Abstract
Dichloroacetate (DCA) and trichloroacetate (TCA) are drinking water chlorination byproducts previously found to induce oxidative stress (OS) in hepatic tissues of B6C3F1 male mice. To assess the effects of mixtures of the compounds on OS, groups of male B6C3F1 mice were treated daily by gavage with DCA at doses of 7.5, 15, or 30 mg/kg/day, TCA at doses of 12.5, 25, or 50 mg/kg/day and three mixtures of DCA and TCA (Mix I, Mix II and Mix III), for 13 weeks. The concentrations of the compounds in Mix I, II and III corresponded to those producing approximately 15, 25 and 35%, respectively, of maximal induction of OS by individual compounds. Livers were assayed for production of superoxide anion (SA), lipid peroxidation (LP) and DNA single strand breaks (SSB). DCA, TCA and the mixtures produced dose-dependent increases in the three tested biomarkers. Mix. I and II effects on the three biomarkers, and Mix. III effect on SA production were found to be additive, while Mix. III effects on LP and DNA-SSB were shown to be greater than additive. Induction of OS in livers of B6C3F1 mice after sub-chronic exposure to DCA and TCA was previously suggested as an important mechanism in chronic hepatotoxicity/hepatocarcinogenicity induced by these compounds. Hence, there may be rise in exposure risk to these compounds as these agents co-exist in drinking water.
INTRODUCTION
Drinking water chlorination is a disinfection process that is associated with the production of several haloacetate by-products from the reaction of chlorine with organic material present in the surface water [Miller and Uden, 1983]. Dichloroacetate (DCA) and trichloroacetate (TCA) were found to be among the haloacetates formed during that process [Richardson et al., 2008]. The compounds are also formed as metabolites of the widely used industrial solvent, trichloroethylene [Dekant et al., 1985; Fang et al., 2013; Lash et al, 2000] that contaminates the surface waters in certain locations. Hepatocarcinogenic and hepatotoxic effects were found to be the predominant effects produced in rodents after acute and chronic exposure to DCA and TCA [Bull et al., 1990; Daniel et al., 1992; DeAngelo et. al., 1989; 1991; 1999; Herren-Freund et al., 1987; Pereira, 1996]. Recently, chronic studies on DCA and TCA demonstrated induction of various biomarkers of oxidative stress (OS) and modulation of antioxidant enzyme activities and glutathione (GSH) levels in livers of mice, and that these biomarkers were significantly induced earlier than induction of any hepatotoxic/ hepatocarcinogenic effects leading to them [Hassoun et al., 2010a; Hassoun and Cearfoss, 2011]. Dose-dependent increases in tinduction of hepatic superoxide anion (SA), lipid peroxidation (LP) levels and DNA damage in response to subchronic doses of DCA and TCA ranging from 7.7–410 mg/kg/day mg/kg/day, with maximal elevation in induction of those biomarkers achieved by DCA and TCA daily doses of 154 and 410 mg/kg/day, respectively [Hassoun et al, 2010a]. The induction of hepatic OS after sub-chronic exposure of mice to DCA and TCA was also shown to be associated with induction of phagocytic activation in the same animals [Hassoun et al., 2010b]. Since humans experience life-time exposure to mixtures rather than individual compounds, and the outcome of toxicity levels in response to mixtures may vary from those induced by individual compounds, studies on chronic effects of mixtures of the compounds is important. However, investigations on the adverse effects of mixtures of haloacetates are currently scarce. Pereira et al. [1997] studied promotion by mixtures of DCA and TCA of N-methyl-N-nitrosourea-initiated cancer in liver of female B6C3F1 mice and noted that the proliferative lesions promoted by DCA were different from those of TCA, but the mixture effects were at least additive and the lesions were predominately similar to those promoted by DCA. Bull et al [2002], indicated differences in immunoreactivity to a c-Jun antibody of tumors induced by DCA and TCA in mice. When the compounds were administered in various combinations they produced a few tumors that were c-Jun+, many that were c-June-, but a number with a mixed phenotype that rose with the relative dose of DCA. Bull et al (2002) also reported additivity in tumor numbers when low doses of DCA were combined with high doses of TCA without the use of tumor initiator. Narotsky et al. [2011] examined developmental toxicity of mixtures of 5 haloacetates in pregnant rats and reported elevation in resorption rates and eye malformation in the surviving litters.
Since previous studies suggested OS as an early biomarker of DCA and TCA-induced toxicity chronic hepatotoxicity, this study was designed to (1) examine the additivity model on production of various biomarkers of OS-related effects in livers of B6C3F1 mice after subchronic exposure to well-defined mixtures of DCA and TCA, and (2) provide a basis for future testing of more complex mixtures containing other haloacetates.
METHODS
Chemicals
All chemicals and reagents used for this study including sodium dichloroacetate (DCA) and sodium trichloroacetate (TCA), were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA) and were at the highest grade available.
Animals and Treatments
This study was conducted on B6C3F1 male mice according to a protocol that had been approved by the Institutional Animal Care and Handling Committee at the University of Toledo. Animals were purchased from Harlan Teklad (Indianapolis, Indiana) at 6 weeks of age and weighing approximately 20 g when they were received. Mice were allowed to acclimate for three days before the start of the experiment and had free access to food and water while caged in a room at 21° C with a 12 hr light/dark cycle. Groups of animals (6/ group) were randomly assigned for the different treatments. DCA and TCA were dissolved in distilled water (pH adjusted to 7), and solutions of the compounds were administered to the animals by gavage at doses of 7.5, 15, or 30 mg DCA/kg/day, and 12.5, 25, or 50 mg TCA/kg/day for 13 weeks. The doses of DCA and TCA were chosen based on a previous study and corresponded approximately to those producing 15, 25 and 35% of maximal induction of OS in livers of B6C3F1 mice after subchronic exposure [Hassoun et al., 2010a]. Three different mixtures of DCA and TCA were prepared (Mix I, Mix II and Mix III) in distilled water at DCA: TCA ratios that corresponded to 7.5:12.5, 15:25, and 30:50 mg/kg/day, in Mix I, II and II respectively. After adjusting the pH of the mixture solutions to 7 with NaOH, chemicals were administered to groups of mice by gavage daily for 13 weeks. Control animals were administered distilled water (pH adjusted to 7) daily by gavage for 13 weeks. The animals were monitored for signs of toxicity and unusual behaviors on daily basis. No toxicity or changes in the behaviors were observed in any of the treatment groups. The body weights of the animals were also monitored on weekly basis. No significant changes were observed when comparing any of the treated groups with control. All groups were euthanized 24 hr after the last dose using carbon dioxide anesthesia followed by cervical dislocation. The livers were collected, weighed and used for the determination of superoxide anion (SA), lipid peroxidation (LP) levels, and DNA-single strand breaks (SSB) production.
Sample preparation for SA and LP assays
A part of each liver was removed, weighed and homogenized in Tris-KCl buffer (0.05 M Tris-HCl and 1.15% KCl, pH adjusted to 7.4) to produce 10% (w/v) homogenate that was used for the determination of SA and LP production.
Determination of Superoxide anion (SA)
SA measurements in the tissue homogenates were based on the cytochrome c reduction method of Babior et al. [1973]. This method has been extensively used in our lab and in several other labs, and results were found to be reproducible. Further, the specificity of this method for SA production in liver homogenates was previously tested by adding superoxide dismutase (SOD) to the assay mixture of liver homogenate, where a 91.1% inhibition of cytochrome c reduction was reported [Hassoun and Dey, 2008]. SOD is an antioxidant enzyme that acts specifically on SA, resulting in SA dismutation to hydrogen peroxide [Davies, 1995]. In brief, the reaction tubes contained 50 µl Tris-KCl liver homogenate mixed with 1.5 ml of 45 nmol cytochrome c solution in phosphate buffered saline (PBS), pH 7.2, and incubated for 15 min at 37°C. After stopping the reactions by placing tubes in crushed ice for 2 min, reaction tubes were centrifuged at 2500g for 15 min and absorbance of supernatant measured at 550nm, using a µQuant™ spectrophotometer (BioTek, Winooski, VT). Absorbance values were converted to nmoles of cytochrome c reduced/min using the extinction coefficient 2.1 × 104 M−1 cm−1.
Determination of lipid peroxidation (LP)
The thiobarbituric acid reactive substances (TBARS) method was used to determine LP production in the homogenates [Halliwell and Chirico, 1993; Uchiyama and Mihara, 1977], with modification. The assay involved mixing 0.5 ml Tris-KCl liver homogenate, 3 ml 1% phosphoric acid, 1 ml 0.6% thiobarbituric acid. Butylated hydroxyanisol solution (30 mg/ml) was also added to each sample at a volume of 100 µl to limit the formation of additional oxidation products that may generate during the heating process. The reaction tubes were heated in a water bath at 100°C, for 45 min and then left to cool at room temperature. TBARS were extracted with 4 ml of n-butanol that were added to each reaction tube, and tubes were then vortexed and centrifuged at 2500 g for 10 min. The butanol layer was separated and absorbance measured at 535 nm using a µQuant™ spectrophotometer (BioTek, Winooski, VT). Absorbance values were converted to nmoles of TBARS formed using the extinction coefficient 1.556 × 105 M−1 cm−1.
Determination of DNA Single Strand Breaks (DNA SSB)
Portions of livers were weighed and homogenized in the buffer of White et al. [1981] containing 120 mM KCl, 30 mM NaCl, 0.3 mM spermine HCl, 1mM spermidine HCl, 0.25 M sucrose, 4 mM EDTA, 1mM EGTA, 15mM Tris-HCl, 1mM PMSF and 15mM 2-mercaptoethanol to produce 25% w/v homogenate. The homogenate was centrifuged at 3000 g for 15 min and nuclear fraction re-suspended in half the buffer volume that was originally used for each sample. The nuclear suspensions were used for the determination of DNA SSB using the method of Wahba et al. [1988] with modification. In brief, 0.1 ml of the nuclear suspensions was layered onto 5 µm SMWP Millipore filters (Millipore Corporation, Bedford, MA) attached through tubes to a monostat cassette pump. The nuclear fractions were lysed at a speed of 200 µl/ min, using a lysing solution containing 2% (w/v) sodium dodecyl sulfate (SDS) and 25 mM EDTA, pH10.3, adjusted with NaOH. After 20 min, the lysing solution was exchanged with an elution solution containing 0.1% (w/v) SDS and 20 mM EDTA, pH 12.3, adjusted with disodium salt of tetraethyl ammonium hydroxide, and the elution rate was adjusted to 100 µl/ min. Using an Isco fraction collector (Isco, Lincoln, NE), 7 eluate fractions, 3 ml each, were collected from each nuclear sample. To determine the total amount of DNA-SSB that corresponds to the amount in each nuclear sample layered on the filters, 0.1 ml of each of the nuclear homogenates was mixed with 3 ml of elution buffer and samples were treated similar to collected fractions for the rest of the procedure. A solution of bovine serum albumin (BSA) containing 2.5 mg BSA/ml was prepared and added at a volume of 0.1 ml together with 1 ml 40% trichloroacetic acid solution to each tube, and the tubes were incubated at 4°C overnight. The tubes were then centrifuged at 2500g for 15 min, supernatants removed, and pellets in tubes were washed with 3 ml of 36:1, ethanol:HCl mixture. After centrifugation of the tubes for 10 min at 2500g, supernatants were removed, pellets were allowed to dry overnight in a fume hood, and 100 µl 3,5-diaminobenzoic acid (270 mg/ml) was added. The tubes were incubated in a water bath at 70°C for 35 min and then cooled down to room temperature. Three ml of 1M HCl was added to each tube and fluorescence was measured at excitation 436 nm and emission 521 nm in a Shimadzu RF 5000U spectrofluorometer (Kyoto, Japan). The log10 of the %DNA remaining on the filter after the collection of each fraction was plotted against the cumulative volume of the eluate and the elution rate constant (k) was determined by multiplying slope of the plot of each sample by −2.3.
Protein Determination
The method of Lowry et al. [1951] was used to determine the amount of protein in hepatic tissue. BSA was used as a standard, and data for various biomarkers were expressed per mg protein.
Statistical Analysis
Data were analyzed using Microsoft Excel data analysis package and GraphPad Prism®. Data are expressed as means of 6 samples (animals) ± S.D. A one-way analysis of variance (ANOVA) was used to determine the statistical differences between groups followed by Tukey’s post-hoc test. A significance level of ≤ 0.05 was employed. Pearson’s correlation coefficients and regression were calculated using Microsoft Excel data analysis package.
RESULTS
The effects of treatment of mice with various doses of DCA, TCA and mixtures on liver/body weight rations are presented in Table 1. Data show no significant changes in any of those ratios compared with control.
Table 1.
The liver/ body weight ratios of control (C) animlas, and animals treated with different doses of DCA, TCA and mixtures. Values indicate the mean ± SD.
| Treatment | Liver/body weight ratio |
|---|---|
| C | 0.052 ± 0.006 |
| DCA(mg/kg/day) | |
| 7.5 | 0.054 ± 0.009 |
| 15 | 0.051 ± 0.002 |
| 30 | 0.053 ± 0.007 |
| TCA (mg/kg/day) | |
| 12.5 | 0.057 ± 0.003 |
| 25 | 0.055 ± 0.005 |
| 50 | 0.049 ± 0.005 |
| Mixtures | |
| I | 0.058 ± 0.004 |
| II | 0.051 ± 0.004 |
| III | 0.063 ± 0.002 |
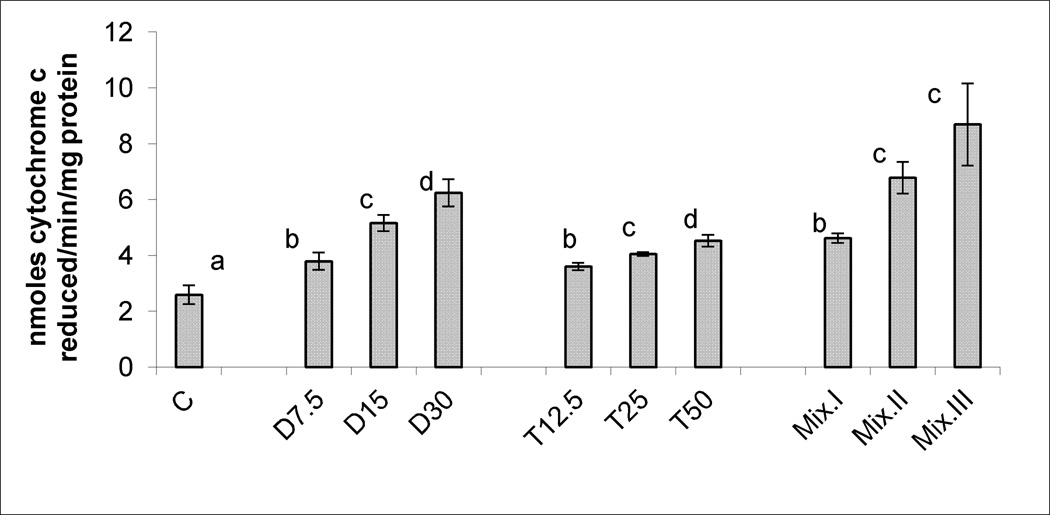
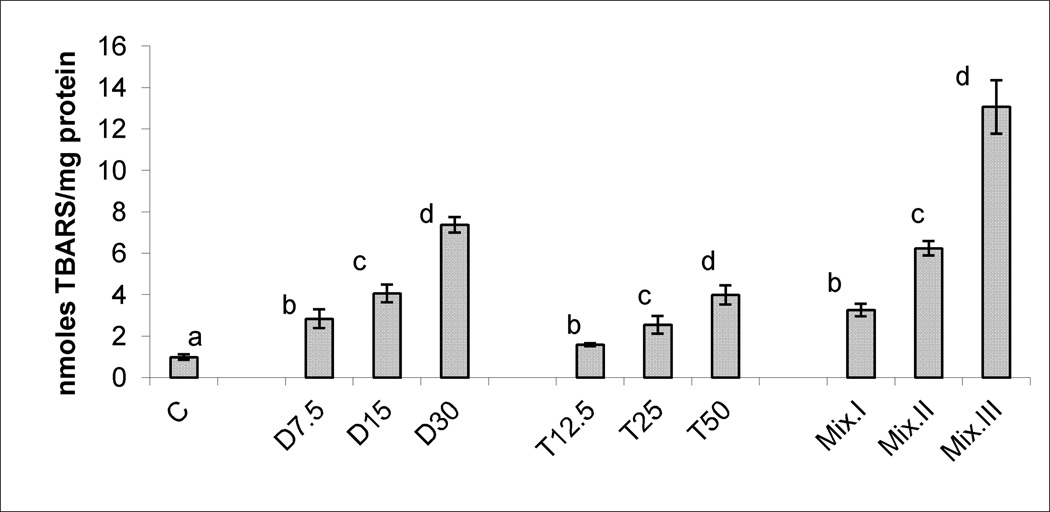
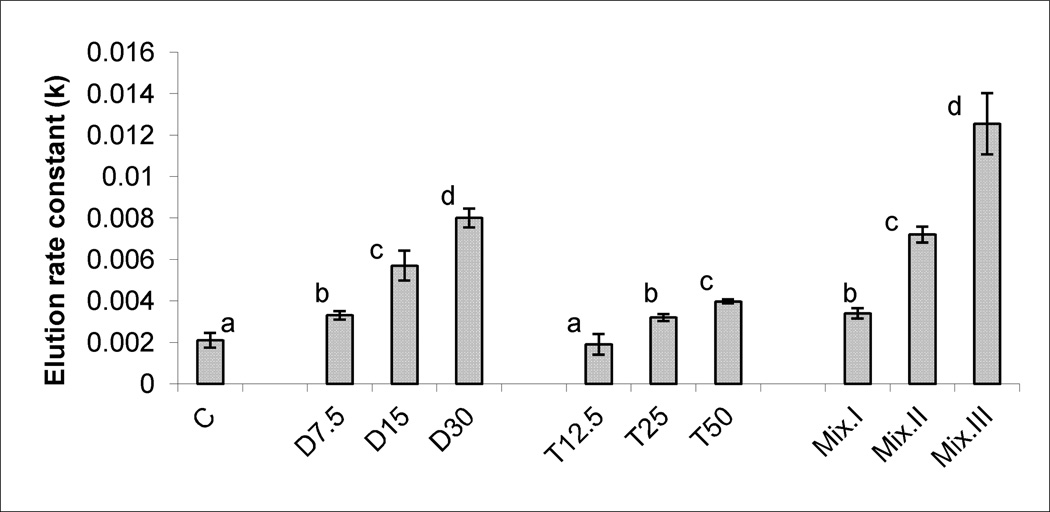
Figures 1, 2, and 3, demonstrate the production of SA, LP levels and DNA damage, respectively, in response to treatments with different doses of DCA, TCA and the three mixtures. Significant and dose-dependent increases in the three tested biomarkers were observed in the three treatment groups. The r2 values and parameters of regression analysis of the dose-response curves of the three tested biomarkers of OS induced by DCA and TCA are presented in Table 2. The values in the Table indicate sufficient linearity of the induced biomarkers with the doses of the individual compounds.
Figure 1.
SA production determined as cytochrome c reduced/min/mg protein in the livers of mice treated subchronically with 7.5, 15 and 30 mg DCA/kg/day, 12.5, 25 and 50 mg TCA/kg/day, and mixture I, II and III of DCA/TCA ratios that correspond to 7.5/12.5, 15/25 and 30/50 mg/kg/day, respectively. Columns with non- identical superscript within each of the treatment groups are significantly different (p<0.05) when compared against each other, and also against the control.
Figure 2.
LP, indicated by TBARS production in the livers of mice treated subchronically with 7.5, 15 and 30 mg DCA/kg/day, 12.5, 25 and 50 mg TCA/kg/day, and mixture I, II and III of DCA/TCA ratios that correspond to 7.5/12.5, 15/25 and 30/50 mg/kg/day, respectively. Columns with non identical superscript are significantly different (p<0.05) when compared against each other and also against the control.
Figure 3.
DNA SSBs production, indicated by the elution rate constant (k), in the livers of mice treated sub-chronically with 7.5, 15 and 30 mg DCA/kg/day, 12.5, 25 and 50 mg TCA/kg/day, and mixture I, II and III of DCA/TCA ratios that correspond to 7.5/12.5, 15/25 and 30/50 mg/kg/day, respectively. Columns with non- identical superscript within each of the treatment groups are significantly different (p<0.05) when compared against each other, and also against the control.
Table 2.
Regression analysis (r2) of dose-reponse curves for induction of SA, LP and DNA-SSBs by DCA and TCA
| Biomarker | Compound | r2 | Parameters |
|---|---|---|---|
| SA | |||
| DCA | 0.9347 | SA* = 0.1036 . Dose + 3.250 | |
| TCA | 0.9688 | SA*= 0.0237 . Dose + 3.365 | |
| LP | DCA | 0.9951 | LP**= 0.2041 . Dose + 1.185 |
| TCA | 0.9944 | LP** = 0.0631. Dose + 0.870 | |
| DNA-SSBs | |||
| DCA | 0.9596 | DNA-SSBs***= 0.000201 . Dose + 0.00215 | |
| TCA | 0.8907 | DNA-SSBs***= 0.000052 . Dose + 0.00152 |
SA production determined as nmoles cytochrome c reduced/ min/ mg protein
LP production determied as nmoles TBARS/mg protein
DNA-SSBs assessed as elution rate constant k
The correlations between SA induction and production of LP and DNA damage were assessed by determining Pearson’s correlation coefficients that are given in Table 3. Data for SA induction by three doses of each of DCA, TCA or the mixtures were pooled and compared with pooled data for LP or DNA-SSBs induction by the three corresponding doses of each compound or mixtures. Data in the Table illustrate strong correlations between SA induction and production of LP and DNA-SSBs by DCA, TCA and the mixtures.
Table 3.
Correlations between SA induction and LP and DNA-SSBs production in response to different doses of DCA, TCA, and mixtures I, II and III. Numbers indicate Pearson’s correlation coefficients.
| Biomarkers | DCA | TCA | Mixtures |
|---|---|---|---|
| SA and LP | 0.947 | 0.995 | 0.966 |
| SA and DNA-SSBs | 0.998 | 0.987 | 0.991 |
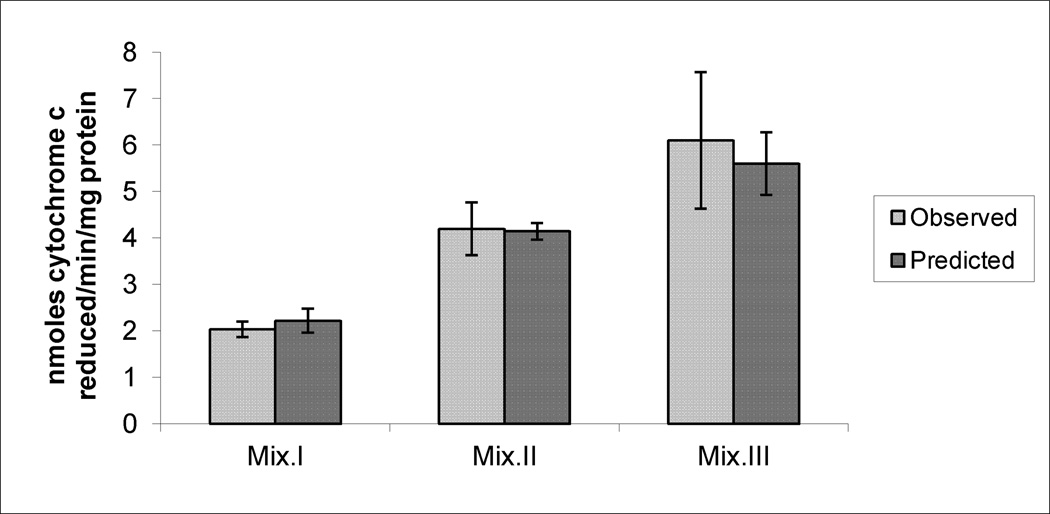
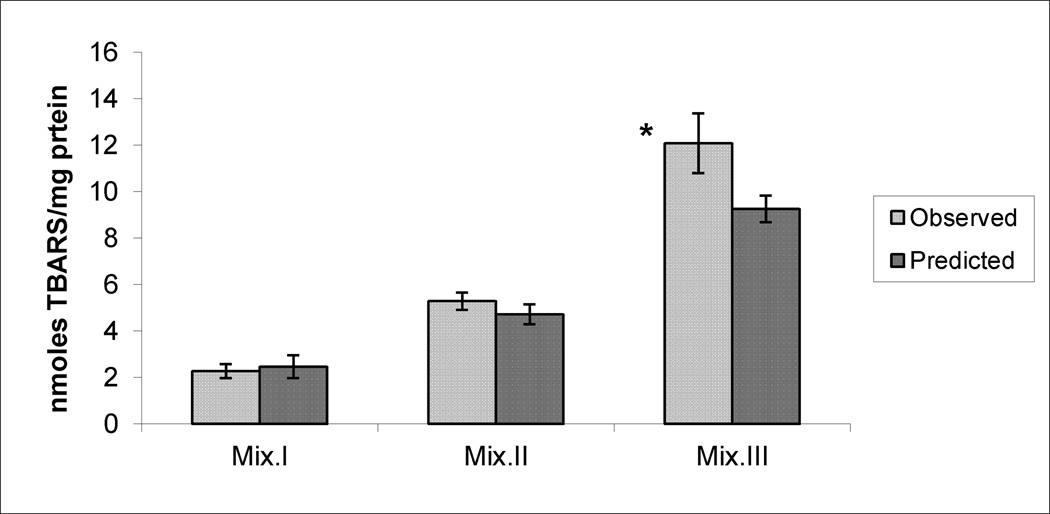
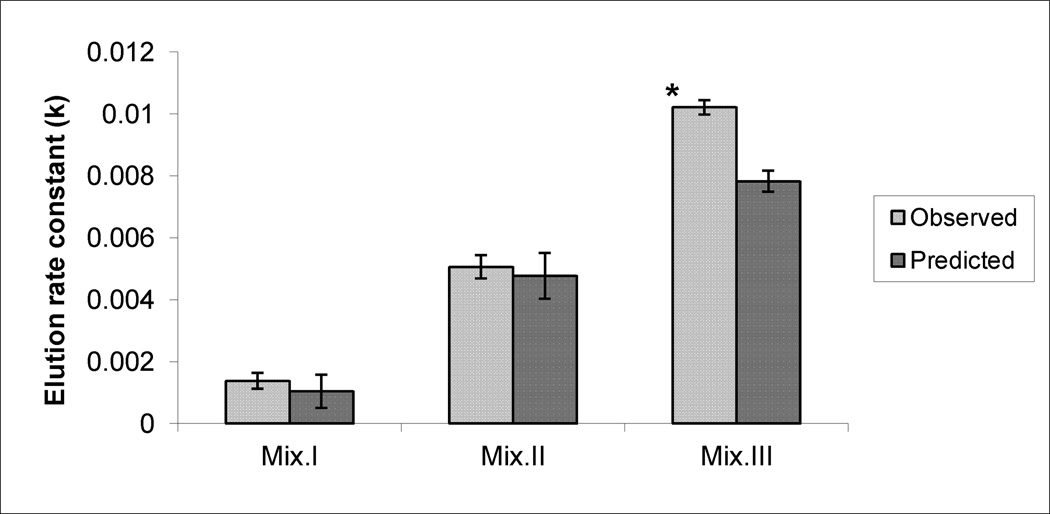
Figures 4, 5 and 6 demonstrate the observed (experimentally-determined) effects on the production of SA, LP and DNA damage, respectively, after subtracting the corresponding control values from them. Data also illustrate the predicted effects of the mixtures that were mathematically calculated by adding the effects produced by each compound’s dose present in a mixture, after subtracting control values. While there were no significant differences between observed and predicted effects of Mix. I and II in any of the tested biomarkers and with Mix. III on SA production, significantly higher levels were observed with LP and DNA damage production in response to Mix.III.
Figure 4.
The observed and the predicted effects of mixtures (Mix) I, II and III on SA production in the livers of mice. * indicates significant difference between the observed and predicted effects (p< 0.05), by Student’s t-test.
Figure 5.
The observed and the predicted effects of mixtures (Mix) I, II and III on LP production in the livers of mice. * indicates significant difference between the observed and predicted effects (p< 0.05), by Student’s t-test.
Figure 6.
The observed and the predicted effects of mixtures (Mix) I, II and III on DNA-SSBs production in the livers of mice. * indicates significant difference between the observed and predicted effects (p< 0.05), by Student’s t-test.
DISCUSSION
Previously Hassoun et al (2010a) demonstrated the effects of different doses of DCA and TCA on the induction of SA, LP and DNA-SSBs in hepatic tissues of male B6C3F1 mice, after subchronic exposure The doses used for this study corresponded to 15, 25 and 35% of maximal effects (100%) on the tested biomarkers induced by 154 mg DCA/kg/day and 410 mg TCA/kg/day [Hassoun et al., 2010a], and were estimated to be 7.5, 15 and 30 mg DCA/kg/day, and 12.5, 25, and 50 mg TCA/kg/day, respectively. Berenbaum [1989] indicated that the summation effects for agents are based on the linearity of the dose-response curves, where linearity is produced in the low-dose/low-effect region for many agents, and that when agents display linear dose-response curves, the effect of a zero-interactive combination is the sum of the effects of its constituents. The study design was based on this assumption so that the interactivities between DCA and TCA at 15, 25 and 35% of maximal effects of each compound would be expected to produce 30, 50 and 70% of maximal responses, respectively when administered as mixtures. The assumption was found to be sufficient since linearity of the compounds dose-effects relationship were confirmed by regression analysis of the dose-response curves, and the ranges of their observed effects were noted to be within the response ranges of the mixtures. Linearity is expected to hold for higher concentrations in mixture III, since previous studies demonstrated continuous increases in induction of those biomarkers by higher doses than those used in this study [Hassoun et al. 2010a].
Lack of significant differences between observed and predicted effects of Mix I and II on SA, LP and DNA-SSB, indicated production of additive effects by those mixtures, while the significantly higher elevations observed with Mix. III on LP and DNA damage suggested greater than additive effects. Although the dose regimens and periods of treatments used in this study are different form previous studies testing effects of combinations of DCA and TCA on tumor promotion and proliferation [Pereira et al., 1997; Bull et al., 2002], results of this study are in agreement with the reported additive promoting effects on N-methyl-N-nitrosourea-initiated cancer in liver of female B6C3F1 mice [Pereira et al. 1997], and observed additivity in induced tumor numbers [Bull et al. 2002].
The contribution of SA induction to the observed production of LP and DNA damage was noted by the strong correlation coefficients demonstrated in Table 3. Further, previous in vitro studies demonstrating strong correlations between SA production and reduction of cellular viability in response to DCA and TCA [Hassoun and Ray, 2003], and those investigations showing significant increases in viability of DCA- and TCA-treated cells when the antioxidant enzyme SOD was also added to the cells [Hassoun and Kini, 2004] support the role of SA to haloacetate compounds-induced cellular toxicity. While the observed effect of Mix. III on SA production was equivalent to 70% of maximal response, its effects on LP and DNA damage were approximately equivalent to maximal induction (100%) of those biomarkers previously noted with threshold doses for hepatotoxicity in subchronic studies (154 and 410 mg/kg/day of DCA and TCA, respectively) [Hassoun et al. 2010a].Therefore, the observed effects of Mix. III are suggested to place the liver at a threshold for development of subchronic hepatotoxic effects.
Hepatomegaly is a prominent toxic effect associated with higher doses of DCA and TCA in mice than used in this study [DeAngelo et al., 1989; 1991; Herren-Freund et al., 1987; Nelson et al., 1989]. Our results did not reveal significant changes in the liver/body weight ratios among any of the treated groups compared with control, but a 1.2 fold rise in liver/ body weight ratio was found in response to Mix.III compared with control. The reported elevation was not statistically significant, but was close to the 1.3 fold increase previously shown to be associated with hepatomegaly and reduction in biomarkers of OS induced by 410 mg DCA/kg/day in subchronic studies [Hassoun et al, 2010a]. Previously Hassoun et al (2010b) indicated a protective role of phagocytic activation against chronic DCA- and TCA-induced hepatotoxicity and that mixtures of compositions similar to Mix I and II produced additive effects on this mechanism. However, a mixture of similar composition to Mix III resulted in less than additive effects [Hassoun et al., 2013]. It is possible the less than additive effects of Mix. III on phagocytic activation may be a decline in protection against mixture-induced hepatotoxicity [Hassoun et. al, 2013], further suggesting placement of liver at a threshold state for development of hepatotoxicity by Mix.III.
SA production by Mix. III was equivalent to that produced by Mix.II. Preliminary studies in our lab indicated induction of SOD by Mix.III. SOD is an antioxidant enzyme that results in SA dismuation, converting it to the more oxidizing species, hydrogen peroxide [Davies, 1995]. This may also indicate that in addition to SA, other ROS may have contributed to the observed LP and DNA damage generated by Mix. III. Studies are being conducted in our lab to assess the role of antioxidant enzymes and contribution of other ROS to the outcome of mixtures effects.
The changes from additive to greater than additive effects on the biomarkers of OS observed with increasing the mixtures concentrations is not known at this point, but may be attributed to possible changes in DCA and TCA toxicokinetics. For example, DCA was shown to inhibit its own metabolism to glyoxylate through inhibition of glutathione –S- transferase zeta, resulting in a decrease in its elimination (Cornett et al., 1999; Schultz et al., 2002), and prior treatment of mice with DCA, but not TCA produced a significant increase in blood concentration–time profile of DCA (Gonzalez-Leon et al., 1999). Stacpoole et al. [1990, 1998] proposed a reductive dechlorination pathway for the metabolism of DCA to monochloroacetate (MCA). However, Larson and Bull [1992] provided evidence for the existence of this pathway for DCA and TCA metabolism through identification of MCA and DCA, respectively, as metabolites in urine of mice treated with the compounds, and also proposed transient generation of free radicals through that pathway. Merdink et al. [2002] further confirmed that, at least, for TCA there was a trapping of DCA radical in rodent microsomes incubated with TCA as a substrate. Hence, possible accumulation of DCA due to suicide inhibition of its own metabolism by the higher mixture concentration may have favored a reductive dehalogenation pathway that was associated with over production of free radicals and disturbance of the oxidant-antioxidant balance of cells leading to generation of more ROS. Consequently, that disturbance may have enhanced TCA-induced production of ROS leading to the observed increases in effects of that mixture. Future studies on the compounds metabolism when administered as mixtures to mice are required to confirm that suggestion.
ACKNOWLEDGEMENTS
The project was supported by Grant Number R15ES013706 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences.
REFERENCES
- Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms: The production by leukocytes of superoxide, a potential bacteriocidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MC. What is synergy? J Pharamcol. Exp. Ther. 1989;41:93–141. [PubMed] [Google Scholar]
- Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ. Liver tumor induction in B6C3F1mice by dichloroacetate and trichloroacetate. Toxicology. 1990;63:341–359. doi: 10.1016/0300-483x(90)90195-m. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Orner GA, Cheng RS, Stillwell L, Stauber AJ, Sasser LB, Lingohr MK, Thrall BD. Contribution of dichloroacetate and trichloroacetate to liver tumor induction in mice by trichloroethylene. Toxicol. Appl. Pharamcol. 2002;182:55–65. doi: 10.1006/taap.2002.9427. [DOI] [PubMed] [Google Scholar]
- Cornett R, James MO, Henderson GN, Cheung J, Shroads AL, Stacpoole PW. Inhibition of glutathione S-trnasferase zeta and tyrosine metabolism by dichloroacetate: A potential unifying mechanism for its altered biotransformation and toxicity. Biochem. Biophys. Res. Commun. 1999;262:752–756. doi: 10.1006/bbrc.1999.1287. [DOI] [PubMed] [Google Scholar]
- Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP. Hepatocarcinogenicity of chloral hydrate, 2-chloroaldehyde and dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1992;19:159–168. doi: 10.1016/0272-0590(92)90147-a. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Oxidative stress: the paradox of aerobic life. In: Rice-Evans C, Halliwell B, Lunt GG, editors. Free Radical and Oxidative Stress: Environment, Drugs and Food Additives. London: Portland Press Ltd; 1995. pp. 3–31. [Google Scholar]
- DeAngelo AB, Daniel F, Stober J, Olson G. The carcinogenicity of dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1991;16:337–347. doi: 10.1016/0272-0590(91)90118-n. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, McMillan L, Wernsing P, Savage RJ., Jr. Species and strain sensitivity to the induction of peroxisome proliferation by chloroacetic acids. Toxicol. Appl. Pharmacol. 1989;101:285–298. doi: 10.1016/0041-008x(89)90277-9. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, George MH, House DE. Hepatocarcinogenicity in the male B6C3F1 mouse following a lifetime exposure to dichloroacetic acid in the drinking water: dose-response determination and modes of action. J. Toxicol. Environ. Health A. 1999;58:485–507. doi: 10.1080/009841099157115. [DOI] [PubMed] [Google Scholar]
- Dekant W, Metzler M, Henschler D. Novel metabolites of trichloroethylene through dechlorination reaction in mice and humans. Biochem. Phramcol. 1984;33:2021–2027. doi: 10.1016/0006-2952(84)90568-9. [DOI] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Tanaka N, Li F, Qu A, Idle JR, Gonzalez FJ. Metabolomics reveals trichloroacetate as major contributor to trichloroethylene-induced metabolic alterations in mouse urine and serum. Arch Toxicol. 2013;87:1975–1987. doi: 10.1007/s00204-013-1053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Leon A, Merdink JL, Bull RJ, Schultz IR. Effect of pretreatment with dichloroacetic or trichloroacetic acid in drinking water on the pharmacokinetics of a subsequent challenge dose in B6C3F1 mice. Chem-Biol. Interact. 1999;123:239–253. doi: 10.1016/s0009-2797(99)00140-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Cearfoss J. Dichloroacetate- and trichloroacetate-induced modulation of superoxide dismutase, catalase and glutathione peroxidase activities and glutathione level in the livers of mice after subacute and subchronic exposure. Toxicol. Environ. Chem. 2011;93:332–344. doi: 10.1080/02772248.2010.509602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun EA, Cearfoss J, Musser B, Krispinsky S, Al-Hassan N, Liu M-C. The induction of phagocytic activation by mixtures of the water chlorination by-products, dichloroacetate and trichloroacetate, in mice after subchronic exposure. J. Biochem. Mol. Toxicol. 2013;27:237–242. doi: 10.1002/jbt.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun E, Cearfoss J, Spildener J. Dichloroacetate- and trichloroacetate-induced oxidative stress in the hepatic tissues of mice after long term exposure. J. Appl. Toxicol. 2010a;30:450–456. doi: 10.1002/jat.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun EA, Dey S. Dichloroacetate- and trichloroacetate-induced phagocytic activation and production of oxidative stress in the hepatic tissues of mice after acute exposure. J. Biochem. Mol. Toxicol. 2008;22:27–34. doi: 10.1002/jbt.20210. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Kini V. Effects of superoxide dismutase and polyclonal tumor necrosis factor–alpha antibodies on chloroacetate-induced cellular death and superoxide anion production by J774.A1 macrophages. Comp. Biochem. Physiol. 2004;138:113–120. doi: 10.1016/j.cca.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Ray S. The induction of oxidative stress and cellular death by drinking water disinfection by-products, dichloroacetate and trichloroacetate in J774.A1 cells. Comp. Biochem. Physiol. 2003;135:119–128. doi: 10.1016/s1532-0456(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Spildener J, Cearfoss J. The induction of tumor necrosis factor, superoxide anion, myeloperoxidase, and superoxide dismutase in the peritoneal lavage cells of mice after prolonged exposure to dichloroacetate and trichloroacetate. J. Biochem. Mol. Toxicol. 2010b;24:136–144. doi: 10.1002/jbt.20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herren-Freund SL, Pereira MA, Khoury MD, Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol. Appl. Pharmacol. 1987;90:183–189. doi: 10.1016/0041-008x(87)90325-5. [DOI] [PubMed] [Google Scholar]
- Larson JL, Bull RJ. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol. Appl. Pharmacol. 1992;115:268–277. doi: 10.1016/0041-008x(92)90332-m. [DOI] [PubMed] [Google Scholar]
- Lash LH, Fisher JW, Lipscomb JC, Parker JC. Metabolism of trichloroethylene. Environ. Helath. Persp. 2000;108(Suppl 2):177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Merdink JL, Bull RJ, Schultz IR. Trapping and identification of the dichloroacetate radical from the reductive dehalogenation of trichloroacetate by mouse and rat liver microsomes. Free. Radic. Biol. Med. 2000;29:125–130. doi: 10.1016/s0891-5849(00)00330-0. [DOI] [PubMed] [Google Scholar]
- Miller JW, Uden PC. Characterization of non-volatile aqueous chlorination products of humic substances. Environ. Sci. Technol. 1983;17:150–157. doi: 10.1021/es00109a006. [DOI] [PubMed] [Google Scholar]
- Narotsky MG, Best DS, McDonald A, Godin EA, Hunter ES, 3rd, Simmons JE. Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids. Reprod. Toxicol. 2011;31:59–65. doi: 10.1016/j.reprotox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Narotsky MG, Best DS, Rogers EH, McDonald A, Sey YM, Simmons JE. Integrated disinfection by-products mixtures research: Assessment of developmental toxicity in Sprague-Dawley rats exposed to concentrates of water disinfected by chlorination and ozonation/postchlorination. J. Toxicol. Environ. Health A. 2008;71:1216–1221. doi: 10.1080/15287390802182623. [DOI] [PubMed] [Google Scholar]
- Nelson MA, Lansing AJ, Sanchez IM, Bull RJ, Springer DL. Dichloroacetic acid and trichloroacetic acid-induced DNA strand breaks are independent of peroxisome proliferation. Toxicology. 1989;58:239–248. doi: 10.1016/0300-483x(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Pereira MA. Carcinogenic activity of dichloroacetic acid and trichloroacetic acid in the liver of female B6C3F1 mice. Fundam. Appl.Toxicol. 1996;31:192–199. doi: 10.1006/faat.1996.0091. [DOI] [PubMed] [Google Scholar]
- Preira MA, Li K, Kramer PM. Promotion by mixtures of dichloroacetic acid and trichloroacetic acid of N-methyl-N-nitrosourea-initiated cancer in the liver of female B6C3F1 mice. Cancer Lett. 1997;115:15–23. doi: 10.1016/s0304-3835(97)04699-5. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Thruston AD, Jr., Krasner SW, Weinberg HS, Miltner RJ, Schenck KM, Narotsky MG, McKague AB, Simmons JE. Integrated Disinfection byproducts mixtures research: Comprehensive characterization of water concentrates prepared from chlorinated and ozonated/postchlorinated drinking water. J. Toxicol. Environ. Health A. 2008;71:1165–1186. doi: 10.1080/15287390802182417. [DOI] [PubMed] [Google Scholar]
- Schultz IR, Merdink JL, Gonzalez-Leon A, Bull RJ. Dichloroacetate toxicokinetics and disruption of tyrosine catabolism in B6C3F1 mice: dose-response relationships and age as a modifying factor. Toxicology. 2002;173:229–147. doi: 10.1016/s0300-483x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Harwood HJ, Jr., Cameron DF, Curry SH, Samuelson DA, Cornwell PE, Sauberlich HE. Chronic toxicity of dichloroacetate: Possible relation to thiamine deficiency in rats. Fundam. Appl. Toxicol. 1990;14:327–337. doi: 10.1016/0272-0590(90)90212-3. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Henderson GN, Yan Z, James MO. Clinical pharmacology and toxicology of dichloroacetate. Environ Health Persp. 1998;106:989–994. doi: 10.1289/ehp.98106s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1977;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Wahba ZZ, Lawson TA, Stohs SJ. Induction of hepatic DNA-single strand breaks in rats by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Cancer Lett. 1988;29:281–286. doi: 10.1016/0304-3835(88)90071-7. [DOI] [PubMed] [Google Scholar]
- White RD, Sipes IG, Gandolfi AJ, Bowden GT. Characterization of the hepatic DNA Damage caused by 1,2-bromoethane using the alkaline elution techniques. Carcinogenesis. 1981;2:839–843. doi: 10.1093/carcin/2.9.839. [DOI] [PubMed] [Google Scholar]