Summary
Recent studies have identified the proton-coupled folate transporter (PCFT) as the mechanism by which folates are absorbed across the apical brush-border membrane of the small intestine and across the basolateral membrane of the choroid plexus into the cerebrospinal fluid. Both processes are defective when there are loss-of-function mutations in this gene as occurs in the autosomal recessive disorder hereditary folate malabsorption. Because this transporter functions optimally at low pH, antifolates are being developed that are highly specific for PCFT in order to achieve selective delivery to malignant cells within the acidic environment of solid tumors. PCFT has a spectrum of affinities for folates and antifolates that narrows and increases at low pH. Residues have been identified that play a role in folate and proton binding, proton coupling, and oscillation of the carrier between its conformational states.
Introduction
Studies on the mechanism of transport of folates date back more than a half-century providing a clear characterization of the properties of the various folate-specific transporters in a variety of different tissues and cell lines. However, information on the molecular basis for these activities emerged more recently and a full understanding of important aspects of transport, in particular, vectorial transport across epithelia, has not as yet been achieved.
There are two folate-specific members of the superfamily of solute carriers, the reduced folate carrier (RFC-SLC19A1) and the proton-coupled folate transporter (PCFT-SLC46A1). The former was cloned in 1994 [1], the latter was identified in 2006 [2]. PCFT, like other proton-coupled processes, is expressed at the acidic microenvironment of the apical brush-border membrane of the proximal small intestine and mediates the intestinal absorption of folates. PCFT is highly specific for folates and folate analogs. This is unlike the proton-coupled amino acid transporter (SLC36A1; hPAT1), the monocarboxylic acid transporter (SLC16A1; MCT1), and the peptide transporter (PEPT1; SLC15A1) that mediate the intestinal absorption of a diverse spectrum of substrates and, hence, are of considerable potential utility as drug transporters [3]. PCFT is also expressed in a variety of malignant cells as well as normal tissues [2,4,5]. Hence, the pharmacological potential of PCFT is focused on its role in the intestinal absorption of antifolates and its potential for the delivery of antifolates to tumor cells for the treatment of cancer. This paper will review the physiological role of PCFT, illustrating its functional properties, and the status of studies that address the pharmacological potential of this transporter. Transport of folates has been the subject of recent reviews [6–8].
The physiological role of PCFT as established by loss of function mutations in this gene in humans and mice
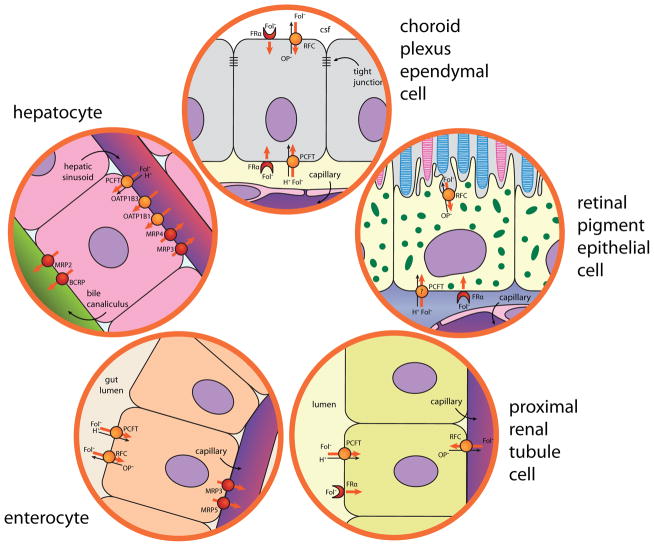
The physiological role of PCFT has been established by the phenotype of humans with the rare autosomal recessive disorder, hereditary folate malabsorption (HFM), in which the function of this transporter is lost or severely impaired [2,9]. The pathophysiological consequences are due to two defects: (i) Impaired transport across the apical brush-border membrane of the proximal small intestine where PCFT is highly expressed (Figure 1) resulting in severe systemic folate deficiency with anemia, sometimes pancytopenia, hypo-immunoglobulinemia and gastrointestinal defects. This fully corrects with pharmacological doses of oral folates or low doses of parenteral folate. (ii) Impaired transport of folates across the blood-brain barrier into the cerebrospinal fluid (CSF) apparently due to a defect in transport across the choroid plexus where PCFT is expressed at the basolateral membrane (Figure 1). Infants and children with HFM have very low CSF folate levels even when blood folate levels are corrected. Much higher blood folate levels are required to normalize CSF folate levels [9,10].
Figure 1.
The expression pattern of folate-specific and other transporters in epithelia. (i) Enterocyte: Both PCFT and RFC are expressed at the apical brush-border membrane. However, RFC does not contribute significantly to folate absorption under physiological conditions due, at least in part, to its neutral pH optimum and the low pH at the microenvironment of the surface of the proximal small intestine. Hence, when PCFT is absent, as occurs in HFM and PCFT-null mice, but RFC is expressed, there is a failure of intestinal folate absorption. Transport across the serosal membrane is likely mediated by several multidrug resistance-associated proteins (MRPs). (ii) Hepatocyte: PCFT is expressed at the sinusoidal membrane. RFC is also expressed but its location not defined. Their role in blood to bile transport is not clear. A variety of other transporters, that are not folate-specific, play a role in this process based upon studies in knock-out mice and the consequences of polymorphisms and mutations in these transporter genes. (iii) Choroid Plexus: FRα is highly expressed at the apical membrane in apposition to the cerebrospinal fluid (CSF), to a much lesser extent at the basolateral membrane in apposition to the capillaries. PCFT is expressed at the basolateral membrane. RFC is also expressed at the apical membrane. RFC is a bidirectional transporter and since it is an organic anion antiporter driven by the organic phosphate gradient, it favors transport from CSF into the ependymal cells. Vectorial transport of folate from blood to CSF requires both PCFT and FRα since deletion of either one leads to a marked decrease in CSF folate (6). (iv) Retinal Pigment Epithelium: All three folate-specific transporters are expressed in this epithelium. The location of PCFT has not been confirmed. Neither PCFT nor FRα is required for visual function since vision is intact in humans who have lost either transporter. (v) Proximal Renal Tubule: PCFT and FRα are expressed in the apical membrane. Folate clearance is increased in FRα-null mice. The roles of PCFT and RFC are not clear. Again, RFC’s location favors transport into the cells (6). Cancer cells express both RFC and PCFT. FRs are also expressed in most malignant cells but to varying degrees.
A mouse model of HFM in which this gene has been targeted reproduces the pathological changes observed in humans with this disorder [11]. PCFT-null mice appear to have the same phenotype as humans. The neurological deficiency dominates the clinical picture in mice when the anemia and other systemic signs of folate deficiency are corrected. There are many unanswered questions regarding the biological role of PCFT and the pathophysiological changes that occur when PCFT function is lost, such as: (i) What is the mechanism of intestinal absorption of folate in the absence of PCFT? (ii) What is the role of PCFT in folate transport across the choroid plexus? (iii) What is the role of PCFT in transport of folates across the basolateral membrane of hepatocytes? (iv) Does PCFT have a functional role in other tissues in which it is expressed such as the proximal renal tubule (apical) and the retinal pigment epithelium (basolateral) [12]. While it is unclear as to the mechanism of folate transport across the basolateral membrane of the small intestine, there is evidence that MRP3 and other multidrug resistance-associated proteins are involved [6,13].
The secondary structure of PCFT
Based largely on the substituted cysteine accessibility method, along with hemagglutin tagging, PCFT has been shown to consist of twelve transmembrane domains with both N- and C-termini directed to the cytoplasm [14–16]. The human protein is glycosylated at two sites (N58 and N68 in the 1st extracellular loop); neither is required for trafficking nor function in individual cells [15]. PCFT contains seven Cys residues, three are within transmembrane domains. Two of the Cys residues are in extracellular loops, (C66 1st and C298 4th extracellular loops), and form a disulfide bond [14]. None of these residues, nor the bond that links two extracellular loops, are required for function. However, the loss of the Cys residues makes the protein less tolerant of additional amino acid substitutions [17]. PCFT appears to form an oligomer based upon detection of a high molecular weight species on protein electrophoresis [18,19]. However, the monomeric structure may represent the functional unit in the plasma membrane [20].
Residues that play a role in folate and proton binding, proton coupling, and oscillation of the carrier
Notable findings that relate to the impact of protons on PCFT function are emerging: His 281 in the 7th TMD is an important determinant of proton binding. When mutated to Ala there is a marked increase in the folate influx Kt (concentration at which influx is one-half the maximum rate). This is reversed when the pH is decreased [21]. This is in contrast to Glu185 in the 5th TMD which appears to be required for proton coupling. In this case, when mutated to Ala, there is impaired function at low pH but no change in function at pH 7.4 in the absence of a pH gradient. This mutation results in a marked fall in the influx Vmax without any change in the influx Kt. The defect here was attributed to impaired proton dissociation from the carrier at the inner cell membrane interface, a rate-limiting step in carrier cycling [22]. A variety of residues and domains have been identified recently that play a role in folate substrate binding and oscillation of the carrier between its conformational states [19,23–28].
Regulation of PCFT expression
The basal PCFT promoter has been defined [29,30]. Transcription of the PCFT gene is regulated by the nuclear respiratory factor (NRF1) and NRF1 binding sites are present in the PCFT promoter [31]. PCFT expression is increased by vitamin D3 in Caco-2 cells and in rat duodenal biopsies and at least one vitamin D receptor response element has been identified in the PCFT gene [32]. Hypermethylation of the GC-rich PCFT promoter appears to be responsible for the lack of PCFT expression in T leukemia cell lines and in methotrexate-resistant HeLa cells [29,33]. PCFT mRNA in the small intestine of folate deficient mice is markedly increased, as are the other folate-specific transporters; however, the underlying molecular basis is not known [16,34].
The pharmacological role of PCFT
Four folate analogs are currently employed for the treatment of cancer. All these antifolates form active polyglutamate derivatives, mediated by folylpolyglutamate synthetase (FPGS). This results in: (i) their retention and build up within cells and (ii) broadens and intensifies their inhibitory effects on tetrahydrofolate-cofactor dependent enzymes. Methotrexate and pralatrexate are dihydrofolate reductase inhibitors that deplete cellular tetrahydrofolate cofactors. This results in the cessation of downstream tetrahydrofolate cofactor-dependent purine, thymidylate, and methionine synthesis. Pemetrexed, raltitrexed and pralatrexate have much higher affinities for FPGS than methotrexate; the longer polyglutamates of the former two are potent inhibitors of thymidylate synthase and, in the case of pemetrexed, inhibition of one of the enzymes required for formation of the purine ring [7]. These antifolates, similar to naturally-occurring tetrahydrofolate cofactors, are negatively charged molecules that diffuse poorly across cell membranes. Hence, they require folate transporters to enter cells, reach their intracellular targets and achieve their pharmacological effects. These antifolates are excellent substrates for RFC with influx Kts in the 0.5–5 μM range. While transport efficiency mediated by PCFT varies considerably among folates and antifolates at neutral pH, this divergence decreases as the pH is decreased to 5.8–6.0, levels found at the intestinal absorptive surface [35,36]. The PCFT-mediated influx Kt for these drugs decreases, and influx Vmax increases, further as the pH is decreased to 5.5. Methotrexate and raltitrexed have low affinity for PCFT at neutral pH (influx Kt ≥ 100 μM); however, pemetrexed retains an influx Kt of ~ 15 μM at pH 7.4 and only a 50% decrease in the influx Vmax from its maximal level at pH 5.5 [2,35,37].
Antifolates are administered intravenously within the context of their use in cancer treatment. Methotrexate is also utilized for the treatment of autoimmune/inflammatory disorders and in this context is usually administered orally. Methotrexate and other antifolates are good substrates for PCFT at low pH as assessed in cell systems in vitro; hence, PCFT is assumed to be the mechanism by which methotrexate is, and other antifolates could be, absorbed from the intestine. Since pemetrexed is the favored substrate for this transporter, it should be highly bioavailable by the oral route if it is stable following passage through the stomach.
PCFT as an alternative route for antifolate transport
All the antifolates noted above are transported into tumor cells via RFC. When there is a loss of RFC expression, or mutations in the carrier that result in loss-of-function, there is resistance to methotrexate and raltitrexed [35,38]. This is not the case for pemetrexed. While PCFT is not sufficient to sustain a normal rate of pemetrexed transport in the absence of RFC, the residual transport at neutral pH is sufficient to sustain full activity of the drug in vitro because there is concurrent depletion of cellular folates when RFC function is lost which is not compensated by PCFT [35,38]. This enhances the rate of polyglutamation of the drug by decreasing the level of folates that compete with pemetrexed at the level of FPGS [35,39]. This has enormous pharmacological ramifications because it means that resistance to pemetrexed due to impaired transport would require the loss of two genetically distinct transporters. Indeed, transport-associated resistance to pemetrexed has not, as yet, been observed.
A role for PCFT in receptor-mediated endocytosis
Folates are also transported by an endocytic process mediated by folate receptors; FRα (in epithelial tissues and tumors) and FRβ (hematopoietic malignancies, some normal hematopoietic cells, and hepatoma) [40]. There is evidence that PCFT plays a role in the export of folates from acidified endosomes, where it co-localizes with FRα, into the cytoplasm [41,42]. However, the endocytic process for at least some folate substrates is functional in the absence of PCFT [43,44]. Another proton-coupled transporter, DMT1, plays a similar role in the endocytosis of dimetal ions [45].
The potential impact of PCFT on the pharmacokinetics of antifolates through its role in the enterohepatic circulation
Methotrexate and other folates/antifolates participate in the entero-hepatic circulation and this impacts on their pharmacokinetics. This process involves a role for other transporters that are not specific for folates/antifolates as illustrated in Figure 1. Excretion in the bile requires transport across two epithelial surfaces: (i) the sinusoidal (basolateral) membrane from blood to hepatocyte and (ii) across the bile canalicular (apical) membrane from hepatocyte to the biliary duct. OATP1B1 and OATP1B3 are expressed at the basolateral membrane with Kt‘s for MTX of 25–40 μM. The role for OATPB1 in biliary excretion of methotrexate is supported by the observation that when this transporter is over-expressed in mice, clearance of methotrexate from the blood is increased [46]. PCFT is also expressed in the basolateral membrane; however, its role in transport across this interface is not clear. MRP2 and the breast cancer resistance protein (BCRP) are expressed at the bile canalicular membrane and loss of function of these proteins also results in decreased methotrexate clearance from the blood [47]. The intestinal reabsorptive component of this cycle is mediated by PCFT. Substrates with a low affinity for this transporter would likely have increased fecal excretion, and a more rapid rate of clearance from the blood than methotrexate or pemetrexed, when administered intravenously.
The development of drugs designed specifically as substrates selective for PCFT
Of particular interest is a new class of folate analogs being developed as highly specific substrates for PCFT. The rationale here is that RFC is known to be the vehicle that delivers antifolates to normal tissues and is therefore responsible for the major antifolate toxicities to replicating bone marrow and intestinal cells. While PCFT is widely expressed, it has limited function within the neutral pH that surrounds normal tissues, but would be active within the acidic environment of solid tumors due to the comprised blood supply that results in hypoxia which, along with the glycolytic shift in tumors (Warburg Effect), results in increased production of lactate and a local acidosis [48]. Accordingly, antifolates are being developed as anticancer agents that have a very low affinity for RFC but a high affinity for PCFT. The agents currently in development are inhibitors of glycinamide ribonucleotide formyltransferase, one of the two enzyme required for the synthesis of the purine ring [5,8,44,49]. These drugs result in a marked fall in cellular ATP levels that, if selective for tumor cells alone, could have considerable therapeutic potential.
PCFT-associated drug interactions
While PCFT is highly specific for folates and their analogs, a number of molecules have been found to be inhibitory when present at sufficiently high concentrations. Of particular interest is the inhibitory effect of bicarbonate which, at physiological concentrations at neutral pH is markedly inhibitory. Other univalent anions such as bisulfite and nitrite are also inhibitory but this is due largely to their PCFT-independent diffusion into cells causing the collapse of the transmembrane pH gradient. On the other hand, sulfate and nitrate are not inhibitory [50]. A number of drugs inhibit PCFT-mediated transport when present at high concentrations, such as sulfasalazine [51]. Since this agent is co-administered with methotrexate in the treatment of rheumatoid arthritis and other inflammatory disorders, it could impact on the oral bioavailabiity of methotrexate. There is also evidence that proton pump inhibitors decrease the expression of PCFT [52].
Highlights.
PCFT is the mechanism of intestinal absorption of folates and antifolates
Inactivating mutations of the pcft gene result in hereditary folate malabsorption
PCFT activity is enhanced within the acidic microclimate of solid tumors
PCFT is a potential route for the selective delivery of antifolates to tumor cells
PCFT plays a role in folate transport across the choroid plexus
Acknowledgments
This work is supported by a grant from National Institutes of Health National Cancer, CA82621. The authors wish to thank Dr. Lorenzo Agoni for composing the figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dixon KH, Lanpher BC, Chiu J, Kelley K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. J Biol Chem. 1994;269:17–20. [PubMed] [Google Scholar]
- 2**.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. This is the first report identifying PCFT as the mechanism of transport of folates across the apical brush-border membrane of the small intestine, and demonstrating that loss-of-function mutations in this gene are the molecular basis for hereditary folate malabsorption. [DOI] [PubMed] [Google Scholar]
- 3.Thwaites DT, Anderson CMH. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007;92:603–619. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: Contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res. 2004;10:718–727. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- 5*.Desmoulin SK, Wang L, Hales E, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, et al. Therapeutic Targeting of a Novel 6-substituted Pyrrolo[2,3-d]Pyrimidine Thienoyl Antifolate to Human Solid Tumors Based on Selective Uptake by the Proton-coupled Folate Transporter. Mol Pharmacol. 2011;80:1096–1107. doi: 10.1124/mol.111.073833. This report demonstrated that an antifolate targeted specifically for transport mediated by PCFT is pharmacologically active in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of Membrane Transport of Folates into Cells and Across Epithelia. Annu Rev Nutr. 2011;31:177–201. doi: 10.1146/annurev-nutr-072610-145133. This is the most recent comprehensive review of the properties of the various folate-specific and other processes that transport folates and antifolates across cell membranes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Goldman ID, Chattopadhyay S, Zhao R, Moran RG. The Antifolates: Evolution, New Agents in the Clinic, and How targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Curr Opin Investig Drugs. 2010;11:1409–1423. This is a review of the current status of understanding of the pharmacological properties and biological consequences of antifolates addressing, in particular, new generation antifolates pemetrexed and pralatrexate that are approved for cancer treatment. [PubMed] [Google Scholar]
- 8*.Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther. 2012;13:1355–1373. doi: 10.4161/cbt.22020. This is a review of the properties of PCFT that addresses, in particular, therapeutic targeting of solid tumors with PCFT-selective antifolates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diop-Bove N, Kronn D, Goldman ID. Hereditary Folate Malabsorption. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews [Internet] Seattle (WA): Unverisity of Washington, Seattle; 2011. [Google Scholar]
- 10.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 2002;81:51–68. doi: 10.1097/00005792-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Salojin KV, Cabrera RM, Sun W, Chang WC, Lin C, Duncan L, Platt KA, Read R, Vogel P, Liu Q, et al. A mouse model of hereditary folate malabsorption: deletion of the PCFT gene leads to systemic folate deficiency. Blood. 2011;117:4895–4904. doi: 10.1182/blood-2010-04-279653. [DOI] [PubMed] [Google Scholar]
- 12.Gnana-Prakasam JP, Reddy SK, Veeranan-Karmegam R, Smith SB, Martin PM, Ganapathy V. Polarized distribution of heme transporters in retinal pigment epithelium and their regulation in the iron-overload disease hemochromatosis. Invest Ophthalmol Vis Sci. 2011;52:9279–9286. doi: 10.1167/iovs.11-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura Y, Hirouchi M, Kusuhara H, Schuetz JD, Sugiyama Y. Increasing systemic exposure of methotrexate by active efflux mediated by multidrug resistance-associated protein 3 (mrp3/abcc3) J Pharmacol Exp Ther. 2008;327:465–473. doi: 10.1124/jpet.108.140475. [DOI] [PubMed] [Google Scholar]
- 14.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry. 2010;49:2925–2931. doi: 10.1021/bi9021439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochim Biophys Acta. 2008;1178:1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 17.Zhao R, Shin DS, Goldman ID. Vulnerability of the cysteine-less proton-coupled folate transporter (PCFT-SLC46A1) to mutational stress associated with the substituted cysteine accessibility method. Biochim Biophys Acta. 2011;1808:1140–1145. doi: 10.1016/j.bbamem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Z, Desmoulin SK, Etnyre E, Olive M, Hsiung B, Cherian C, Wloszczynski PA, Moin K, Matherly LH. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. J Biol Chem. 2011 doi: 10.1074/jbc.M111.306860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao R, Shin DS, Fiser A, Goldman ID. Identification of a functionally critical GXXG motif and its relationship to the folate binding site of the proton-coupled folate transporter (PCFT-SLC46A1) Am J Physiol Cell Physiol. 2012;303:C673–C681. doi: 10.1152/ajpcell.00123.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duddempudi PK, Nakashe P, Blanton MP, Jansen M. The monomeric state of the proton-coupled folate transporter represents the functional unit in the plasma membrane. FEBS J. 2013;280:2900–2915. doi: 10.1111/febs.12293. [DOI] [PubMed] [Google Scholar]
- 21.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem. 2009;284:17846–17857. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol. 2009;297:C66–C74. doi: 10.1152/ajpcell.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT; SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood. 2010;116:5162–5169. doi: 10.1182/blood-2010-06-291237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Shin DS, Zhao R, Yap EH, Fiser A, Goldman ID. A P425R mutation of the proton-coupled folate transporter causing hereditary folate malabsorption produces a highly selective alteration in folate binding. Am J Physiol Cell Physiol. 2012;302:C1405–C1412. doi: 10.1152/ajpcell.00435.2011. A mutant PCFT associated with HFM had sufficient residual function to characterize its properties. There was generalized impaired oscillation of the carrier for all substrates but selective preservation of pemetrexed binding to the carrier in contrast to methotrexate and reduced folates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin DS, Zhao R, Fiser A, Goldman DI. Functional roles of the A335 and G338 residues of the proton-coupled folate transporter (PCFT-SLC46A1) mutated in hereditary folate malabsorption. Am J Physiol Cell Physiol. 2012;303:C834–C842. doi: 10.1152/ajpcell.00171.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahadeo K, Diop-Bove N, Shin D, Unal E, Teo J, Zhao R, Chang MH, Fulterer A, Romero MF, Goldman ID. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol. 2010;299:C1153–C1161. doi: 10.1152/ajpcell.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (PCFT, SLC46A1), clustering of mutations and the bases for associated losses of function. J Biol Chem. 2011;286:24150–24158. doi: 10.1074/jbc.M111.236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin DS, Zhao R, Fiser A, Goldman ID. The Role of the Fourth Transmembrane Domain in Proton-Coupled Folate Transporter (PCFT) Function as Assessed by the Substituted Cysteine Accessibility Method. Am J Physiol Cell Physiol. 2013;304:C1159–C1167. doi: 10.1152/ajpcell.00353.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Molecular Cancer Therapeutics. 2009;8:2424–2431. doi: 10.1158/1535-7163.MCT-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark M, Gonen N, Assaraf YG. Functional elements in the minimal promoter of the human proton-coupled folate transporter. Biochem Biophys Res Commun. 2009;388:79–85. doi: 10.1016/j.bbrc.2009.07.116. [DOI] [PubMed] [Google Scholar]
- 31**.Gonen N, Assaraf YG. The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1. J Biol Chem. 2010;285:33602–33613. doi: 10.1074/jbc.M110.135640. This paper provides evidence that transcriptional expression of PCFT is regulated by nuclear respiratory factor-1, NRF1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eloranta JJ, Zair ZM, Hiller C, Hausler S, Stieger B, Kullak-Ublick GA. Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol. 2009;76:1062–1071. doi: 10.1124/mol.109.055392. [DOI] [PubMed] [Google Scholar]
- 33.Gonen N, Bram EE, Assaraf YG. PCFT promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun. 2008;376:787–792. doi: 10.1016/j.bbrc.2008.09.074. [DOI] [PubMed] [Google Scholar]
- 34.Liu M, Ge Y, Cabelof DC, Aboukameel A, Heydari AR, Mohammad R, Matherly LH. Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. J Biol Chem. 2005;280:5588–5597. doi: 10.1074/jbc.M412662200. [DOI] [PubMed] [Google Scholar]
- 35.Zhao R, Hanscom M, Chattopadhyay S, Goldman ID. Selective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier; association with the presence of a secondary transport pathway. Cancer Res. 2004;64:3313–3319. doi: 10.1158/0008-5472.can-03-3953. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res. 2004;10:6256–6264. doi: 10.1158/1078-0432.CCR-04-0645. [DOI] [PubMed] [Google Scholar]
- 37.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities as compared to the reduced folate carrier. Mol Pharmacol. 2008;74:854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–449. doi: 10.1158/1535-7163.MCT-05-0243. [DOI] [PubMed] [Google Scholar]
- 39.Desmoulin SK, Wang L, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. Functional loss of the reduced folate carrier enhances the antitumor activities of novel antifolates with selective uptake by the proton-coupled folate transporter. Mol Pharmacol. 2012;82:591–600. doi: 10.1124/mol.112.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53:6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 41.Bozard BR, Ganapathy P, Duplantier JN, Mysona B, Ha Y, Roon P, Smith R, Goldman ID, Prasad PD, Martin PM, et al. Molecular and biochemical characterization of folate transport proteins in retinal Muller cells. Invest Ophthalmol Vis Sci. 2010;51:3226–3235. doi: 10.1167/iovs.09-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT - SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009;284:4267–4274. doi: 10.1074/jbc.M807665200. This paper demonstrated that PCFT plays a role in the endocytosis of folates mediated by folate receptors by augmenting the export of folates from acidified endosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Cherian C, Desmoulin SK, Polin L, Deng Y, Wu J, Hou Z, White K, Kushner J, Matherly LH, et al. Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. J Med Chem. 2010;53:1306–1318. doi: 10.1021/jm9015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Desmoulin SK, Cherian C, Polin L, White K, Kushner J, Fulterer A, Chang MH, Mitchell-Ryan S, Stout M, et al. Synthesis, Biological, and Antitumor Activity of a Highly Potent 6-Substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Antifolate Inhibitor with Proton-Coupled Folate Transporter and Folate Receptor Selectivity over the Reduced Folate Carrier That Inhibits beta-Glycinamide Ribonucleotide Formyltransferase. J Med Chem. 2011;54:7150–7164. doi: 10.1021/jm200739e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 46.van de SE, van der Kruijssen CM, Wagenaar E, Burggraaff JE, Mesman E, Kenworthy KE, Schinkel AH. Methotrexate pharmacokinetics in transgenic mice with liver-specific expression of human organic anion-transporting polypeptide 1B1 (SLCO1B1) Drug Metab Dispos. 2009;37:277–281. doi: 10.1124/dmd.108.024315. [DOI] [PubMed] [Google Scholar]
- 47.Vlaming ML, Pala Z, van EA, Wagenaar E, de Waart DR, van de Wetering K, van der Kruijssen CM, Oude Elferink RP, van TO, Schinkel AH. Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin Cancer Res. 2009;15:3084–3093. doi: 10.1158/1078-0432.CCR-08-2940. [DOI] [PubMed] [Google Scholar]
- 48.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 49.Cherian C, Kugel DS, Wang L, Polin L, White K, Kushner J, Stout M, Hou Z, Gangjee A, Matherly LH. Therapeutic targeting malignant mesothelioma with a novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate via its selective uptake by the proton-coupled folate transporter. Cancer Chemother Pharmacol. 2013;71:999–1011. doi: 10.1007/s00280-013-2094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao R, Visentin M, Suadicani SO, Goldman ID. Inhibition of the proton-coupled folate transporter (PCFT-SLC46A1) by bicarbonate and other anions. Mol Pharm. 2013;84:95–103. doi: 10.1124/mol.113.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, Otagiri M, Hayashi Y, Yuasa H. Functional characterization of human PCFT/HCP1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther. 2007;322:469–476. doi: 10.1124/jpet.107.122606. [DOI] [PubMed] [Google Scholar]
- 52.Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB. The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G248–G254. doi: 10.1152/ajpgi.00224.2009. [DOI] [PubMed] [Google Scholar]