioB gene (Berrelha et al., 2009). Each sample of DNA was screened for the Bartonella genus using qPCR with a Taqman probe that targeted the 16S/23S RNA intergenic spacer (ITS) (Varagnol et al., 2009). In each test, we included a negative control of DNA extracted from uninfected laboratory ticks and a positive control of DNA from R. felis and B. elizabethae (ITS PCR) (one positive control for every 20 samples). The flea DNA samples that tested positive for Bartonella by qPCR were confirmed via standard PCR of the ITS gene fragments (Rolain et al., 2003) using the GeneAmp PCR System 2400 and 9700 thermal cyclers (PerkinElmer, Waltham, MA, USA). Amplification products were analysed by electrophoresis on a 1% agarose gel that was stained with ethidium bromide. To identifythe detected Bartonella spp., PCR products were purified and sequenced in duplicate (both forward and reverse) using the BigDye Terminator v1.1 Cycle Sequencing Kit (ABI PRISM, PE Applied Biosystems, Coignieres, France) and primers specific for the ITS fragments (Rolain et al., 2003). The sequences were resolved on an ABI 3100 capillary sequencer (Applied Biosystems). All sequences were assembled and edited with the Auto Assembler software version 1.4 (PerkinElmer). We used BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi) to compare our sequences with those in the GenBank database.
RESULTS
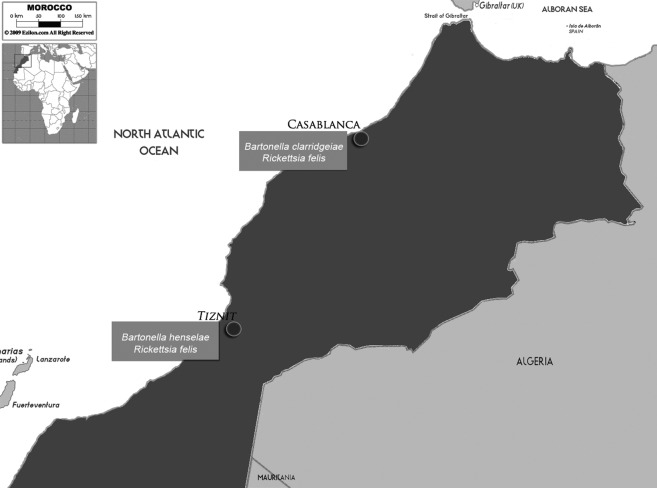
A total of 554 fleas were collected, including 391 Ctenocephalides felis, 130 Pulex irritans and 33 Ctenocephalides spp. In all qPCR and PCR experiments, the expected results were obtained for the positive and negative controls. R. felis DNA was detected in 112 (20%) of 554 fleas. Bartonella DNA was detected by qPCR in 24 (4.3%) of 554 fleas. Seven of the 24 positive fleas (seven Ctenocephalides felis collected in the Casablanca and Tiznit regions) tested positive for Bartonella spp. using the standard ITS PCR. Sequencing identified Bartonella clarridgeiae (100% similarity) in three C. felis samples collected from cats in Casablanca and Bartonella henselae in four C. felis (99.08% similarity) samples collected from goats and sheep in the Tiznit region. Table summarizes our results, and Fig. 1 shows a map of the Moroccan regions used in this survey.
Flea collection and detection of Rickettsia spp. and Bartonella spp., Morocco 2007–2008.
| Flea collection | Results | ||||||||
| Detection of Rickettsia spp. | Detection of Bartonella spp. | ||||||||
| Collection sites (number of fleas collected) | Host | Species of fleas collected | Fleas number | Positive samples using qPCR of a partial sequence of gltA | Positive samples using qPCR of a R. felis gene | Positive samples using PCR of the ITS | Identification by gene sequence (% similarity with the corresponding sequence available in GenBank) | Co-infection | |
| Tiznit | Douar Afaibork (367) | Goats and sheep (3 herds) | Ctenocephalides spp. | 22 | 0 | 0 | 0 | Bartonella henselae | |
| C. felis | 277 | 4 | 4 | 4 | (99.08 % with L35101.1) | ||||
| Pulex irritans | 68 | 0 | 0 | 0 | |||||
| Douar Lahlate (48) | Goats and sheep (1 herd) | Pulex irritans | 48 | 0 | 0 | 0 | |||
| Douar Tazmourt (25) | Sheep (1 herd) | Pulex irritans | 14 | 0 | 0 | 0 | |||
| Ctenocephalides spp. | 11 | 0 | 0 | 0 | |||||
| Casablanca | (114) | Dogs and sheep | C. felis | 105 | 102 | 102 | 0 | ||
| Cats | C. felis | 9 | 6 | 6 | 3 | Bartonella clarridgeiae(100% with AF312497.1) | 2 | ||
Fig. 1.
Map of Moroccan regions of the fleas collection.
DISCUSSION
Our results provide the first evidence of R. felis, B. henselae and B. clarridgeiae in Morocco, and they suggest the presence of the related flea-borne diseases.
R. felis is the agent of flea-borne spotted fever. R. felis was first detected (as ‘R. ctenocephali’) in C. felis in Europe in 1918, was rediscovered in 1990 and was definitively characterized in 2002 (Bitam et al., 2010). This rickettsia has been associated with a large variety of flea species; however, C. felis is currently the only known biological vector of R. felis and serves as the main reservoir. This flea species, likely plays a central role in transmitting human illness (Pérez–Osorio et al., 2008; Reif and Macaluso, 2009). In North Africa, R. felis has been identified in fleas from Algeria (Bitam et al., 2006b). Flea-borne spotted fever is an emerging disease with few confirmed cases throughout the world. The first human case of R. felis infection was reported from Texas in 1994 (Reif and Macaluso, 2009). Since then, additional human cases have been reported from Spain, Germany, France, Egypt, Brazil, Mexico, Thailand, Taiwan, South Korea, Laos, Tunisia and Australia (Parola et al., 2003; Kaabia et al., 2006; Znazen et al., 2006; Williams et al., 2011). More recently, a study conducted over 9 months in two Senegalese villages, found that up to 6% of 134 indigenous febrile non-malaria patients were infected with R. felis (Socolovschi et al., 2010). The major clinical symptoms of these patients were fever associated with weakness, headache with sleep disorders and digestive and respiratory symptoms. The lack of cutaneous rash or inoculation eschar was noted; however, a cutaneous rash might be imperceptible in patients with pigmented skin.
In Morocco, eight different rickettsiae of the spotted fever group have been identified, including seven pathogens: R. conorii, R. aeschlimannii, R. massiliae, R. slovaca, R. monacensis, R. raoultii and R. helvetica (Sarih et al., 2008; Boudebouch et al., 2009a; Boudebouch et al., 2009b).
Our results revealed an important R. felis infestation of Ctenocephalides fleas, which were collected from cats and dogs as well as from a herd of sheep from the same location. Pulex irritans were not found infected, although these fleas have already been found elsewhere to be infected by R. felis (Azad et al., 1997; Sackal et al., 2008).
Our results suggest that R. felis infections are prevalent in Morocco and that fleas are potential vectors of flea-borne spotted fever in this country. Further studies are needed to elucidate and describe the epidemiology of R. felis infections (Pérez–Osorio et al., 2008). However, clinicians in Morocco, and those elsewhere who may see patients who have travelled to Morocco, need to be aware of possible R. felis infections in patients who present signs of spotted fever and/or an eschar. Although it may be misdiagnosed as and treated similarly to tick-borne Mediterranean spotted fever, the risk of exposure and methods of prevention of flea-transmitted diseases are different and warrant consideration.
We further report the first detection of Bartonella clarridgeiae and B. henselae in fleas from Morocco. qPCR appeared to be more sensitive for the detection of these bacteria compared with regular PCR. Bartonella are fastidious, gram-negative bacteria that have been identified on a variety of domestic and wild mammals. Currently, more than 20 known species or subspecies of Bartonella are known. Among the 13 species and subspecies that are known or suspected to be human pathogens, four have been isolated from cats (Chomel and Kasten, 2010). Fleas appear to play an important role in the maintenance and transmission of many Bartonella species among cat and rodent populations (Chomel et al., 2009). B. henselae is the causative agent of cat scratch disease (CSD), which is characterized by benign regional lymphadenopathy, low-grade fever, malaise and aching. In addition, headache, anorexia and splenomegaly can occur, while abscessed lymph nodes are reported occasionally. Five to nine percent of CSD cases may develop atypical manifestations, including Parinaud’s oculoglandular syndrome, encephalitis, endocarditis, hemolytic anemia, hepatosplenomegaly, glomerulonephritis, pneumonia, relapsing bacteremia and osteomyelitis (Chomel and Kasten, 2010). CSD is more frequently observed in persons who are younger than 20 years old and in people who own a young cat or who have been scratched or bitten by a cat (Chomel and Kasten, 2010). B. henselae is also involved in other clinical situations, such as endocarditis, bacillary angiomatosis and peliosis hepatitis in immunocompromised patients (Rolain et al., 2003). Domestic cats are the principal reservoir for B. henselae. Several serotypes have been identified, such as the two main genotypes designated Houston-1 and Marseille. The respective prevalences of these two genotypes vary considerably among cat populations in different geographical areas (Bouchouicha et al., 2009; Angelakis et al., 2010; Chomel and Kasten, 2010).
Bartonella clarridgeiae is known to infect domestic cats. It was first isolated in the USA from the pet of an HIV-positive patient (Chomel and Kasten, 2010). It has been suspected to be an agent of CSD in humans, but its pathogenic role in humans has yet to be definitively demonstrated (Anderson and Neuman, 1997). The transmission of B. clarridgeiae is associated with the inoculation of wounds with infected cat flea (Ctenocephalides felis) feces (Breitschwerdt and Kordick, 2000).
These results suggest the presence of infection with these organisms; however, more studies are needed to precise the prevalence of these agents in arthropods in Morocco to estimate the risk of human exposure.
The incidence of flea-borne diseases in the world is much greater than is generally recognized by physicians and health authorities. As a result, diagnosis and treatment are often delayed by health care professionals who are unaware of the presence of these infections and thus do not take them into consideration during diagnosis.
REFERENCES
- Anderson BE, Neuman MA.(1997)Bartonella spp. as emerging human pathogens. Clinical Microbiology Reviews 10203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E, Billeter SA, Breitschwerdt EB, Chomel BB, Raoult D.(2010)Potential for tick-borne bartonelloses. Emerging Infectious Diseases 16385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM.(1997)Flea-borne rickettsioses: ecologic considerations. Emerging Infectious Diseases 3319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucournu JC, Launay H.(1990)Les Puces de France et du Bassin Méditerranéen Occidental. Faune de France, Vol. 76, Paris: FFSSN [Google Scholar]
- Berrelha J, Briolant S, Muller F, Rolain JM, Marie JL, Pagés F, Raoult D, Parola P.(2009)Rickettsia felis and Rickettsia massiliae in Ivory Coast, Africa. Clinical Microbiology and Infection 15251–252. [DOI] [PubMed] [Google Scholar]
- Bitam I, Baziz B, Rolain JM, Belkaid M, Raoult D.(2006a)Zoonotic focus of plague, Algeria. Emerging Infectious Diseases 121975–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitam I, Dittmar K, Parola P, Whiting M, Raoult D.(2010)Fleas and flea-borne diseases. International Journal of Infectious Diseases 14e667–e676. [DOI] [PubMed] [Google Scholar]
- Bitam I, Parola P, De La Cruz KD, Matsumoto K, Baziz B, Rolain JM.(2006b)First molecular detection of Rickettsia felis in fleas from Algeria. American Journal of Tropical Medicine and Hygiene 74532–535. [PubMed] [Google Scholar]
- Blanc G, Pouponneau A, Baltazard M, Carbou A.(1936)Existence du Typhus murin chez l’homme au Maroc: première observation faite à Casablanca. Maroc Médical 165 [Google Scholar]
- Bouchouicha R, Durand B, Monteil M, Chomel BB, Berrich M, Arvand M, Birtles RJ, Breitschwerdt EB, Koehler JE, Maggi R, Maruyama S, Kasten R, Petit E, Boulouis HJ, Haddad N.(2009)Molecular epidemiology of feline and human Bartonella henselae isolates. Emerging Infectious diseases 15813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudebouch N, Sarih M, Socolovschi C, Amarouch H, Hassar M, Raoult D, Parola P.(2009a)Molecular survey for spotted fever group rickettsiae in ticks from Morocco. Clinical Microbiology and Infection 15259–260. [DOI] [PubMed] [Google Scholar]
- Boudebouch N, Sarih M, Socolovschi C, Fatihi T, Chakib A, Amarouch H, Hassar M, Rolain JM, Parola P, Raoult D.(2009b)Spotted fever group rickettsioses documented in Morocco. Clinical Microbiology and Infection 15257–258. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Kordick DL.(2000)Bartonella infections in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clinical Microbiology Reviews 13428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, Birtles RJ, Koehler JE, Dehio C.(2009)Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Veterinary Research 4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel BB, Kasten RW.(2010)Bartonellosis, an increasingly recognized zoonosis. Journal of Applied Microbiology 109743–750. [DOI] [PubMed] [Google Scholar]
- Kaabia N, Rolain JM, Khalifa M, Ben Jazia E, Bahri F, Raoult D, Letaïef A.(2006)Serologic study of rickettsioses among acute febrile patients in central Tunisia. Annal New York Academy of Science 1078176–179. [DOI] [PubMed] [Google Scholar]
- Parola P, Sanogo YO, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez JP, Miller RS, Telford SR, 3rd, Wongsrichanalai C, Raoult D.(2003)Identification of Rickettsia spp. and Bartonella spp. in fleas from the Thai-Myanmar border. Annal New York Academy of Science 990173–181. [DOI] [PubMed] [Google Scholar]
- Pérez-Osorio CE, Zavala-Velázquez JE, Arias León JJ, Zavala-Castro JE.(2008)Rickettsia felis as emergent global threat for humans. Emerging Infectious Diseases 141019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif KE, Macaluso KR.(2009)Ecology of Rickettsia felis: a review. Journal of Medical Entomology 46723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain JM, Franc M, Davoust B, Raoult D.(2003)Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerging Infectious Diseases 9338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackal C, Laudisoit A, Kosoy M, Massung R, Eremeeva ME, Karpathy SE, Van WK, Gabitzsch E, Zeidner NS.(2008)Bartonella spp. And Rickettsia felis in fleas, Democratic Republic of Congo. Emerging Infectious Diseases 141972–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarih M, Socolovschi C, Boudebouch N, Hassar M, Raoult D, Parola P.(2008)Spotted fever group rickettsiae in ticks, Morocco. Emerging Infectious Diseases 141067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolovschi C, Mediannikov O, Sokhna C, Tall A, Diatta G, Bassene H, Trape J.-F, Raoult D.(2010)Rickettsia felis-associated uneruptive fever, Senegal. Emerging Infectious Diseases 161140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagnol M, Parola P, Jouan R, Beaucournu J.-C, Rolain JM, Raoult D.(2009)First detection of Rickettsia felis and Bartonella clarridgeiae in fleas from Laos. Clinical Microbioly and Infection 15334–335. [DOI] [PubMed] [Google Scholar]
- Williams M, Izzard L, Graves SR, Stenos J, Kelly JJ.(2011)First probable Australian cases of human infection with Rickettsia felis (cat-flea typhus). Medical Journal of Australia 19441–43. [DOI] [PubMed] [Google Scholar]
- Znazen A, Rolain JM, Hammami A, Jemaa MB, Raoult D.(2006)Rickettsia felis infection, Tunisia. Emerging Infectious Diseases 12138–140. [DOI] [PMC free article] [PubMed] [Google Scholar]