Abstract
In three years, four drugs have gained regulatory approval for the treatment of metastatic and unresectable melanoma with at least seven other drugs having recently completed, currently in, or soon to be in phase III clinical testing. This amazing achievement has been made following a remarkable increase of knowledge in molecular biology and immunology that led to the identification of high-valued therapeutic targets and the clinical development of agents that effectively engage and inhibit these targets. The discovery of either effective molecularly targeted therapies or immunotherapies would have led to dramatic improvements to the standard of care treatment of melanoma. However, through parallel efforts that have showcased the efficacy of small molecule BRAF and MEK inhibitors as well as the immune checkpoint inhibitors, namely ipilimumab and the anti-PD1/PDL1 antibodies (lambrolizumab, nivolumab, MPDL3280), an opportunity exists to transform the treatment of melanoma specifically and cancer generally by exploring rational combinations of molecularly targeted therapies, immunotherapies, and molecular targeted therapies with immunotherapies. This overview presents the historical context to this therapeutic revolution, reviews the benefits and limitations of current therapies, and provides a look ahead at where the field is headed.
Keywords: Melanoma, BRAF inhibitor, MEK inhibitor, PI3K, PD1/PDL1, combined molecular targeted and immunotherapy
Introduction
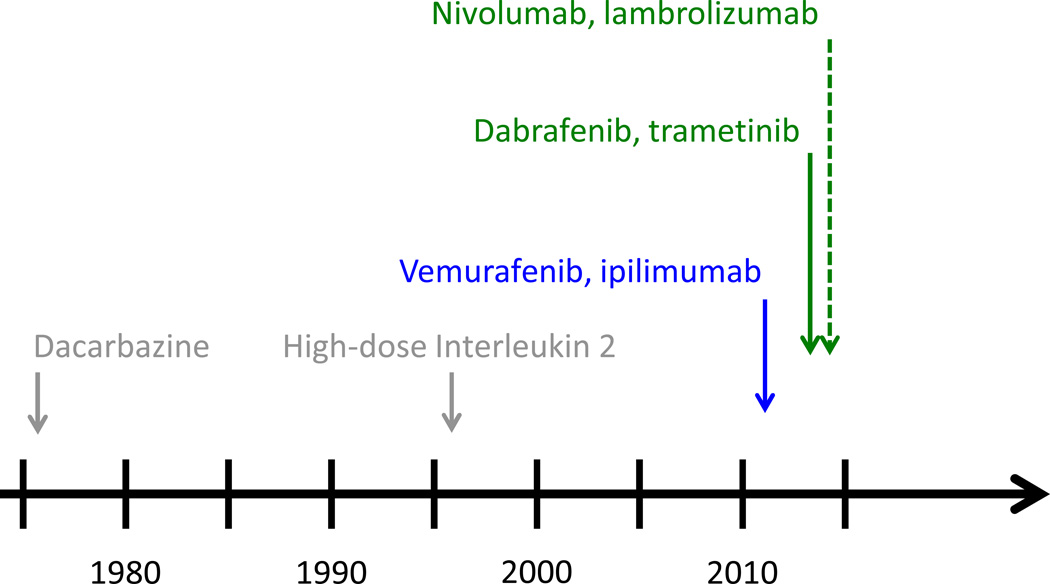
Melanoma is a deadly disease that is rising in incidence. In 2013, an estimated 76,000 new cases and 9400 deaths are expected worldwide.(1) Still, despite these stark numbers, there is great optimism in the melanoma research and treatment communities due to a number of breakthroughs that are transforming the way this disease is being treated. To highlight the dramatic progress being made to treat patients with melanoma, it is very possible that for the second time in three years the number of therapies approved by the FDA for the treatment of advanced melanoma could double in a 12-month period (Figure 1). These advances have been the result of extraordinary scientific discovery combined with robust clinical and translational efforts. What follows is an overview of the past, current, and near future states of melanoma therapeutics and an introduction to the topics covered in this Clinical Cancer Research Focus section.
Figure 1. FDA-approval timeline for metastatic melanoma.
Dacarbazine (1976) and high-dose interleukin 2 (1998) were the only approved agents between 1976 and 2011. In 2011, both vemurafenb and ipilimumab were approved, thereby doubling the number of approved agents. In 2013, dabrafenib and trametinib were approved and based on the emerging data with nivolumab and lambrolizumab, regulatory approved is expect in the near future; thereby setting up the possibility that the number of approved agents will double again within a 12–18 month time period.
Immunotherapy and Melanoma
Melanoma has long been considered a malignancy that has a complex and unique interaction with the immune system. The first description of immune infiltrates in primary tumors was made decades ago, as was the definition of the prognostic significance of these infiltrates.(2, 3) Further interactions between the immune system and melanoma have been posited as the explanation of two fascinating phenomenon: 1) The long latency from primary melanoma resection of early stage disease to the development of widespread metastases and 2) The spontaneous regression of metastatic melanoma in a small number of patients.(4, 5) Due to these findings and beliefs, immunotherapy has a long history in the treatment of melanoma starting with injections of immune stimulants (i.e. BCG), moving to treatment with mediators of immune responses (i.e. cytokines) with or without “educated” immune effectors such as primed T-lymphocytes (adoptive cell transfer), and more recently monoclonal antibodies that target critical immune check points and thereby lead to T-lymphocyte (T-cell) activation. (6–11)
Cytokine therapy
In the early days of tumor immunology, it was evident that T-cell activation, in particular cytotoxic T-lymphocyte (CTL) activation, was required.(12) While the understanding of how T-cells become active has evolved over the past four decades, one of the first major discoveries was that a number of substances were produced and secreted by immune cells and could interact with receptors on other immune cells as well as tumor cells.(13–15) The substances known as cytokines were initially grouped as one of two types – Type 1 associated with CTL activation (so-called Cellular Immunity), and Type 2 associated with antibody formation (so-called Humoral Immunity).(16) Interestingly, these two types of cytokines were typically antagonistic, such that Type 1 cytokines would inhibit Humoral Immunity and Type 2 cytokines would inhibit Cellular Immunity. Not surprisingly, a number of Type 1 cytokines were tested as antineoplastic therapies for melanoma among other malignancies; only interferon alpha-2B (IFN2B) and interleukin 2 (IL-2) demonstrated sufficient benefit to support regulatory approval for melanoma.(17)
High-dose IFN2B is approved for the adjuvant treatment of patients with intermediate to high-risk melanoma (defined as AJCC Stage IIB, IIC, IIIA, IIIB, and IIIC) based on data that showed an improvement in relapse/disease free survival (RFS) and overall survival (OS).(18) Since this initial report, a number of studies have been performed with high-dose IFN2B showing a consistent improvement in RFS, yet not necessarily in OS. (19) Similar data has been seen with pegylated-IFN2B, an agent that received approval in 2011.(20) While the data with IFN2B led to its FDA approval as adjuvant therapy for patients with intermediate and high-risk melanoma, given its toxicity profile and underwhelming efficacy, its use in this setting is more by default due to a lack of more promising options than an endorsement of its effectiveness.
High-dose IL-2 is a highly-toxic therapy that leads to a capillary leak syndrome associated with hypotension/shock, massive fluid retention, and renal failure necessitating that it be given in an inpatient, ICU-level care setting.(8, 21) Its use is associated with a 16–23% response rate with 5–10% of patients treated achieving a durable response that can last for decades.(8, 22) Given the high toxicity and low response rate, IL-2 is only given in a small number of centers; though the potential for decades long response is compelling and the reason why this therapy is still considered for highly-selected and motivated patients.
Adoptive immunotherapy
Another therapy associated with long-term remissions is adoptive T-cell therapy.(23) This involves the harvesting of tumor infiltrating lymphocytes (TIL) from metastatic tumors, ex vivo expansion and administration with IL-2 following non-ablating lympho-depleting chemotherapy.(24) In small series of patients, this has resulted in complete remissions in up to 40% of cases, even when other immunotherapies have failed.(24, 25) This approach remains investigational but is being explored at an increasing number of centers. In addition, further manipulations or genetic modifications of TILs (new culture conditions, altered cytokine secretion) and co-administration with other immune cells (i.e., Natural Killers cells) or Dendritic cells) and/or vaccines are being explored.(26–30) Alternatively, tumor reactive T-cells for clinical administration are also being engineered from peripheral blood by the introduction of receptors specific for tumor associated antigens. And, most recently, the potential for molecularly targeted therapy to augment tumor infiltrates and enhance effector T-cell function following administration is being explored (reviewed by Kwong and colleagues in this issue).(31)
Immune checkpoint inhibition
Over the past three decades, the complexities of immune activation, and T-lymphocyte activation specifically, have been elucidated. While cytokines play an important role in directing immune effectors, the process of T-cell activation requires two major signals: 1) TCR recognition of antigen in the context of major histocompatibility complex (MHC) expressed on a professional antigen presenting cell (APC), and 2) Co-stimulation in the form of T-cell receptor interactions between the T-cell and the APC. (32–34) This second step of co-stimulation involves a number of so-called “check-points” that regulate whether this process occurs.(35) The two check points that have garnered the most attention to date are the cytotoxic T-lymphocyte antigen 4 (CTLA4) and the Program Death 1 (PD1) molecule.
CTLA4 is a surface protein on T-cells that interacts with the APC membrane bound co-stimulatory molecules B7-1 (CD80) and B7-2 (CD86) and functionally competes with the T-cell co-stimulatory molecule CD28.(35, 36) After its identification as a potent, negative regulator of T-cell activation, it became an attractive target for monoclonal antibody therapy.(37) Ipilimumab is a fully humanized monoclonal antibody that binds and inhibits CTLA4 function, thereby releasing a critical brake to T-cell co-stimulation.(10) The preclinical discoveries of CTLA4 and its role in T-cell co-stimulatory regulation and subsequent clinical development of ipilimumab offers an amazing example of translational research, as ipilimumab was the first agent to be proven to prolong overall survival in patients with metastatic melanoma and the first agent since IL-2 to achieve FDA-approval for this treatment indication.(10, 38) Clinical activity of ipilimumab has also been associated with the induction of serious immune mediated adverse events, most prominently colitis, implying a broad role for CTLA4 in suppressing autoimmunity. Notably, an agonist CD28 antibody proved to induce a life-threatening cytokine release syndrome and highlights the delicate balance immune cell activation/inactivation that must be respected in designing safe and effective immune checkpoint targeted therapies. (39)
A second example of the increased knowledge of immune checkpoint biology leading to clinical improvement is the development of antagonists of PD1 and one of its ligands, PDL1. Following chronic T-cell activation, the inhibitory receptor PD-1 is induced on T-cells and expression of one of its ligands, PDL1, on tissue-based macrophages and tumor cells can offer protection from immune destruction.(40) As a result, targeting either PD1 or PDL1 offers an opportunity to disable a major mechanism of tumor-mediated immune evasion. The clinical development of monoclonal antibodies that inhibit either PD1 or PDL1 is underway, and the results of early stage clinical trials of the PD1 antibodies nivolumab and lambrolizumab, as well as the PDL1 antagonists MDX-1107 and MPDL1-3280 are impressive. Tumor responses (at least 50% appearing to be durable) are seen in a sizable minority of patients, while toxicity appears to be less prominent as compared to ipilimumab.(11, 41–44) It is not an understatement to say that these agents are the most promising for the treatment of melanoma that have ever been developed given the high clinical benefit rate, reasonable tolerability, and potential for being added to other standard and experimental agents. This is reviewed in greater detail in the Immunotherapy article herein by Ott and colleagues.(45)
Molecular Signaling and Melanoma
In parallel to the amazing developments in the field of immunotherapy, there has been a remarkable advancement in the understanding of the molecular biology of tumor cells. Perhaps the most profound discovery as it relates to the field of targeted therapy development is the identification that tumors generally, and melanoma specifically, co-opt and then become dependent upon a small number of signal transduction pathways to stimulate cell cycle progression and angiogenesis, prevent apoptosis, and abrogate host defense responses. In melanoma, the mitogen activated protein kinase (MAPK) and phosphoinositol-3-kinase (PI3K) pathways are the two major pathways that mediate growth and survival signals (Figure 2).(46, 47) The role of the PI3K pathway is reviewed in detail by Kwong and Davies in this issue.(48)
Figure 2. Molecular and Immunologic signaling in melanoma.
Melanoma cells utilize a diverse set of cell surface receptors and intracellular signaling molecules to promote growth, cell cycle progression, cell survival, angiogenesis, and immune evasion.
Molecular Classification of Melanoma
The MAPK pathway is almost always overexpressed in melanoma and is constitutively activated through genetic aberrations, most commonly via specific point mutations, in the great majority of cases (Figure 3). The most common of these genetic aberrations is mutation at the 600 position of BRAF (V600), present in 40–50% of melanomas.(49, 50) Mutations of the N-isoform of RAS are found in another 15–25% of cases and tend to occur at either position 12 or 61.(51) NRAS and BRAF mutations are mutually exclusive (the co-occurrence rate is <<1%), lead to hyperactivation of the MAPK pathway, and are associated with a worse prognosis than melanomas with wild-type NRAS and BRAF.(52, 53) A loss-of-function mutation in the tumor suppressor gene, neurofibromatosis 1 (NF1) was recently identified in approximately 10–15% of cases and also is associated with abnormal MAPK signaling.(54) When mutated, NF1 is no longer capable of keeping RAS in its inactive RAS-GDP form and thus leads to constitutive activation of RAS and the pathways downstream.(55) Genetic mutations or amplifications are seen in other genes leading to upregulation of the MAPK pathway as well. These include cKIT mutations, seen in less than 1% of all melanomas, although in upwards of 10–30% of acral or mucosal melanomas, and mutations in small g-protein subunits called GNAQ and GNA11 that are present in over 80% of ocular melanomas, though rarely seen in other melanoma subtypes.(56–58)
Figure 3. Oncogenic mutations in melanoma.
Oncogenic mutations and molecular aberrations are regularly present in the MAPK pathway in melanoma and include in order of frequency BRAF, NRAS, PTEN loss, NF1 loss, CKIT mutation or amplification, and GNAQ/GNA11 (<1% of all melanoma, >80% of ocular melanoma).
In addition to the oncogenic mutations that lead to hyperactivation of the MAPK pathway (BRAF, NRAS, CKIT, NF1, and GNAQ/GAQ11), there are a number of other genes that may be altered in melanoma and serve to complement the primary oncogenic mutations described above. In particular, abnormalities of genes involved in cell cycle regulation including cyclin dependent kinases (CDK), CDK inhibitors (such as P16Ink4a [CDKN2A]), and cyclin D are seen in over 70% of patients (This is reviewed in detail by Sheppard and McArthur in this issue).(59, 60) Also, the function of the negative regulator of the PI3K pathway, phosphatase and tensin homolog (PTEN), is either lost or impaired in up to 30% of cases.(61, 62) This is most commonly seen in a subset of patients with BRAF mutations, thereby allowing for unregulated signaling of both the MAPK pathway, via BRAF mutation, and the PI3K pathway, though PTEN loss of function. (52, 63)
MAPK inhibition and resistance
With the first description of oncogenic BRAF mutations in melanoma, efforts were made to identify and develop clinically inhibitors of both BRAF specifically and the MAPK in general. The initial targeted therapy studies in melanoma were with agents that are now considered non-specific inhibitors of BRAF, sorafenib and RAF265, and lower potency inhibitors of MEK1/2 such as selumetinib, PD-325901, and CI-1040.(64–72) It is important to note that these studies were open to any patient with melanoma independent of mutational status. Thus, these trials were doomed for failure as the agents were not able to inhibit the MAP kinase pathway sufficiently at tolerable doses and the patients treated were not preselected to include only those most likely to benefit. Mechanisms of resistance to these agents were impossible to determine given the fact that the pathway was suboptimally inhibited and so few patients received benefit.
BRAF-directed therapy in BRAF mutants
The first so-called targeted therapy to show substantial efficacy in melanoma was vemurafenib.(73) In the initial phase I study, it was determined early on that only patients with oncogenic (i.e V600) mutations experienced clinical benefit and that almost every one of these patients had some evidence of tumor regression with treatment. Further, responses occurred early with improvement of symptoms within days and near complete FDG-PET responses within two weeks from the onset of therapy. A subsequent phase II study confirmed the remarkable response dynamics and frequency of vemurafenib and a phase III study confirmed that vemurafenib conferred a survival advantage compared with chemotherapy.(50, 74) A second, potent and specific mutant BRAF inhibitor, dabrafenib, has been associated with very similar clinical efficacy as vemurafenib and joined it as the second BRAF inhibitor to achieve FDA-approval.(75) A third such BRAF inhibitor, LGX818, has shown responses at every dose level tested.(76)
While the advantages of BRAF inhibitor therapy are the rapid onset and high frequency of responses, the disadvantage is the limited duration of clinical benefit.(74, 75) Specifically, BRAF inhibitor treatment is associated with a progression free survival (PFS) of only 5–7 months as a result of the development of cellular resistance over this relatively short period of time. The mechanisms of this resistance can be subdivided into those that can be predicted based on pretreatment analysis of tumors and those that clearly were not identified at baseline but rather developed as a result of selective pressure placed upon the tumor cells by BRAF inhibitor treatment.
A number of identifiable pretreatment factors have been described as being associated with either a poorer response and/or a shorter PFS to BRAF inhibitor treatment. These include stromal hepatocyte growth factor (HGF) production, BCL2A1 (an anti-apoptotic B-cell leukemia 2 (BCL-2) family member) expression, activation of Cyclin D1, and loss of PTEN.(77–81) It is interesting that each of these examples is associated with critical regulation of either growth, survival, or cell cycle regulation: the activation of PI3K pathway (stromal HGF production leads to CMET activation of the pathway; PTEN loss leads to dysregulation of the pathway), resistance to apoptosis (BCL2A1), or cell cycle progression (Cyclin D1). (Figure 2)
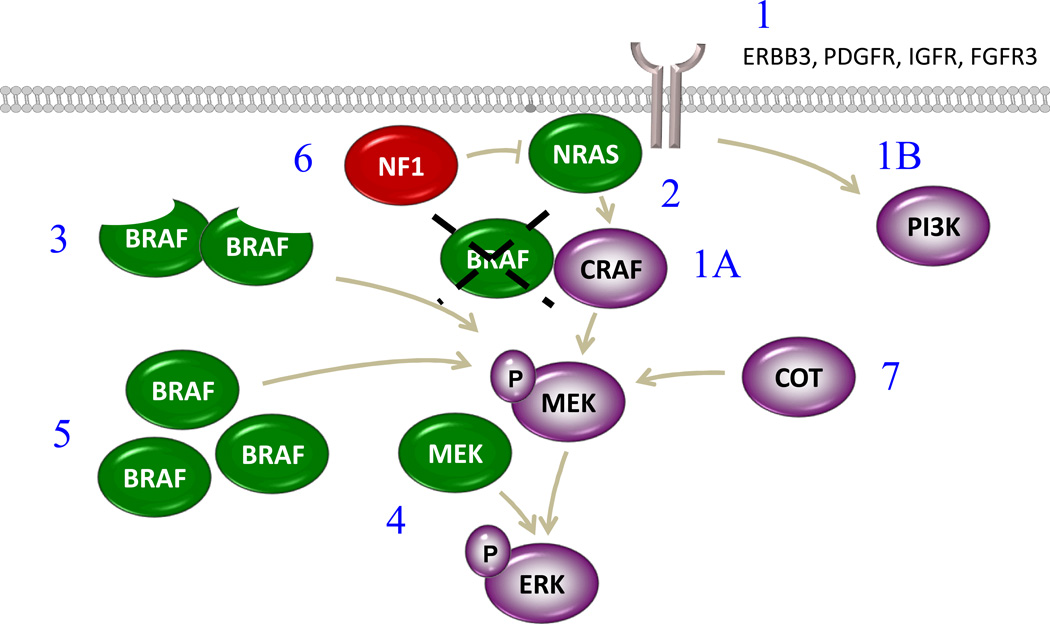
Acquired resistance to BRAF inhibitors, defined here as the development of cellular resistance by a mechanism not identified in pretreatment tumors, is associated with reactivation of the MAPK pathway approximately two thirds of the time.(82) One of the first described mechanisms of resistance (MOR) to BRAF inhibitors was the upregulation of receptor tyrosine kinases (RTK) such as insulin like growth factor receptor 1 (IGFR1), platelet derived growth factor (PDGF), as well as human epidermal growth factor receptor 3 (HER3) that can signal through PI3K (IGFR1) or signal through the MAPK by activating the C-isoform of RAF.(83–86) While BRAF inhibitors potently inhibit BRAFV600, a mutant isoform that signals through a constitutively active kinase in a monomeric form, they paradoxically facilitate RAF dimerization thereby leading to activation of the MAPK pathway.(87, 88) This so-called “BRAF-inhibitor paradox” explains how RTK activation upstream of RAF can reactivate the MAPK pathway, through CRAF homo- or hetero-dimerization, and how other MOR’s to BRAF inhibitor therapy activate the pathway. For example, concomitant mutation of NRAS and BRAF is seen in over 20% of resistance samples driving MAPK signaling and an alternative splice variant of BRAFV600E that can dimerize in the context of BRAF mutation emerges in 20–25% of resistance samples; loss of NF-1 leading to NRAS activation has also been described.(86, 89, 90) Additionally, other MOR’s that do not rely on paradoxical activation also are seen and include increased expression of BRAFV600, downstream oncogenic mutation of MEK, and alternative MAPK activation (COT) leading to activation of MEK.(91–94) (MORs are summarized in Figure 4)
Figure 4. Mechanisms of Acquired Resistance to BRAF inhibitors.
1) Receptor Tyrosine Kinase Activation that can signal either through CRAF (1A) or the PI3K pathway (1B); 2) Concomitant NRAS mutation; 3) Emergence of a truncated BRAFV600E variant from alternative splicing; 4) Concomitant MEK mutation; 5) BRAFV600 overexpression; 6) Loss of NF1; 7) COT, an alternative MAPK, activation.
MEK-directed therapy
The clinical development of more selective MEK1/2 inhibitors, such as tremetinib and MEK162, has led to the proof of principle that MEK is a legitimate target in melanoma, both in BRAF mutant melanoma and, to a lesser degree, in BRAF wild-type melanoma (harboring NRAS or NF1 mutations).(95, 96) In fact, trametinib has recently been FDA-approved for the treatment of BRAF-mutant metastatic melanoma based on a randomized phase III study demonstrating that treatment with tremetinib is associated with a survival advantage compared with conventional chemotherapy (RR 22%, PFS 4.8 months [MO], 6-mo OS 81% for trametinib vs RR 8%, PFS 1.5 mo, .6-MO OS 67% FOR chemo).(96) MEK162 has also shown clinical efficacy in both BRAF and NRAS mutant melanoma in a phase II study (BRAF mutants – RR 20%, PFS 3.6 mo; NRAS mutants – RR 20%, PRS 3.7 mo).(97) A phase III study is underway to explore whether MEK162 is more effective than chemotherapy in NRAS mutant melanoma. (NCT01763164) Lastly, selumetinib was shown to have modest efficacy in patients with uveal melanoma. In a randomized, phase II study, treatment with selumetinib was associated with a two-fold improvement in PFS compared to patients who received chemotherapy; though notably, overall survival was not different in the two treatment groups.(98) Based on the results of all of these studies, it appears clear that MEK inhibition is associated with modest benefit (20% RR, PFS 4–5 months) in subsets of patients with melanoma and will have a role as a single agent in the treatment of this disease. The MOR’s of single-agent MEK inhibitor therapy has not been well elucidated.
The future of “Targeted Therapy” in melanoma
It is important to acknowledge that targeted therapy in melanoma remains in its infancy. Only four years have passed since initial clinical data with vemurafenib were presented. During this time, the collective knowledge regarding both mechanisms of action and resistance of BRAF and MEK inhibitor therapy has grown nearly exponentially. As these data have emerged, so too have clinical trial ideas using BRAF and MEK inhibitor therapy as the backbone to combinatorial regimens that have been rationally designed from our scientific understanding of how these agents change tumor cells.
The first example of this second wave of trials focusing on combination regimens is a phase I/II combination of dabrafenib and trametinib.(99) It was predicted that reactivation of the MAPK pathway would occur in the setting of BRAF inhibitors.(82) Therefore, inhibition of the pathway downstream of BRAF by targeting either MEK or ERK was considered an approach that may lead to further clinical benefit and perhaps more remarkably, improvement in severity of toxicity. Interestingly, the sequential administration of a BRAF inhibitor followed by a MEK inhibitor is ineffective and exposes patients to the potential toxicities seen with each single agent, yet the concurrent treatment of both agents is associated with an improvement of response rate, response depth (i.e. greater maximal response), response duration, PFS, and a reduction in toxicity severity.(99, 100) This peculiar safety signal is based on the fact that BRAF inhibitors paradoxically activate the MAPK pathway through facilitation of RAF dimerization.(87, 88) As discussed above, many MOR’s to BRAF inhibitors emerge as a result of this phenomenon, however the toxicity of BRAF inhibitors is also likely explained by this. Namely, BRAF inhibitor toxicity is likely a result of upregulation of the MAPK in non-melanoma cells; the best described example is the development of squamous cell carcinomas of the skin secondary to RAS mutations in skin cells.(101) This phenomenon is seen at a much lower frequency with the treatment of BRAF-mutant melanoma with MEK inhibitors.(99) Therefore, in BRAF mutant cells BRAF and MEK inhibitors both inhibit the pathway leading to augmented inhibition but exert differential effects on the MAPK pathway in non-BRAF mutant cells such as squamous cells of the skin, leading to an attenuation of toxicity. There are now two additional phase I combinations of BRAF plus MEK inhibitors showing similar improvements in efficacy and abrogation of toxicity severity.(102, 103) Phase III studies of each of these combinations are underway (NCT01689519; NCT01597908; NCT01584648) or being planned. It is expected that over the coming 5 years, triple and quadruple drug regimens will be studied in the clinic to treat BRAF mutant melanoma with the BRAF and MEK inhibitor combination at the core.
In NRAS mutant melanoma, it is anticipated that two events will occur in the near future that will hopefully lead to the dramatic improvement in how these patients are treated. First, a phase III study of MEK162 (NCT01763164) has been launched to determine the efficacy of this agent compared with chemotherapy. If successful, regulatory approval would be expected. Second, a number of combination regimens are expected based on preclinical data showing that a number of agents may augment MEK inhibitor toxicity in patients with NRAS mutant melanoma.(104, 105) Two examples include the combination of a MEK inhibitor with a CDK4/6 inhibitor (NCT01781572) and the second a combination of a MEK inhibitor with an HDM2 antagonist; though many more doublet and triplet combinations are expected in the near future.
Grand unification: The intersection of MAPK inhibition and immunotherapy
When ipilimumab and vemurafenib were approved by the US FDA within months of each other in 2011, a great deal of advocacy was directed towards the makers of each drug to support a combination trial. It turns out that there is actually a compelling rationale to combine BRAF inhibitors with immunotherapies that goes beyond the fact that they are both effective in patients with melanoma. In particular, emerging evidences suggests that oncogenic BRAF is immunosuppressive.(106, 107) Further, treatment with MAPK inhibitors is associated with enhanced expression of melanocytic antigens, antigen-recognition by T-cells, and an influx of CTLs.(108–112) These findings offer compelling evidence for the development of combined targeted and immune therapies, although based on the early attempts at combining BRAF inhibitors with checkpoint inhibitors, clinical trials may not be so simple. As an example, the phase I trial of vemurafenib plus ipilimumab was closed due to toxicity concerns, namely a high rate of severe hepatic toxicity.(113) Still, a number of trials have opened exploring various BRAF-directed therapies (single agent, BRAF inhibitor plus MEK inhibitor combinations, etc.) with checkpoint inhibitors and cytokines alike. The great hope is that the ideal combination will be identified that will be associated with a very high rate of durable clinical response and no untoward toxicity.
Conclusions/Future directions
From the bleakness of the recent past to the great promise of the near future, the development of melanoma therapeutics has always relied on a strong connection with hard-core molecular biology and immunology laboratories to drive the clinical progress. This is more important than ever as critical issues remain regarding the ideal sequences and combinations of the various agents that have proven preclinical and clinical efficacy.
Footnotes
Conflicts of interest:
K. Flaherty, Consultant/Advisory Board, Glaxo Smith Kline, Roche/Genentech, Novartis
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Morton D, Eilber FR, Malmgren RA, Wood WC. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970;68:158–163. discussion 63–4. [PubMed] [Google Scholar]
- 4.Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361–2370. [PubMed] [Google Scholar]
- 5.Baker HW. Spontaneous Regression of Malignant Melanoma. Am Surg. 1964;30:825–829. [PubMed] [Google Scholar]
- 6.Morton DL, Eilber FR, Holmes EC, Hunt JS, Ketcham AS, Silverstein MJ, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180:635–643. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkwood JM, Ernstoff MS. Interferons in the treatment of human cancer. J Clin Oncol. 1984;2:336–352. doi: 10.1200/JCO.1984.2.4.336. [DOI] [PubMed] [Google Scholar]
- 8.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Mule JJ. Immunotherapy of cancer with lymphokine-activated killer cells and recombinant interleukin-2. Surgery. 1985;98:437–444. [PubMed] [Google Scholar]
- 10.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman LR, Cerottini JC, Brunner KT. In vivo studies of the role of cytotoxic T cells in tumor allograft immunity. J Immunol. 1972;109:1371–1378. [PubMed] [Google Scholar]
- 13.Grossberg SE. The interferons and their inducers: molecular and therapeutic considerations. 3. N Engl J Med. 1972;287:122–128. doi: 10.1056/NEJM197207202870305. [DOI] [PubMed] [Google Scholar]
- 14.Grossberg SE. The interferons and their inducers: molecular and therapeutic considerations. 2. N Engl J Med. 1972;287:79–85. doi: 10.1056/NEJM197207132870206. [DOI] [PubMed] [Google Scholar]
- 15.Grossberg SE. The interferons and their inducers: molecular and therapeutic considerations. 1. N Engl J Med. 1972;287:13–19. doi: 10.1056/NEJM197207062870104. [DOI] [PubMed] [Google Scholar]
- 16.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan RJ, Atkins MB. Cytokine therapy in melanoma. J Cutan Pathol. 2010;37(Suppl 1):60–67. doi: 10.1111/j.1600-0560.2010.01507.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 20.Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 21.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 22.Joseph RW, Sullivan RJ, Harrell R, Stemke-Hale K, Panka D, Manoukian G, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. 2012;35:66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–175. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Tian R, Xiu B, Yan J, Jia R, Zhang L, et al. Antitumor activity of T cells generated from lymph nodes draining the SEA-expressing murine B16 melanoma and secondarily activated with dendritic cells. Int J Biol Sci. 2009;5:135–146. doi: 10.7150/ijbs.5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwong MM, Neyns B, Yang JC. Adoptive T-cell transfer therapy and oncogene targeted therapy for melanoma: the search for synergy. Clin Cancer Res. 2013:19. doi: 10.1158/1078-0432.CCR-13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sondel PM, Bach FH. Recognitive specificity of human cytotoxic T lymphocytes. I. Antigen-specific inhibition of human cell-mediated lympholysis. J Exp Med. 1975;142:1339–1348. doi: 10.1084/jem.142.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 36.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 37.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 38.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 39.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013 Jun 2; doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamid O, Sosman J, Lawrence D, Sullivan RJ, Ibrahim N, Kluger H, et al., editors. J Clin Ocol. Chicago: ASCO; 2013. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) [Google Scholar]
- 44.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 Jun 2; doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan RJ, Flaherty K. MAP kinase signaling and inhibition in melanoma. Oncogene. 2012 Sep 3; doi: 10.1038/onc.2012.345. [DOI] [PubMed] [Google Scholar]
- 47.Davies MA. The role of the PI3K-AKT pathway in melanoma. Cancer J. 2012;18:142–147. doi: 10.1097/PPO.0b013e31824d448c. [DOI] [PubMed] [Google Scholar]
- 48.Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res. 2013:19. doi: 10.1158/1078-0432.CCR-13-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 50.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–6488. [PubMed] [Google Scholar]
- 52.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jakob JA, Bassett RL, Jr, Ng CS, Curry JL, Joseph RW, Alvarado GC, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2011 Dec 16; doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maertens O, Johnson B, Hollstein P, Frederick DT, Cooper ZA, Messiaen L, et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 2013;3:338–349. doi: 10.1158/2159-8290.CD-12-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibney GT, Smalley KS. An unholy alliance: cooperation between BRAF and NF1 in melanoma development and BRAF inhibitor resistance. Cancer Discov. 2013;3:260–263. doi: 10.1158/2159-8290.CD-13-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 57.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McArthur GA, Young RJ, Sheppard KE, Mar V, Waldeck K, Fox SB, et al. Clinical significance of genomic alterations of the CDK4-pathway and sensitivity to the CDK4 inhibitor PD 0332991 in melanoma. J Clin Oncol. 2012;30 (Suppl; abstr 8520) [Google Scholar]
- 60.Sheppard KE, McArthur GA. The cell cycle regulator CDK4 an emerging therapeutic target in melanoma. Clin Cancer Res. 2013:19. doi: 10.1158/1078-0432.CCR-13-0259. [DOI] [PubMed] [Google Scholar]
- 61.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 62.Tsao H, Zhang X, Benoit E, Haluska FG. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene. 1998;16:3397–3402. doi: 10.1038/sj.onc.1201881. [DOI] [PubMed] [Google Scholar]
- 63.Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 2000;60:1800–1804. [PubMed] [Google Scholar]
- 64.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase, III. randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 65.Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31:373–379. doi: 10.1200/JCO.2012.42.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 68.Delord JNHAA, Taamma A, Faivre SJ, Besse-Hammer T, Italiano A, Vignaud C, Donica M, Raymond E, editors. J Clin Oncol. Chicago: ASCO; 2010. First-in-human phase I safety, pharmacokinetic (PK), and pharmacodynamic (PD) analysis of the oral MEK-inhibitor AS703026 (two regimens [R]) in patients (pts) with advanced solid tumors. [Google Scholar]
- 69.Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 70.Boasberg PD, Redfern CH, Daniels GA, Bodkin D, Garrett CR, Ricart AD. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer Chemother Pharmacol. 2011;68:547–552. doi: 10.1007/s00280-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 71.LoRusso PM, Krishnamurthi SS, Rinehart JJ, Nabell LM, Malburg L, Chapman PB, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin Cancer Res. 2010;16:1924–1937. doi: 10.1158/1078-0432.CCR-09-1883. [DOI] [PubMed] [Google Scholar]
- 72.Sharfman WH, Hodi FS, Lawrence DP, Flaherty KT, Amaravadi RK, Kim KB, et al., editors. J Clin Oncol. Chicago, IL: ASCO; 2011. Results from the first-in-human (FIH) phase I study of the oral RAF inhibitor RAF265 administered daily to patients with advanced cutaneous melanoma. [Google Scholar]
- 73.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 76.Dummer R, Robert C, Nyakas M, McArthur GA, Kudchadkar RR, Gomez-Roca C, et al., editors. J Clin Oncol. Chicago, IL: ASCO; 2013. Initial results from a phase I, open-label, dose escalation study of the oral BRAF inhibitor LGX818 in patients with BRAF V600 mutant advanced or metastatic melanoma. [Google Scholar]
- 77.Haq R, Yokoyama S, Hawryluk EB, Jonsson GB, Frederick DT, McHenry K, et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc Natl Acad Sci U S A. 2013;110:4321–4326. doi: 10.1073/pnas.1205575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nathanson KLAM, Letrero R, D'Andrea KP, O'Day S, Infante JR, Falchook GS, Millward M, Curtis CM, Ma B, Gagnon RC, Lebowitz PF, Long RF, Kefford RF, editors. J Clin Oncol. Chicago: ASCO; 2011. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor GSK2118436 (GSK436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7:2876–2883. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–223. doi: 10.1038/nm.2609. [DOI] [PubMed] [Google Scholar]
- 84.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whittaker SR, Theurillat JP, Van Allen E, Wagle N, Hsiao J, Cowley GS, et al. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov. 2013;3:350–362. doi: 10.1158/2159-8290.CD-12-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang H, Daouti S, Li WH, Wen Y, Rizzo C, Higgins B, et al. Identification of the MEK1(F129L) activating mutation as a potential mechanism of acquired resistance to MEK inhibition in human cancers carrying the B-RafV600E mutation. Cancer Res. 2011;71:5535–5545. doi: 10.1158/0008-5472.CAN-10-4351. [DOI] [PubMed] [Google Scholar]
- 95.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med. 2012;10:107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N Engl J Med. 2012 Jun 4; doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 97.Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 98.Carvajal RD, Sosman JA, Quevedo F, Milhem M, Joshua AM, Kudchadkar RR, et al., editors. J Clin Oncol. Chicago, IL: ASCO; 2013. Phase II study of selumetinib (sel) versus temozolomide (TMZ) in gnaq/Gna11 (Gq/11) mutant (mut) uveal melanoma (UM) [Google Scholar]
- 99.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N Engl J Med. 2012 Sep 29; doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez R, Ribas A, Daud A, Pavlick A, Gajewski TF, Puzanov I, et al., editors. Phase IB Study of Vemurafenib in Combination with the MEK inhibitor, GDC-0973, in Patients (pts) with Unresectable or Metastatic BRAFV600 Mutated Melanoma (BRIM7); European Society for Medical Oncology; 2012. [Google Scholar]
- 103.Kefford R, Miller WH Jr, Tan DS-W, Sullivan RJ, Long GV, Tai WMD, et al., editors. Preliminary results from a phase Ib/II, open-label, dose-escalation study of the oral BRAF inhibitor LGX818 in combination with the oral MEK1/2 inhibitor MEK162 in BRAF V600-dependent advanced solid tumors. Chicago: ASCO; 2013. [Google Scholar]
- 104.Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. 2012;18:1503–1510. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji Z, Njauw CN, Taylor M, Neel V, Flaherty KT, Tsao H. p53 rescue through HDM2 antagonism suppresses melanoma growth and potentiates MEK inhibition. J Invest Dermatol. 2012;132:356–364. doi: 10.1038/jid.2011.313. [DOI] [PubMed] [Google Scholar]
- 106.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khalili JS, Liu S, Rodriguez-Cruz TG, Whittington M, Wardell S, Liu C, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 109.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donia M, Fagone P, Nicoletti F, Andersen RS, Hogdall E, Straten PT, et al. BRAF inhibition improves tumor recognition by the immune system: Potential implications for combinatorial therapies against melanoma involving adoptive T-cell transfer. Oncoimmunology. 2012;1:1476–1483. doi: 10.4161/onci.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 113.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–136. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]