Abstract
To study the prevalence of leptospira in acute hepatitis syndrome and to assess interleukin (IL)-8 and tumour necrosis factor (TNF)-alpha levels in the pathogenesis of hepatitis due to leptospiral infection.
Two hundred and forty-seven consecutive cases with symptoms of acute hepatitis and 30 healthy controls were enrolled in the study and detailed clinical history was elicited from them. Enzyme-linked immunosorbent assays (ELISAs) for HAV, HBV, HCV and HEV were performed to rule out common viral aetiology of hepatitis. IgM antibodies to leptospira were detected by ELISA. IL-8 and TNF-alpha levels were estimated in leptospira-positive cases and healthy controls by ELISA.
Out of 247 cases of acute hepatitis, 46 (18.62%) were observed to be positive for IgM antibodies for leptospira. The mean age of these patients was 31.99±0.28 years (25 males and 21 females; M/F ratio: 1.19∶1). The mean ALT, AST and ASP were raised in the majority of patients. IL-8 was found to be elevated (130.81 pg/ml) in a large majority of cases 41/46, 89.1% (P<0.001). Patients with more severe symptoms were associated with higher levels of IL-8. One mortality was observed due to leptospira. Unpredictably, TNF-alpha level was largely suppressed (45.63 pg/ml) in most of the leptospira-positive patients in comparison with healthy controls.
Leptospira-induced hepatitis should be actively looked for in patients negative for A–E viral hepatitis. IL-8 appears to play an important role in the pathogenesis of leptospiral hepatitis. High TNF-alpha should alert clinicians for aggressive in hospital management of patients.
INTRODUCTION
Leptospirosis, a spirochaetal zoonosis, is a globally re-emerging infectious disease that is gradually disseminating from its habitual rural base and is making inroads into urban areas (Sarkar et al., 2002; Dias et al., 2007; Magalhaes et al., 2010). It is considered the most widespread worldwide zoonosis with possible fatal outcomes. The infection may be transmitted to humans by direct contact with infected tissues, contaminated urine or indirectly by contact with water or moist soil contaminated with the urine of mammalian reservoirs. Lack of basic sanitation has produced conditions favouring rodent borne transmission of leptospirosis.
The incidence rates of leptospirosis are usually underestimated partly due to the lack of awareness of the disease and due to the paucity of easy, rapid and accurate tests to permit early diagnosis of the disease. Protean and non-specific presentations of leptospirosis have often led to misdiagnosis, especially in malaria, dengue and viral hepatitis endemic regions of South Asia, South America and the Caribbean (Levett et al., 2000; Flannery et al., 2001; LaRocque et al., 2005). Although there have been some reports of leptospirosis from Chandigarh, Delhi and Mumbai (Bharadwaj et al., 2002; Sambasiva et al., 2003; Luzia et al., 2009), leptospirosis continues to remain largely undetected in most parts of India. Moreover, few investigators have conducted a comparative study of incidence and clinical characteristics of leptospirosis and viral hepatitis in patients presenting with acute hepatitis syndrome (Kanti et al., 2002; Ganchevam et al., 2007). We have earlier reported its pre-eminent role in the causation of cryptogenic hepatitis in our region (Rizvi et al., 2011).
Validated prognostic factors to help forecast the evolution of leptospirosis are scarce. They would be invaluable to physicians to decide whether their patients should be advised domiciliary treatment, admitted in the hospital or directed to the intensive care unit. Little data have been published addressing this issue. Besides, direct injury by microbial factors, cytokines produced in response to infection have been proposed to be involved in pathogenesis of leptospirosis. It is thought that an exaggerated immune response may play a pivotal role in the pathophysiology of leptospira. While tumour necrosis factor (TNF)-alpha, interleukin (IL)-6, IL-10 and interferon-gamma have been studied in great detail, not many reports have emerged regarding the role of IL-8 in relation to leptospirosis (Alisa et al., 2010; Wagenaar et al., 2009; Kyriakidis et al., 2011). IL-8 is produced by a host of cells including monocytes, macrophages, kupffer cells and hepatocytes. Serum IL-8 levels are markedly elevated in several conditions like alcoholic hepatitis (Kyriakidis et al., 2011) and non-alcoholic steato-hepatitis (Wagenaar et al., 2009). TNF-alpha is the primary mediator in hepatic inflammation. Elevated TNF-alpha levels have been observed in previous studies (Odeh, 2007). We wanted to assess whether IL-8 and TNF-alpha play a role in the pathogenesis of leptospiral hepatitis.
The purpose of this study was threefold: (1) to measure the relative importance of leptospirosis infection in conjunction with hepatitis like syndrome; (2) to compare the clinical presentation of leptospiral hepatitis with acute viral hepatitis (AVH); and (3) to assess IL-8 and TNF-alpha levels of leptospiral hepatitis and whether they could be used as prognostic markers.
MATERIALS AND METHODS
Two hundred and forty-seven consecutive cases [160 male (65%) and 87 female (35%); mean age: 31.99±14.02 years] with symptoms of acute liver disease attending the Medicine Outpatient Department or admitted in the Medicine Wards of J.N. Medical College were recruited in the study over a 10-month period from June 2009 to March 2010. This study was conducted in the Department of Microbiology, J.N. Medical College, A.M.U., Aligarh. A written informed consent was obtained from each patient. The study was cleared by the Institutional Ethical Committee of J.N. Medical College. The sample size was calculated using standard formula. The numbers of cases and controls taken in the study are adequate. All patients underwent complete physical examination and detailed clinical history was elicited from them. Patients with autoimmune hepatitis, alcoholic hepatitis and drug-induced hepatitis, renal, pulmonary disorders, other acute or chronic inflammatory diseases or patients who had history of surgery, trauma within the preceding 2 months were excluded from the study.
Healthy Control Individuals
The control group consisted of 30 healthy people who had no history of fever in the last 6 months [22 male (73.34%) and 8 female (26.66%); mean age: 37 years] and were selected from the blood bank.
Collection and Transport of Sample
Blood of 7 ml was collected aseptically from all patients with acute liver disease within 1week±3 days of onset of symptoms. Serum was separated by centrifugation, aliquoted and stored at −20°C till further tests were performed. A battery of biochemical and haematological investigations were conducted.
Routine Investigations
Liver function tests, prothrombin time and total bilirubin were performed. Specific investigations like ultrasonographic examination of liver, upper GI endoscopy and liver biopsy were performed wherever feasible.
Serological Tests for Hepatitis Viruses
The sera were first tested for hepatitis A, B, C and E virus markers using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions: HAV IgM and HEV IgM antibodies were detected by ELISA kits (DRG International, Inc., USA). Sera of all patients were subjected to detailed screening for HBV serological markers, i.e. HBsAg (J. Mitra & Co. Pvt. Ltd, New Delhi, India), Anti-HBc (MonolisaTM; Bio-Rad, Steevoorde, France), IgM anti-HBc (DRG International, Inc., Mountainside, NJ, USA), HBeAg (DRG International, Inc.), anti-HBe (Radim, Rome, Italy) and Anti-HCV antibodies were detected by the third-generation immunoassay test (J. Mitra & Co. Pvt. Ltd).
Serological Test for Leptospira
Patients negative for all the hepatitis viruses and 30 healthy controls were tested for evidence of recent leptospiral infections by specific leptospira IgM antibody using the commercially available ELISA kit (DRG International, Inc.). The test procedure was performed according to the protocol provided along with the kit and absorbance was read at 450 nm. The results for leptospira IgM ELISA were interpreted according to the manufacturer’s instructions, i.e. values, 0.0–0.3 optical density (OD) units (DRG ELISA) were considered negative, 0.5<1.0 OD units were equivocal and >1.0 OD units were positive. For samples showing equivocal results, another blood sample was drawn after a period of 10 or more days, and the test was repeated. Negative and positive control sera were provided by the manufacturer and their absorbances were used for the calculation of the cutoff and for determining the validity of the test.
Cytokine Analysis
Serum IL-8 and TNF-alpha levels were estimated in leptospira-infected patients and healthy controls by ELISA obtained from Orgenium (Helsinki, Finland). Absorbance was read at 450 nm in an automated ELISA plate reader. A standard curve of optical densities versus concentrations of IL-8 and TNF-alpha was generated to determine their concentrations in serum samples.
Statistical Analysis
Chi-square test and Student’s t-test were used for leptospira prevalence. The non-parametric Mann–Whitney U test was used for unpaired comparisons of the levels of IL-8 and TNF-alpha in patient sera. Receiver operating characteristics (ROC) curve was used to analyse the cytokines using Med Calc programme. The level of significance in all cases was set at a two-tailed P<0.05.
RESULTS
Among 247 consecutive patients presenting with acute hepatitis, 142 (57.4%) were diagnosed with AVH, of which 41 (16.6%) individuals were HBV-positive, 6 (2.43%) were positive for anti-HCV, 63 (25%) were infected with anti-HAV and 32 (12.9%) were found to be HEV-positive as seen in Table 1 (the mean age of AVH cases was 37.75±0.77 years). Of the remaining 105, 46 (44%; 95%CI: 53.37–34.69%) were positive for IgM antibodies against leptospira. The prevalence of leptospira among 247 patients was 18.6% (95%CI: 67–29.8%). The mean age of leptospira-positive cases was 28.27±0.34 years and the M/F ratio was 1.19∶1. None of the healthy controls were positive for leptospiral IgM antibodies (P<0.001). The OD in the leptospira-positive patients ranged from 1.68 to 2.847, while in the control group, OD was far below the cutoff. Repeat samples were requested in nine cases where the OD was equivocal. In three cases, a fourfold rise in OD was observed. The remaining six samples did not demonstrate a rise and were considered negative for leptospira.
Table 1. Distribution of leptospiral and viral hepatitis (A–E)-positive cases.
| Etiology of acute viral hepatitis | Number | Percentage |
| HBV | 41 | 16.6% |
| HCV | 06 | 2.4% |
| HAV | 63 | 25.5% |
| HEV | 32 | 12.9% |
| Leptospira | 46 | 18.6% |
There was a clustering of both leptospirosis and hepatitis A and E in the rainy season with 31 (67.39%) cases of leptospira and 58 (61%) of A or E hepatitis viruses occurring in the monsoon months of July to September. Thirty-seven (80.4%) leptospirosis cases were from rural areas. Thirty-nine (84.78%) gave history of contact with animals and 43 (93.47%) gave history of frequent contact with unclean ponds.
A comparison of the clinical profile of AVH and leptospirosis is given in Table 2. Oliguria, pain in calf muscles, tachycardia, renal tenderness and conjunctival suffusion were seen almost exclusively in patients with leptospirosis, although they did not represent the dominant signs and symptoms. Serum bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were uniformly raised in all the patients, although in cases of AVH, ALT and AST levels were around four- to fivefold higher than in leptospira-positive patients which exhibited two- to threefold elevations as seen in Table 3. Erythrocyte sedimentation rate (ESR) was significantly higher in leptospirosis (P<0.001). Almost the same level of hypoproteinemia and hypoalbuminemia was observed in both groups.
Table 2. Comparison of clinical features of leptospirosis and acute viral hepatitis (AVH).
| Clinical features | Leptospirosis (%) | AVH (A–E) (%) | P value |
| (n = 46) | (n = 142) | ||
| Jaundice | 35 (76) | 134 (94.36) | |
| Fever | 45 (97.8) | 88 (61.97) | |
| Headache | 21 (45.6) | 22 (15.49) | |
| Anorexia | 27 (58.6) | 115 (80.98) | |
| Nausea/vomiting | 17 (36.9) | 120 (84.5) | <0.05 |
| Myalgia | 16 (34.7) | 39 (27.46) | |
| Hepatomegaly | 17 (36.9) | 142 (100) | <0.001 |
| Splenomegaly | 5 (10.86) | 82 (57.7) | <0.01 |
| Conjunctival suffusion | 6 (13) | … | <0.001 |
| Diarrhoea | 4 (8.6) | … | |
| Abdominal pain | 31 (67.3) | 52 (36.6) | |
| Respiratory symptoms | 9 (19.5) | … | |
| Oliguria | 26 (57.2) | … | <0.01 |
| Chills | 34 (74.3) | 26 (19.7) | <0.001 |
| Calf muscle pain | 37 (80.5) | … | <0.001 |
| Arthralgia | 3 (7.2) | 15 (10.56) | |
| Tachycardia | 30 (64.2) | … | <0.001 |
| Tachypnea | 26 (55.4) | … | <0.001 |
| Renal tenderness | 7 (14.8) | … | |
| Acute onset | 43 (94.2) | 112 (78.87) |
Table 3. Biochemical profile of leptospirosis and acute viral hepatitis (AVH).
| Parameters | Leptospirosis (mean values) | AVH (mean values) | Reference range |
| Serum glutamic oxaloacetic transaminase (SGOT) | 31.3 | 160.33 | 2–20 IU/l |
| Serum glutamic pyruvic transaminase (SGPT) | 32.61 | 127.16 | 2–15 IU/l |
| Alkaline phosphatase (ALP) | 16.46 | 19.91 | 3–13 KAU/100 ml |
| Serum bilirubin | 2.84 | 13.66 | 0.2–1.0 mg/100 ml |
| Albumin | 2.75 | 3.06 | 3.4–5.4 g/dl |
| International normalized ratio (INR) | 1.64 | 2.48 | 0.8–1.2 |
| Erythrocyte sedimentation rate (ESR) | 44 | 18 | M: 12–19 |
| F: 18–21 |
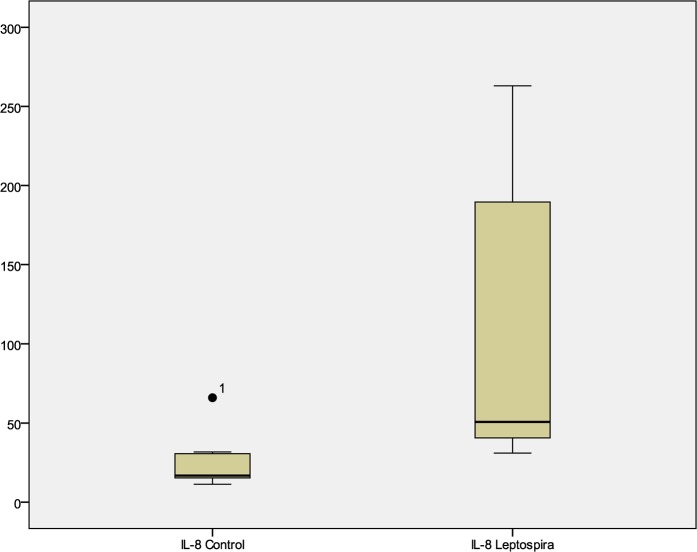
IL-8 levels were elevated in a large majority, 41 (89.1%) of patients with leptospirosis as seen in Fig. 1. The median level of IL-8 was 130.81 pg/ml in them. IL-8 in the control group was 16.738 pg/ml (P<0.001). On examining the ROC curve of IL-8 in relation to leptospiral hepatitis, the significant cutoff level of IL-8 was >31.73 pg/ml. The sensitivity was 77.8%; specificity was 85.7%; positive predictive value was 93.3%; negative predictive value was 60%; area under curve was 0.833 with 95%CI of 0.631–0.951 (P<0.001). Higher levels of IL-8 corresponded with three- to fourfold higher levels of liver enzymes. These patients also presented with a more severe spectrum of clinical signs and symptoms. Seventy-eight percent had hepatomegaly, 84% had jaundice, 90% had nausea and vomiting and 84% complained of abdominal pain.
Fig. 1.
Box plot of interleukin (IL)-8 level in leptospira-infected patients and healthy control.
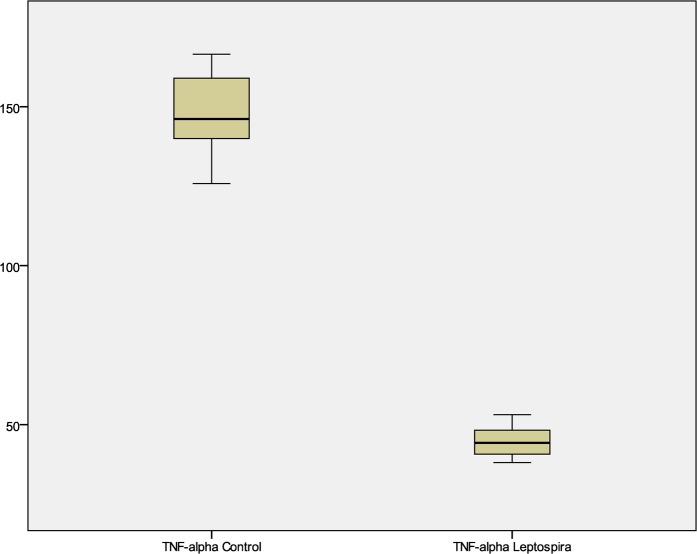
Surprisingly, TNF-alpha levels were largely suppressed in all the patients infected with leptospira-induced hepatitis. Elevated TNF-alpha level was seen only in the patient who expired. The median serum TNF-alpha level was 45.63 pg/ml in patients while in healthy controls was much higher at 146.19 pg/ml (Fig. 2). The significant cutoff for TNF-alpha was ⩽53.16 pg/ml by ROC analysis. Both sensitivity and specificity were 100%; area under curve was 1 with 95%CI of 0.86–1 (P<0.00).
Fig. 2.
Box plot of tumor necrosis factor-alpha level in leptospira-infected patients and healthy control.
DISCUSSION
In much of the developing world, where laboratory capabilities for confirmation of acute leptospiral infection are lacking, leptospirosis as an acute disease is rarely considered in clinical differential diagnosis. Leptospirosis significantly contributes to acute disease in Southeast Asia and can mimic hepatitis, dengue and even febrile illnesses. The non-specific signs and symptoms probably have contributed to significant underreporting, most notably in association with jaundice. High regional endemicity of other diseases, such as malaria, viral hepatitis and dengue hemorrhagic fever, also leads clinicians to assign incorrect presumptive diagnostic status in the absence of alternative diagnostic considerations and supportive laboratory testing. Co-infection of viral hepatitis with leptospira may also occur (Christou et al., 2008).
In our study, 18.6% patients present with acute hepatitis which exhibited serological evidence of recent leptospiral infections. Leptospira appears to be emerging in this region. We first reported its presence in 2010. This region is not endemic for leptospirosis. A higher prevalence of leptospira (28%) in cases of acute jaundice disease has been reported from Indonesia, 22% from Lao people’s Democratic Republic and 20% from Vietnam. A lower prevalence of 8% has been reported from Cambodia (Kanti et al., 2002). The high prevalence of leptospira in cases of acute hepatitis suggests that it should be considered in the differential diagnosis in patients negative for viral hepatitis. Signs and symptoms of leptospiral hepatitis were very similar to that of AVH which suggests that a high degree of suspicion should be maintained. The most common presenting complaints in these patients were fever, jaundice and anorexia. The liver enzymes were moderately raised. Jaundice and bilirubinemia disproportional to hepatocellular damage are common in leptospirosis. Oliguria, renal tenderness, tachycardia, pain in calf muscles and conjunctival suffusion should be specifically looked for as they are useful pointers towards leptospirosis.
Detection of IgM antibodies to leptospira by ELISA is now widely used in diagnosis of leptospirosis, which is more sensitive than microscopic agglutination test (MAT) and most laboratories prefer IgM ELISA formats for the diagnosis of leptospirosis (Sambasiva et al., 2003). Furthermore, this test is reactive even in early cases of leptospirosis when MAT may be negative (Sambasiva et al., 2003). MAT does not have any diagnostic significance in the first week and antibodies peak around the third week. Moreover, it is a very highly complex test to perform, and interpret and therefore can be done only in a reference laboratory. Studies have shown that IgM antibodies could be detected by ELISA from as early as the fifth day to the sixtieth day after acute infection with leptospirosis (Sumathi et al., 1995). However, in highly endemic areas, the prevalence of IgM antibodies may be high and the results should be interpreted with care. In case of other concurrent febrile diseases, cross-reactivity may occur.
IL-8 was observed to be uniformly elevated in all the patients infected with leptospirosis. At present, limited data are available on potential biomarkers that may aid the clinician in monitoring disease progression and identify severe disease at an early stage which might reduce mortality. In this study, we examined the IL-8 and TNF-alpha in their ability to distinguish disease severity. Few studies have reported the role of IL-8 in the pathogenesis of leptospirosis (Kyriakidis et al., 2011). This is a preliminary report on the role of IL-8 in patients with hepatitis induced by leptospirosis. IL-8 appears to play a dominant role in hepatitis as it was raised in 98% patients. One patient died after developing severe hepatitis and acute renal disease. IL-8 levels were extremely raised in this patient. These findings suggest that high IL-8 levels are associated with severe disease. Similar observations were made by Wagenaar et al. (2009), where IL-8 was considered a significant predictor of mortality. Kyriakidis et al. (2011) compared IL-8 levels in survivors and non-survivor of Weil’s disease and reported no significant difference between the two groups. We compared IL-8 levels between healthy controls and leptospira-infected patients. Significantly lower IL-8 levels were observed in healthy controls (P<0.01). The TNF-alpha level, on the other hand, was low in majority of cases except in the one case who expired where it was considerably raised. Low levels of TNF-alpha, a pro-inflammatory cytokine could be the cause of non-progression of disease to Weil’s disease. High TNF-alpha level has been associated with severity of disease and increased mortality among patients with leptospirosis (de Fost et al., 2003; Kyriakidis et al., 2011). Thus, TNF-alpha could serve as a valuable prognostic indicator in leptospirosis for monitoring progression of disease.
Elevated levels of IL-8 with viral and non-viral hepatitis (Bird et al., 1994: Polyak et al., 2001) suggest that this chemokine may play a role in both viral and non-viral pathogenesis of liver disease. We propose that severe disease manifestations with fatal outcome are accompanied with elevated levels of both IL-8 and TNF-alpha. This is a baseline study and it will be useful to assess the levels of cytokines in both mild and severe leptospiral infections. A single point evaluation of IL-8 and TNF-alpha was carried out in our study which is a limitation.
We recommend that a high index of suspicion for leptospirosis should be maintained in patient presenting with acute hepatitis syndrome, especially so in the presence of history of rural background, animal contact with water contaminated with urine. Early diagnosis of this potentially dangerous disease is imperative in preventing fatalities caused by leptospirosis.
Acknowledgments
This work was supported by grants from Department of Science and Technology (DST), Ministry of Science and Technology, INDIA.
REFERENCES
- Alisa L, Sunchai P, Amornpun S, Pattama E, Duangporn P, Yong P, Chintana C.(2010)Expression of TNF-a, TGF-b, IP-10 and IL-10 mRNA in kidneys of hamsters infected with pathogenic Leptospira. Comparative Immunology, Microbiology and Infectious Diseases 33423–434. [DOI] [PubMed] [Google Scholar]
- Bharadwaj R, Bal AM, Joshi SA, Kagal A, Pol SS, Garad G, Arjunwadkar V, Katti R.(2002)An urban outbreak of leptospirosis in Mumbai, India. Japanese Journal of Infectious Diseases 55194–196. [PubMed] [Google Scholar]
- Bird G.(1994)Interleukin-8 in alcoholic liver disease. Acta Gastroenterologica Belgica 57255–259. [PubMed] [Google Scholar]
- Christou L, Kalambokis G, Tsianos EV.(2008)Weil’s disease in a patient with chronic viral hepatitis and history of alcohol abuse. Internal Medicine 47933–937. [DOI] [PubMed] [Google Scholar]
- de Fost M, Hartskeerl RA, Groenendijk MR, van der Poll T.(2003)Interleukin 12 in part regulates gamma interferon releasein human whole blood stimulated with Leptospira interrogans. Clinical and Diagnostic Laboratory Immunology 10332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias JP, Teixeira MG, Costa MC, Mendes CM, Guimarães P, Reis MG, Ko A, Barreto ML.(2007)Factors associated with Leptospira sp. infection in a large urban center in Northeastern Brazil. Revista da Sociedade Brasileira de Medicina Tropical 40499–504. [DOI] [PubMed] [Google Scholar]
- Flannery B, Pereira MM, Velloso LdeF, Carvalho CdeC, De Codes LG, Orrico GdeS, Dourado CM, Riley LW, Reis MG, Ko AI.(2001)Referral pattern of leptospirosis cases during a large urban epidemic of dengue. American Journal of Tropical Medicine and Hygiene 65657–663. [DOI] [PubMed] [Google Scholar]
- Ganchevam G, Tzvetanova C, Ilieva P.(2007)Comparative study in leptospirosis and acute viral hepatitis. Journal of IMAB, Annual Proceeding (Scientific Papers) 1325–28. [Google Scholar]
- Kanti L, Cao BV, Khanthong B, Nguyen TKT, James GO, Sisouk T, Tran NVA, Hoang KL, Narain P, Ha BK, Ung SA, Sithat I, Douglas MW, Beecham HJ, Andrew LC.(2002)The importance of leptospirosis in Southeast Asia. American Journal of Tropical Medicine and Hygiene 67278–286. [DOI] [PubMed] [Google Scholar]
- Kyriakidis I, Samara P, Papa A.(2011)Serum TNF-a, sTNFR1, IL-6, IL-8 and IL-10 levels in Weil’s syndrome. Cytokine 54117–120. [DOI] [PubMed] [Google Scholar]
- LaRocque RC, Breiman RF, Ari MD, Morey RE, Janan FA, Hayes JM, Hossain MA, Brooks WA, Levett PN.(2005)Leptospirosis during dengue outbreak, Bangladesh. Emerging Infectious Diseases 11766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN, Branch SL, Edwards CN.(2000)Detection of dengue infection in patients investigated for leptospirosis in Barbados. American Journal of Tropical Medicine and Hygiene 62112–114. [DOI] [PubMed] [Google Scholar]
- Luzia SC, Roberto V, Antonio AL.(2009)Leptospirosis: a worldwide resurgent zoonosis and important cause of acute renal failure and death in developing nations. Ethnicity & Disease 1937–41. [PubMed] [Google Scholar]
- Magalhaes JV, Teruszkin IB, Sutter de Oliveira F, Costa ADS, Hillen L, Pereira MM.(2010)Multiplex PCR-based detection of leptospira in environmental water samples obtained from a slum settlement. Memórias do Instituto Oswaldo Cruz 105353–355. [DOI] [PubMed] [Google Scholar]
- Odeh M.(2007)Pathogenesis of hepatic encephalopathy: the tumour necrosis factor-α theory. European Journal of Clinical Investigation 34291–304. [DOI] [PubMed] [Google Scholar]
- Polyak SJ, Khabar KS, Rezeiq M, Gretch DR.(2001)Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. Journal of Virology 756209–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi M, Azam M, Shukla I, Malik A, Ajmal MR.(2011)Leptospirosis: an emerging disease in cryptogenic hepatitis patients in North India. Biology and Medicine 398–105. [Google Scholar]
- Sambasiva RR, Naveen G, Bhalla P, Agarwal SK.(2003)Leptospirosis in India and rest of the world. Brazilian Journal of Infectious Diseases 7178–193. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Nascimenrto SF, Barbosa R, Martins R, Nuevo H, Kalafanos I, Grunstein I, Flannery B, Dias J, Riley LW, Reis MG, Ko AI.(2002)Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. American Journal of Tropical Medicine and Hygiene 66605–610. [DOI] [PubMed] [Google Scholar]
- Sumathi G, Subudhi CHPK, Manuel HPS, Shivakumar KS, Rajendran S, Muthusethupathi MA.(1995)Serodiagnosis of leptospirosis — a Madras study. Indian Journal of Medical Microbiology 13192–195. [Google Scholar]
- Wagenaar JFP, Goris MGA, Gasem MH, Isbandrio B, Moalli F, Mantovani A, Boer KR, Hartskeerl RA, Garlanda C, Gorp ECM.(2009)Long pentraxin PTX3 is associated with mortality and disease severity in severe leptospirosis. Journal of Infection 58425–432. [DOI] [PubMed] [Google Scholar]