Abstract
We used the cheek model of itch and pain in rats to determine the dose-response relationships for intradermal injection of serotonin and α methylserotonin on scratching behavior. We also determined the dose-related effects of intracisternally injected morphine on scratching, effects that were greatly reduced by administration of the opiate antagonist naloxone. We then examined the interactions of intradermal injection of serotonin and intracisternal injection of morphine on scratching and found that the two procedures act synergistically to increase itch. These results suggest that morphine applied to the CNS is capable of producing itch and greatly increasing itch originating in the skin (hyperknesis).
Keywords: hyperknesis, itch, serotonin, morphine, intracisternal injection
The unpleasant sensations of itch and pain are each mediated by nociceptive neurons (Davidson and Giesler 2010, Akiyama and Carstens, 2013; LaMotte et al., 2013), yet the sensations are perceived as distinct and induce very different behavioral responses. For example itch causes the desire to scratch and pain causes discomfort which results in guarding or withdrawal from a noxious stimulus. The use of animal models that allow differentiation of itch- versus pain-related behaviors are important in understanding the sensory effects of potential pruritogens or algogens. Until recently, such a model in rodents was not available. However, Shimada and LaMotte (2008) showed that pruritogens and algogens elicit distinct behaviors when applied to the cheek of mice; intradermal injection of histamine in the cheek elicited scratching with the hindlimb whereas capsaicin elicited wiping with the forelimb. Both behaviors were directed to the site of injection. This distinction between itch-evoked scratching and pain-evoked wiping has been replicated using an array of other pruritogens and algogens in mice (Akiyama et al., 2010; Wilson et al., 2011) and rats (Klein et al., 2011; Spradley et al., 2012).
Serotonin is one the most effective pruritogens in the cheek model in rats (Klein et al., 2011). Application of serotonin to the skin can also cause itch in humans (Fjellner and Hägermark, 1979; Weisshaar et al., 1997; Thomsen et al., 2002; Hosogi et al., 2006; Rasul et al., 2012). In several conditions of chronic itch, including allergic contact dermatitis and atopic dermatitis, the skin of patients exhibits increased levels of serotonin (Lundeberg et al., 1999; Soga et al., 2007). Serotonin can also elicit pain in humans (Schmelz et al., 2003). Accordingly, when applied to the rat cheek, serotonin elicits scratching with the hindlimb as well as some wiping with the forelimb (Klein et al., 2011). It has been suggested that serotonin may cause itch in humans via serotonin-induced release of histamine from mast cells (Weisshaar et al. 1997), although administration of antihistamines failed to result in a significant reduction of serotonin-induced itch compared to placebo treatment (Hosogi et al., 2006). In rats, mast cells release large amounts of serotonin (Gustafsson, 1980; Graziano, 1988; Purcell et al., 1989). In addition, topical application of serotonin activated a subpopulation of polymodal nociceptive DRG neurons with C axons and the duration of the responses of some of the recorded fibers matched the duration of scratching or biting evoked by the same stimulus in awake rats (Hachisuka et al. 2010), suggesting that such C fibers play a role in production of itch. Intradermal injection of serotonin was also shown to elicit scratching of the nape of the neck and activity in lumbar dorsal horn neurons (Jinks and Carstens, 2002). In addition, we (Moser and Giesler, 2014) recently reported that intradermal injection of a dose of serotonin that produced scratching for more than 30 min in rats (Klein et al., 2011) activated pruriceptive trigeminothalamic tract neurons with receptive fields on the cheek for a similar period of time. Together, these data support the use of serotonin as a peripheral pruritogen.
In addition to pruritogens which cause itch via activation of peripheral nociceptors, there are a number of agents which cause itch-related behaviors when administered to the CNS in animal models (Koenigstein, 1948; Thomas and Hammond, 1995; Lee et al., 2003; Sun and Chen, 2007; Su and Ko, 2011; Mishra and Hoon, 2013). Morphine is one of the most frequently studied agents of this type. It is commonly prescribed for relief from pain, but side-effects, including severe itch, can limit the amount that be administered, and thus the effectiveness of morphine for producing analgesia. Opioid-induced pruritus is often localized to facial regions of patients (Scott et al., 1980; Baraka et al., 1982; Collier, 1981; Bromage et al., 1982), suggesting the value of using the rodent cheek model of itch to study this phenomenon. The highest incidence of opioid-induced pruritus in human patients (20–100%) occurs following intrathecal administration (Baraka et al,. 1982; Bromage et al., 1982; Ballantyne et al., 1988; Szarvas et al., 2003; Ganesh and Maxwell, 2007). Intracisternal injection of morphine in rats causes robust body and facial scratching (Lee et al., 2003) as does injection of morphine within the spinal trigeminal nucleus (Thomas and Hammond, 1995). In addition, we have found that application of morphine (200 nM) to the dorsal surface of upper cervical segments and the lower medulla activates pruriceptive trigeminothalamic tract neurons (Moser and Giesler, 2013). Thus, the rat trigeminal system appears to be of considerable potential value for studies of the mechanisms underlying opioid-induced pruritus.
Morphine and other pharmacological agents which act at μ-opioid receptors appear to modulate itch caused by other stimuli. Spradley et al. (2012) showed that the μ-opioid receptor antagonist naltrexone reduced scratching caused by facial application of pruritogens, whereas morphine reduced wiping caused by algogens in rats. This finding suggests that μ-opioid receptor activation has opposite effects on processing of itchy versus painful stimuli applied to the cheek. Opioids such as morphine likely also play a role in producing other itch-related sensory phenomena such as hyperknesis (increased itch caused by pruritogens) (Fjellner and Hägermark, 1982; Onigbogi et al., 2000) and alloknesis (itch caused by innocuous mechanical stimuli that normally do not cause itch) (Koenigstein, 1948; Heyer et al., 2002).
We used the cheek model of itch to examine the relationship of itch induced by intradermal injection of serotonin and intracisternal injection of morphine in rats. We report that activation of opioid receptors by intracisternal injection of morphine increases scratching responses to intradermal injection of serotonin and that this interaction is super-additive or synergistic.
Experimental procedures
Animals
Adult male Sprague-Dawley rats (200–300 g) were used according to protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Animals arrived at the university at least one week prior to testing. At least two days prior to testing, animals underwent light anesthesia with isoflurane (3% in 100% oxygen, <5 min) and their cheeks and necks were shaved.
Intradermal injections into the cheeks
For intradermal injections in the cheeks, awake rats were gently restrained using a transparent flexible plastic cone. One experimenter held an animal within the cone while another experimenter performed the injection. Drugs for intradermal injection included serotonin creatinine sulfate complex (9–180 µg, Sigma), α-methylserotonin maleate salt (α-Me-5HT; 3–30 µg, Sigma), or 0.9% normal saline vehicle. Drugs were injected in a 10 µl volume using a 28-gauge hypodermic needle inserted into the skin on the cheek below the eye and caudal to the vibrissal pad. Intradermal injection was confirmed by observation of a small bleb at the injection site.
Intracisternal injections
Rats were lightly anesthetized using isoflurane (3%, <5 min) and intracisternal injections were performed according to Appel and Van Loon (1986). One experimenter held the animal’s head between the thumb and forefinger of the left hand behind the animal’s ears and suspended the animal with the right hand, maximally opening the foramen magnum at the base of the skull. Another experimenter performed the injection by inserting a 25-gauge 5/8-inch needle attached to a 100 µl Hamilton microsyringe through the shaved skin on the back of the neck until the needle came into perpendicular contact with the occipital bone. The needle was then moved ventrally, depressing muscles in the neck, until it could be inserted under the atlanto-occipital membrane through the foramen magnum into the cisterna magna. Drugs for intracisternal injection included morphine sulfate (0.01–333.0 µg, Sigma) or 0.9% normal saline vehicle. Drugs were injected in a 20 µl volume. We wished to evaluate the effectiveness of our methods for producing injections into the cisterna magna. We were reluctant to inject a solution containing morphine and a dye since it was possible that the dye itself might affect the CNS or the diffusion of the morphine. The following approach was used: 15 rats that had been used in intracisternal injection protocols received an injection of methylene blue (2%, 20 µl) using the same injection methods just prior to being euthanized and perfused with 0.9% normal saline. The presence of blue dye under the dura of the caudal medulla and/or rostral cervical spinal cord was used to verify an intracisternal injection. Intraperitoneal (i.p.) or subcutaneous (s.c.) injections of morphine (1 mg/kg, i.p.) or naloxone (1 mg/kg, i.p.; 0.5 mg/kg, s.c.) were administered 10 min prior to subsequent intradermal and/or intracisternal injections. When intradermal serotonin and intracisternal morphine were delivered in combination, intracisternal injections occurred within 5 min prior to intradermal injections.
Behavioral protocol
One week before testing began, animals were habituated for 60 min/day for 5 days to an acrylic chamber (15×15×23 cm) located in a lighted room. On the following test days, before intradermal or intracisternal injection, animals were habituated for 15 min before baseline activity was video-recorded for 30 min. Animals were videotaped from above; chambers were surrounded by angled mirrors to enable viewing of each part of the animals’ body with a single recording. Two animals were tested at a time in separate side-by-side chambers. The mirrors surrounding the chambers prevented animals from viewing one another during testing. Animals were returned to chambers immediately after completion of intradermal or intracisternal injections. Experimenters left the room while behavior was videotaped for ≥60 min.
Data analysis
Observers were blinded to treatment(s) during observation of previously recorded video tapes. Approximately one-third of videotapes was scored by more than one observer and a test for the joint-probability of agreement showed a high degree of inter-rater reliability (Yelton et al. 1977). For videos which were scored by more than one observer, scores determined by the observers were averaged. Scratching, wiping, and grooming behaviors were recorded. Scratching was recorded in bouts because previous studies have demonstrated the number of scratch bouts to be the most robust and reliable measure of scratching behavior (Nojima and Carstens, 2003; Klein et al., 2011). A scratch bout consisted of scratching an area of the head with the hindlimb; a scratch bout began when the hindlimb was first directed toward the head and ended when the hindpaw was placed on the ground or in the animal’s mouth. Individual wipes were recorded, with a wipe beginning when the forelimb was first directed to the face and ending when the forelimb was brought away from the face (often to be placed in or near the mouth). Grooming behavior was defined as bilateral movement of the forelimbs over the head (including the face), with a bout beginning when the forelimbs were first directed toward the face and ending when the forelimbs were held in or near the mouth or oral grooming behavior was directed toward other body areas. For intradermal injections of serotonin or α-Me-5HT, only scratch bouts or wipes directed toward the site of intradermal injection were counted (Fig. 2,3). Data are expressed as mean ± standard error of the mean (SEM). Student’s t-test or one-way ANOVAs with Tukey post tests were used to compare effects across treatments, with p<0.05 considered significant.
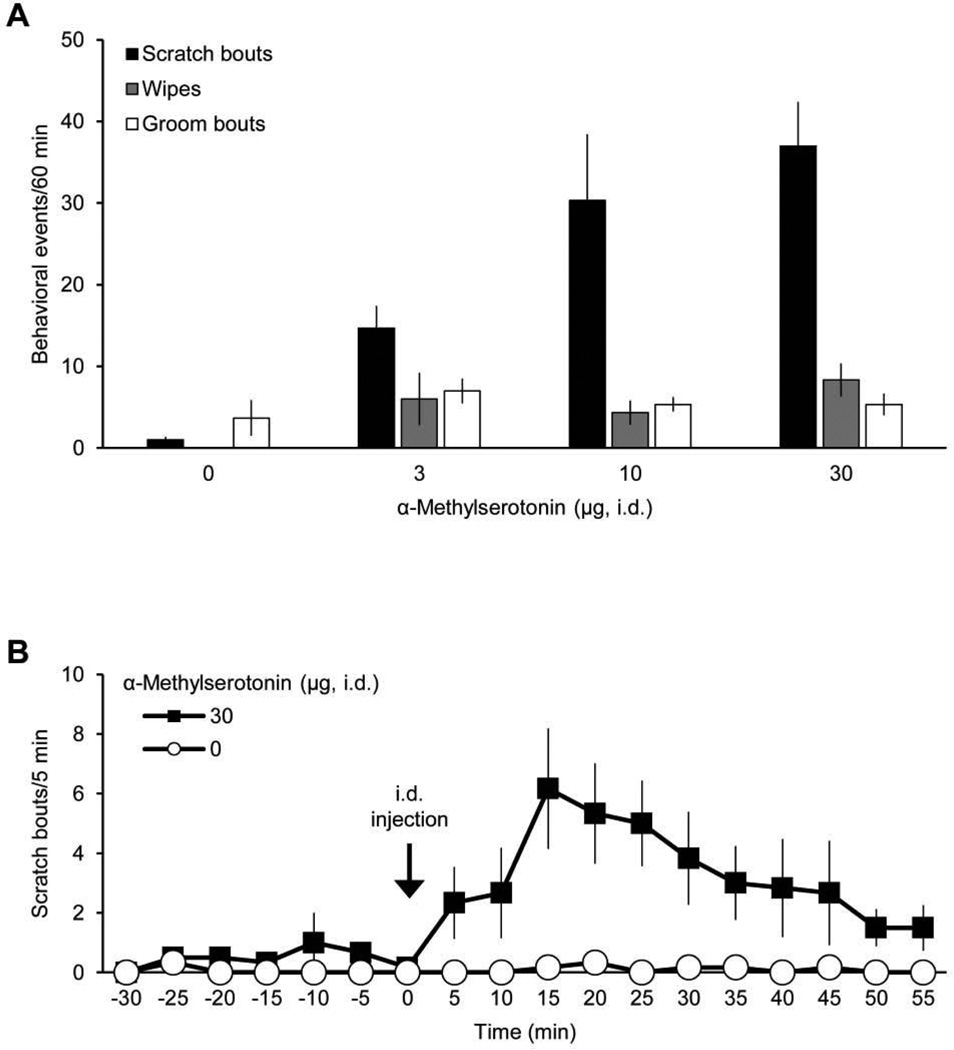
Figure 2.
Effects of intradermal injection of α-methylserotonin on facial scratching, wiping, and grooming. A, dose-response data for scratch bouts, wipes, and groom bouts elicited by intradermal (i.d.) injection of α-Me-5HT in the cheek (n=6). Only scratch bouts and wipes directed to site of i.d. injection are included. B, time course for scratching evoked by α-Me-5HT (data from A).
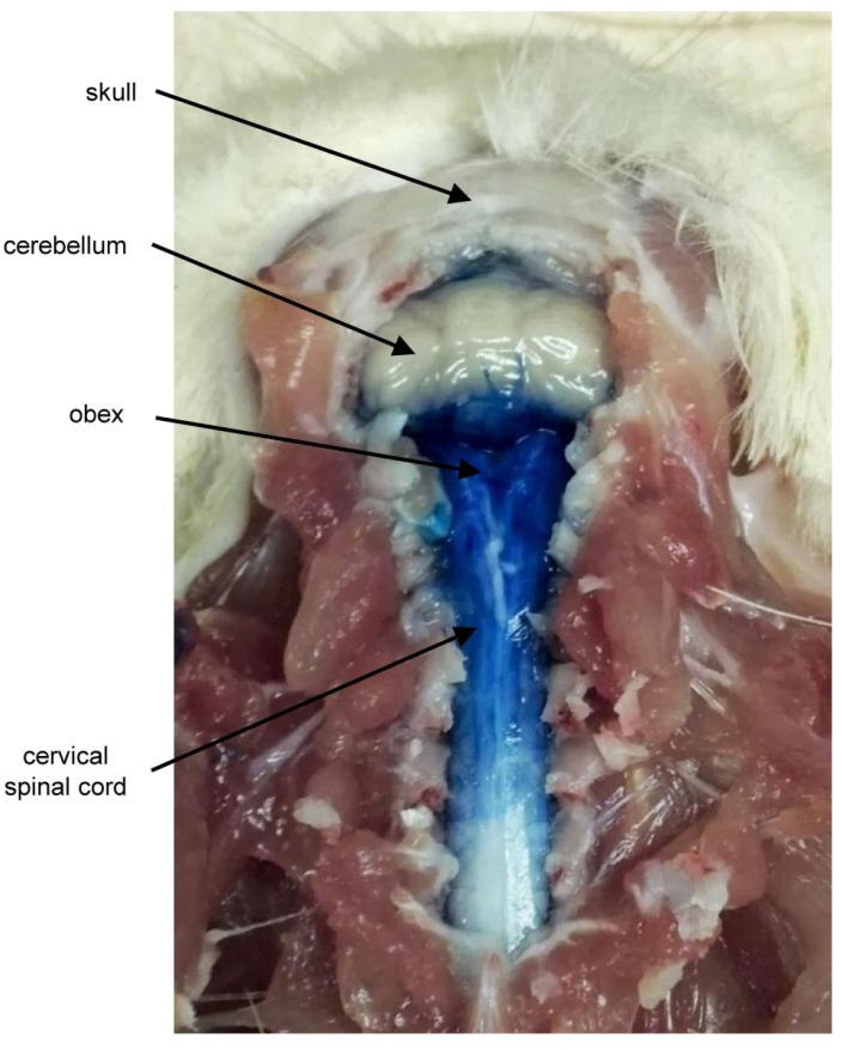
Figure 3.
Distribution of methylene blue dye after intracisternal injection.
Isobolographic analysis (Tallarida, 2001) was used to examine interactions between the effects on itch of intradermal injection of serotonin and intracisternal injection of morphine. Scratch bouts or wipes directed to any area of the head or neck rostral to the shoulders were counted The amount of each drug needed to elicit 50% of the maximum number of scratch bouts in 60 min (ED50) was determined. The theoretical additive line representing the combined amounts of intradermal injection of serotonin and intracisternal injection of morphine expected to yield 50% of the maximum effect was determined. The observed ED50 for combined delivery of serotonin and morphine and confidence interval is also plotted for comparison to the theoretical ED50.
Results
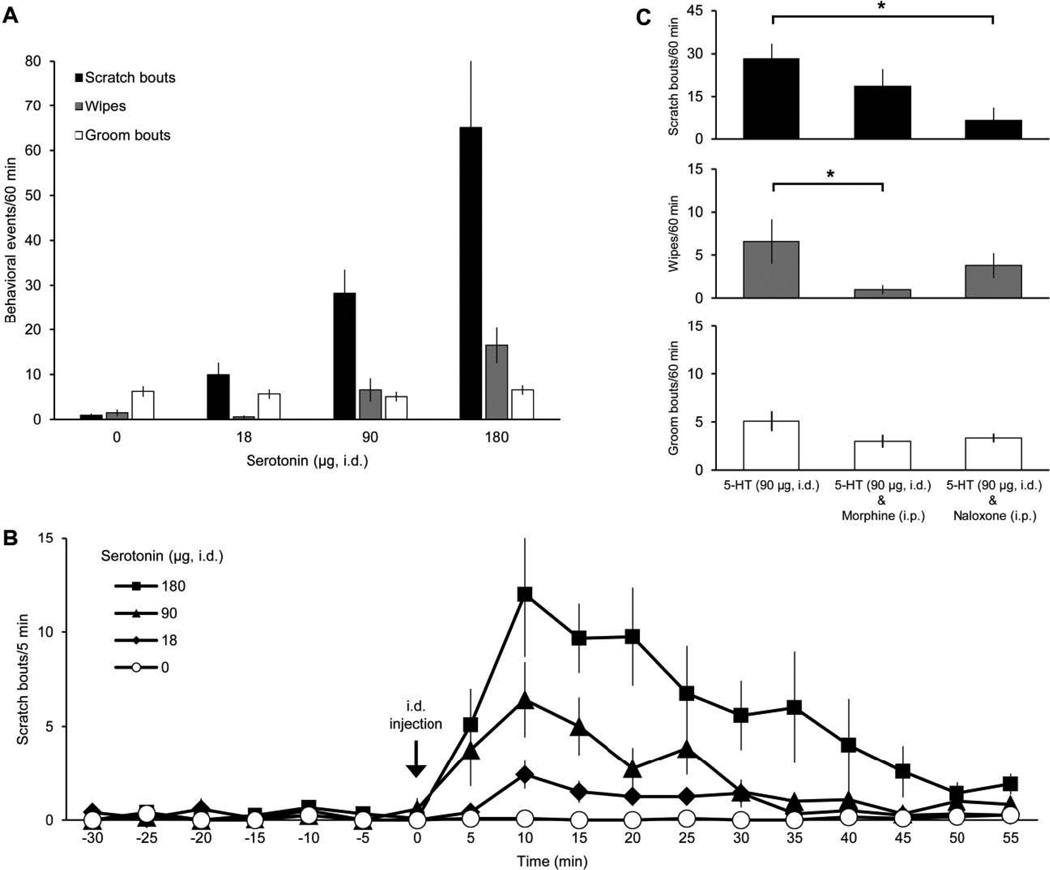
Intradermal injections of serotonin into the cheek of rats elicited dose-dependent scratching with the hindlimb (Hachisuka et al., 2010; Klein et al., 2011) and, to a much lesser degree, wiping with the forelimb directed toward the site of injection (Fig. 1A). The number of grooming bouts did not differ across doses of serotonin. The time course of serotonin-induced scratching is depicted in Figure 1B. For each dose of serotonin tested, scratching peaked 10 min after serotonin injection. For the largest dose tested (180 µg), scratching returned to baseline levels within 50 min. These results are similar to those of Klein et al. (2011) who examined the behavioral effects of serotonin and other pruritogens in the cheek model in rats. In order to test whether scratching induced by serotonin involves activation of opioid receptors, naloxone (1 mg/kg, i.p.) was administered prior to intradermal injection of serotonin (90 µg). Naloxone significantly decreased the number of scratch bouts but did not significantly reduced the number of wipes induced by serotonin (Fig. 1C). Administration of morphine (1 mg/kg, i.p.) prior to injection of serotonin significantly reduced the number of wipes but did not significantly affect the number of scratches elicited by serotonin (Fig. 1C). Neither morphine nor naloxone affected grooming induced by serotonin.
Figure 1.
Effects of intradermal injection of serotonin on facial scratching, wiping, and grooming. A, dose-response data for scratch bouts, wipes, and groom bouts elicited by intradermal (i.d.) injection of serotonin in the cheek (n=12). Only scratch bouts and wipes directed to site of i.d. injection are included. Control application of drug vehicle denoted as 0 µg serotonin. B, time course for scratching evoked by different amounts of serotonin (data from A). Scratch bouts were averaged in 5 min bins beginning 30 min before i.d. injection (“−30”). C, mean number of each behavioral event elicited by serotonin (5-HT) after intraperitoneal (i.p.) administration of morphine (n=6) or naloxone (n=6). * indicates statistically significant difference between groups denoted by black bar (p=0.015 for scratch bouts elicited by 5-HT (i.d.) vs. 5-HT (i.d.) & Naloxone (i.p.), p=0.049 for wipes elicited by 5-HT (i.d.) vs. 5-HT (i.d.) & Morphine (i.p.); one-way ANOVA with Tukey post test).
The 5-HT1/2 receptor agonist α-Me-5HT was also administered intradermally in the cheek to determine its effects on scratching. Unlike serotonin, α-Me-5HT is not enzymatically degraded by monoamine oxidase and therefore has a longer duration of action compared to serotonin (Sourkes et al., 1990). α-Me-5HT produced a dose-dependent increase in scratching and wiping with no significant effect on grooming (Fig. 2A). For α-Me-5HT-induced scratching, the time of peak scratching occurred 15 min after injection and scratching had not returned to baseline by the end of the recording period (60 min after injection) (Fig. 2B).
Morphine was administered intracisternally to determine the effects of opioid receptor activation in the CNS on scratching, wiping, and grooming. After anesthetization with isoflurane and intracisternal injections, animals awoke and returned to normal movements within 2 ± 1.1 min. In each of the 15 animals which received intracisternal injection of methylene blue dye (20 µl), postmortem analysis revealed that the dye was concentrated under the dura over the caudal medulla and rostral cervical spinal cord (Fig. 3). These results indicate that the methods used were capable of producing verified intracisternal injections in each of the cases in which it was attempted These data also suggest that in many, if not all cases, injections of morphine or vehicle using these methods were made into the cisterna magna.
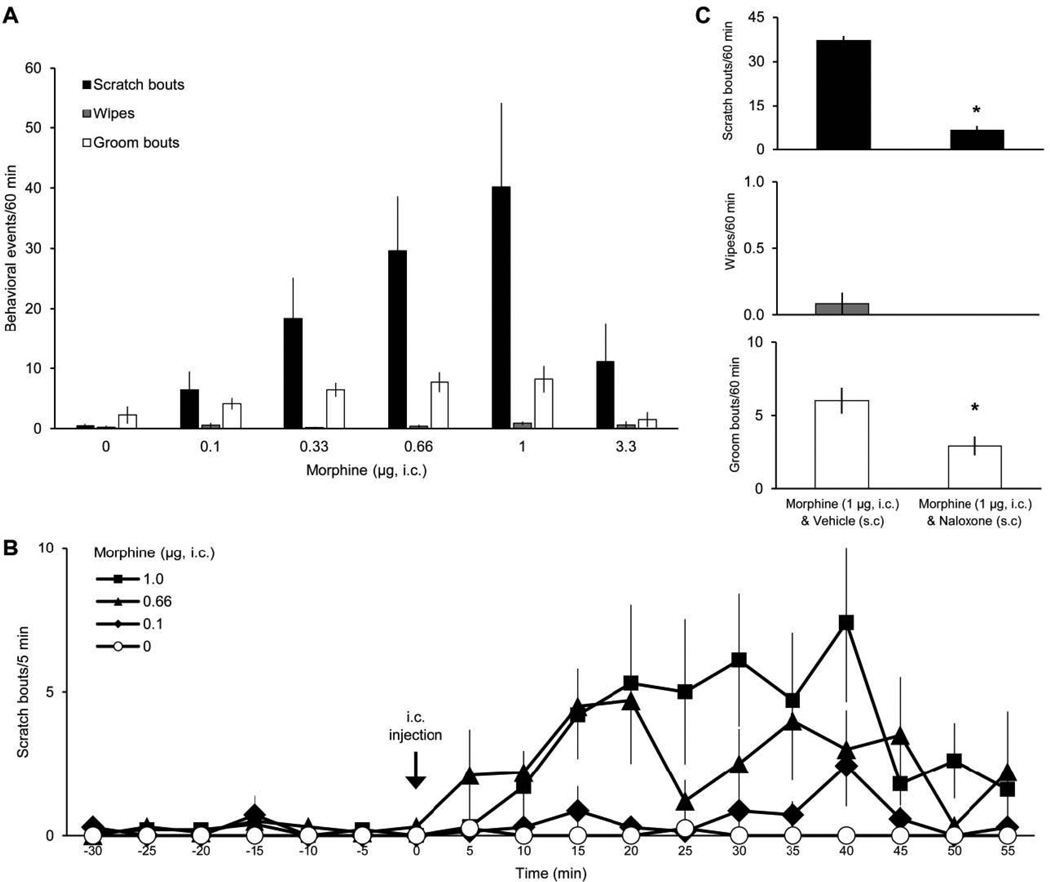
Intracisternal injection of morphine produced a dose-dependent increase in scratching (up to 1 µg) of the head and neck rostral to the shoulders (Fig. 4A). The highest dose of morphine tested (3.3 µg in 20 µl) resulted in a decreased number of scratch bouts compared to lower doses (0.33–1 µg). Grooming was also decreased at the highest dose, suggesting a possible sedative effect. Morphine did not affect wiping compared to baseline levels. Scratching began to increase within 5 min after intracisternal injection of morphine and remained elevated up to 60 min (Fig. 4B). Administration of naloxone (0.5 mg/kg, s.c.) prior to injection of morphine reduced both scratching and grooming behaviors significantly (Fig. 4C), a finding that is consistent with the idea that production of both behaviors involve activation of opioid receptors.
Figure 4.
Effects of intracisternal injection of morphine on facial scratching, wiping, and grooming. A, dose-response data for scratch bouts, wipes, and groom bouts elicited by intracisternal (i.c.) injection of morphine (n=7–11). All scratch bouts and wipes directed to the head or neck rostral to the shoulders are included. B, time course for scratching evoked by different amounts of morphine (data from A). C, mean number of each behavioral event elicited by morphine after subcutaneous (s.c.) administration of naloxone (n=12). * indicates statistically significant difference from Vehicle (s.c.) (p<0.0001 for scratch bouts, p=0.01 for wipes; Student’s t-test).
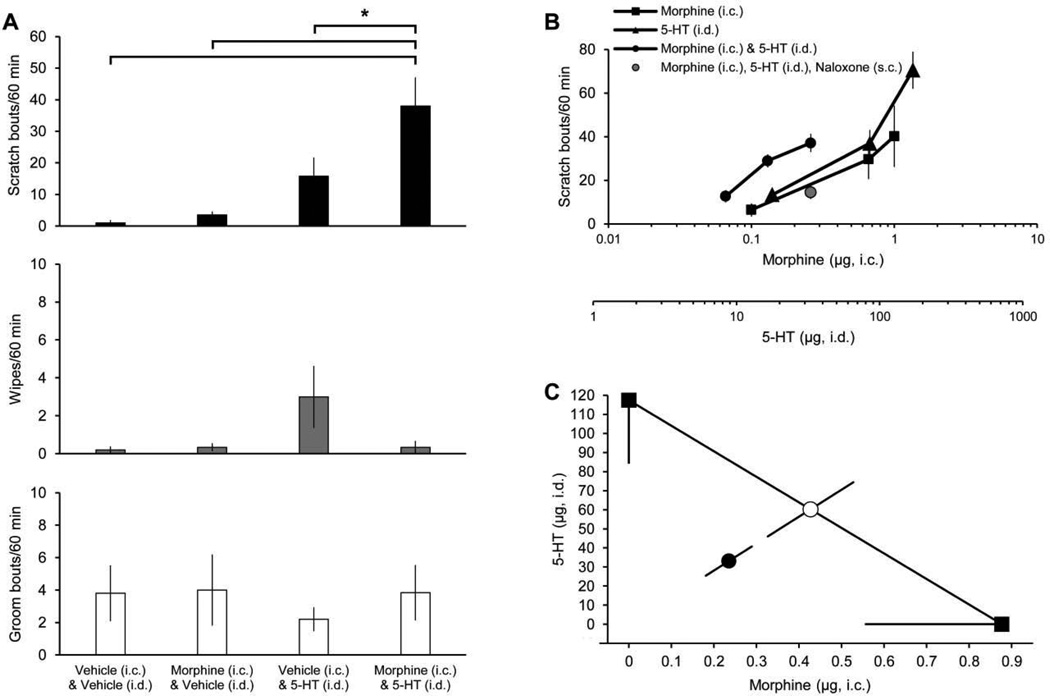
We also examined the possibility that itch produced by intradermal injection of serotonin might be potentiated by intracisternal injection of morphine results (hyperknesis). For these experiments, all scratching with the hindlimb directed to the head and neck rostral to the shoulders was counted. The number of bouts of scratching produced by intradermal injection of serotonin (90 µg) morphine was significantly increased by intracisternal injection of morphine (0.1 µg). Combined injections produced more scratching than did injection of either morphine or serotonin alone (Fig. 5A). We next tested several combinations of intradermal injection of serotonin and intracisternal injection of morphine to establish a dose-response curve for scratching induced by the drugs administered in combination (Fig. 5B). When administered in combination, the amount of either drug necessary to produce a maximal scratching effect was less than the amount of either drug required alone. The increase in scratching caused by combined delivery of serotonin and morphine was attenuated by naloxone (0.5 mg/kg, s.c., Fig 5B).
Figure 5.
Effects of combined delivery of serotonin and morphine on facial scratching, wiping, and grooming. A, scratch bouts, wipes, and groom bouts elicited by intradermal (i.d.) injection of serotonin (5-HT; 90 µg) (or vehicle) combined with intracisternal (i.c.) injection of morphine (0.1 µg) (or vehicle) (n=6). All scratch bouts and wipes directed to the head or neck rostral to the shoulders are included. * indicates statistically significant difference between groups denoted by black bar (p=0.0005 for scratch bouts elicited by Morphine (i.c.) & 5-HT (i.d.) vs. Vehicle (i.c.) & Vehicle (i.d.), p=0.0006 for Morphine (i.c.) & 5-HT (i.d.) vs. Morphine (i.c.) & Vehicle (i.d.), p=0.02 for Morphine (i.c.) & 5-HT (i.d.) vs. Vehicle (i.c.) & 5-HT (i.d.); one-way ANOVA with Tukey post test). B, dose-response curves for scratch bouts elicited by 5-HT (i.d.) (n=12), morphine (i.c.) (n=7–11), or combined delivery of 5-HT (i.d.) and morphine (i.c.) (n=6–8). X-axes denote dose of i.c. injection of morphine (top) and/or i.d. injection of 5-HT (bottom). C, isobolographic analysis using 50% of the maximum scratching effect from the data in B. The y-intercept represents the ED50 for 5-HT (i.d.) while the x-intercept represents the ED50 for morphine (i.c.). The observed ED50 for combined delivery of 5-HT (i.d.) and morphine (i.c.) (●) was significantly lower (p<0.05, t-test) than the theoretical additive ED50 (○), indicating that the increase in scratching was superadditive or synergistic.
We performed an isobolographic analysis (Fig. 5C) to test whether the scratching induced by combined delivery of intradermal injection of serotonin and intracisternal injection of morphine was merely additive or a super-additive, synergistic effect. The ED50 of combined delivery of intradermal injection of serotonin and intracisternal injection of morphine was significantly less than the theoretical additive ED50, indicating that combined the effects of intradermal injection of serotonin and intracisternal injection of morphine super-additively increased scratching.
Discussion
We used the cheek model of itch to characterize the behaviors elicited by intradermal injection of serotonin and α methylserotonin in the cheek and intracisternal application of morphine in rats. Intradermal injection of serotonin produced dose-dependent itch-related scratching and pain-related wiping. Scratching was reduced by naloxone and wiping was reduced by morphine. Intradermal injections of α methylserotonin also produced dose-related increases in scratching that endured for at least 55 min. Intracisternal injection of morphine produced dose-dependent scratching as well as a slight increase in grooming behavior. Scratching and grooming induced with morphine were both reduced by injection of naloxone. When delivered in combination, intradermal injection of serotonin and intracisternal injection of morphine produced a synergistic increase in scratching which was blocked by naloxone. Together, these data suggest that opioid receptor activation is involved in scratching produced by either serotonin or morphine and that systems which contain serotonin receptors and opioid receptors interact to modulate the sensation of itch. Such interactions could occur through a number of mechanisms. For example, dorsal horn neurons, possibly including VTT neurons (Moser and Giesler, 2013), that receive input, directly or indirectly from primary afferent neurons activated by intradermal injections of the pruritogen serotonin might express the excitatory opioid receptor MOR1D (Liu et al., 2011). Alternatively, the hypothesized increase in excitability of these dorsal horn neurons caused by injection of morphine might result from dis-inhibition.
The dose-response curve established here for scratching induced by intradermal injection of serotonin is nearly identical to that reported by Klein et al., (2011), with the maximum effect (65.1 ± 16.5 scratch bouts/60 min for the current data and 60.1 ± 6.3 scratch bouts/60 min by Klein et al.) elicited by 180 µg/10 µl (47 mM) serotonin. Likewise, the time course for serotonin-induced scratching is the very similar to that established by Klein et al (2011). Our finding that scratching induced by α-Me-5HT has a longer duration than scratching induced by serotonin is consistent with α-Me-5HT having a longer duration of action due to not being degraded by monoamine oxidase (Sourkes et al., 1990). Klein et al. (2011) compared the effects of eleven pruritogens on facial scrathing in rats. They found that, with the exception of formalin, serotonin produced the maximum level of scratching. They also found that nearly all chemicals tested, with the exception of chloroquine, that increased scratching also increased wiping. Klein et al. (2011) found no significant difference from vehicle in the number of wipes (4.1 ± 2 /60 min) whereas the present findings show a significant increase in wiping (16.4 ± 4 wipes/60 min) produced by the same dose of serotonin (180 µg/10 µl or 47 mM). The pain-related wiping behavior in response to pruritogens such as serotonin may be analogous to the increase in noxious sensations such as burning and stinging which often accompanies the application of pruritogens in humans (Schmelz et al., 2003; Sikand et al., 2009). Together with these previous studies, our data further support the use of serotonin as a potent pruritogen in rats.
It has previously been demonstrated that morphine causes significantly increased scratching when delivered intracisternally but not intrathecally (at the lumbar level) or intracerebroventricularly in rats (Lee et al., 2003). The highest dose of morphine used by Lee et al. (0.1 µg) corresponds to the lowest dose included on the dose-response curve for scratching induced by intracisternal morphine in our study. At the concentrations tested, morphine did not affect grooming in the study by Lee et al. It has also been shown that injection of morphine within the spinal trigeminal nucleus causes a robust increase in facial scratching in rats (Thomas and Hammond, 1995). The area of the spinal trigeminal nucleus which was injected by Thomas and Hammond (1995) corresponds to the level of the brainstem/spinal cord targeted by intracisternal injections in our study. Thus, it is possible that the scratching induced by morphine in our study is due to effects localized to the spinal trigeminal nucleus, as suggested by Thomas and Hammond.
Our current findings suggest that endogenous opioid systems may be involved in modulating behavioral responses to application of pruritogens to the cheek. We show that naloxone reduces the number of scratch bouts elicited by serotonin, a finding which has been previously shown for opioid receptor antagonists naloxone (Hachisuka et al., 2010) and naltrexone (Spradley et al., 2012). μ-Opioid receptor antagonists have been used to reduce itch produced by several pruritogens in a variety of human conditions (Phan et al., 2010). It has even been suggested that in rodent models, reduction by μ-opioid receptor antagonists be used as a defining criterion for itch-related behaviors while reduction by μ-opioid receptor agonists be used as a defining criterion for pain-related behaviors (Nojima and Carstens, 2003; Akiyama et al., 2010). In the present study, morphine reduced serotonin-induced wiping (however cf. Spradley et al., 2012) and an increased grooming, an effect which was reduced by naloxone. The latter result is in accord with previous work in which treatment with naltrexone attenuated grooming behavior (Spradley et al., 2012).
An isobolographic analysis was performed to study the combined effects of intradermal injection of serotonin and intracisternal injection of morphine on itch. This analysis showed that these two drugs delivered in combination have a super-additive effect on scratching. This result suggests that in addition to serotonin and morphine causing scratching separately, the neural circuits activated by the drugs likely interact at some level to increase scratching behavior. It is likely that serotonin activates peripheral pruriceptors to produce itch (Berendsen and Broekkamp, 1991; Yamaguchi et al., 1999) and that morphine causes itch via actions in the central nervous system (Ko et al., 2004; Kuraishi et al., 2008). It is possible that morphine acts within the central nervous system (perhaps in the spinal trigeminal nucleus) to increase the effects of input from peripheral pruriceptors that respond to intradermal injection of serotonin in rats. This idea is supported by our observation that that application of morphine to the surface of the dorsal surface of the medulla and rostral spinal cord, the areas of the CNS in which morphine was likely concentrated by intracisternal injections in the present study, greatly increased responses of pruriceptive trigeminothalamic tract neurons to intradermal injection of serotonin (Moser and Giesler, 2013). In a related manor, our results suggest that the effects of stimuli that produce itch would likely be heightened, i.e. produce hyperknesis, in patients treated with CNS application of opiates.
In summary, we have established dose-response relationships for scratching induced by intradermal injection of serotonin and α methylserotonin and intracisternal injection of morphine and shown that when these drugs are delivered in combination, the increase in scratching is a super-additive or synergistic. The rat cheek model provides an excellent system for the study of these stimuli, as itch and pain-related behaviors can be readily distinguished. However, the anatomical and physiological substrates of these phenomena in the rat remain poorly understood. Given its involvement in pain processing and modulation by opioids, the spinal trigeminal system appears to be ideal for exploring the mechanisms underlying facial pain and itch and their interactions.
Highlights.
Intradermal injection of serotonin into facial skin of rats causes scratching.
Intracisternal injection of morphine produces scratching and reduces the nociceptive behavior of wiping.
Combining the two types of injections produces a synergistic, super-additive increase in scratching.
The results indicate that application of small doses of morphine to the CNS cause itch and hyperknesis.
Acknowledgements
We thank H. Truong, B. Lipshetz and C. Hosfield for valuable technical assistance, N. Jansen and Drs. C.N. Honda, D.A. Simone and G.L. Wilcox for critically reading an early version of this manuscript. This work was supported by NIH grants P01 NS047399, NINDS Core funding NS062158, and F31 NS077554 to HRM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol. 2010;104:2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel NM, Van Loon GR. β-endorphin-induced stimulation of the central sympathetic outlflow: inhibitory modulation by central noradrenergic neurons. J Pharm Exp Therap. 1986;237:695–701. [PubMed] [Google Scholar]
- Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- Baraka A, Maktabi M, Noueihid R. Epidural meperidine-bupivacaine for obstetric analgesia. Anesth Analg. 1982;61:652–656. [PubMed] [Google Scholar]
- Berendsen HHG, Broekkamp CLE. A peripheral 5-HT1D-like receptor involved in serotonergic induced hindlimb scratching in rats. Euro J Pharmacol. 1991;194:201–208. doi: 10.1016/0014-2999(91)90106-z. [DOI] [PubMed] [Google Scholar]
- Bromage PR, Camporesi EM, Durant PAC, Nielsen CH. Nonrespiratory side effects of epidural morphine. Anesth Analg. 1982;61:490–495. [PubMed] [Google Scholar]
- Collier CB. Epidural morphine. Anaesthesia. 1981;36:67. doi: 10.1111/j.1365-2044.1981.tb08608.x. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ., Jr The multiple pathways for itch and their interactions with pain. Trends in Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellner B, Hägermark Ö. Pruritus in polycythemia vera: treatment with aspirin and possibility of platelet involvement. Acta Derm Venereol. 1979;59:505–512. doi: 10.2340/0001555559505512. [DOI] [PubMed] [Google Scholar]
- Fjellner B, Hägermark Ö. Potentiation of histamine-induced itch and flare responses in human skin by the enkephalin analogue FK 33–824, β-endorphin and morphine. Arch Derm Res. 1982;274:29–37. doi: 10.1007/BF00510355. [DOI] [PubMed] [Google Scholar]
- Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333. doi: 10.2165/00003495-200767160-00003. [DOI] [PubMed] [Google Scholar]
- Graziano Mast cells and mast cell products. Meth Enzymol. 1988;162:501–522. doi: 10.1016/0076-6879(88)62100-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. Cytofluorometric analysis of anaphylactic secretion of 5-hydroxytryptamine and heparin from rat mast cells. Int Arch Allergy Appl Immunol. 1980;63:121–128. doi: 10.1159/000232617. [DOI] [PubMed] [Google Scholar]
- Hachisuka J, Furue H, Frue M, Yoshimura M. Responsiveness of C neurons in rat dorsal root ganglion to 5-hydroxytryptamine-induced pruritic stimuli in vivo. J Neurophysiol. 2010;104:271–279. doi: 10.1152/jn.00938.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer G, Groene D, Martus P. Efficacy of naltrexone on acetylcholine-induced alloknesis in atopic eczema. Exp Derm. 2002;11:448–455. doi: 10.1034/j.1600-0625.2002.110508.x. [DOI] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal hron neurons to intradermal serotonin and other irritatnts: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106:1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Song MS, Edwards T, Lee H, Naughton NN. The role of central μ opioid receptors in opioid-induced itch in primates. J Pharmacol & Exp Thera. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- Koenigstein H. Experimental study of itch stimuli in animals. Arch Derm Syphilol. 1948;57:828–849. doi: 10.1001/archderm.1948.01520180045006. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Yageta Y, Konno M, Andoh T, Yamaguchi-Miyamoto T, Nojima H. Intracisternal, but not intrathecal, injection of naloxone inhibits cutaneous itch-related response in mice. Biol Pharm Bull. 2008;31:2143–2145. doi: 10.1248/bpb.31.2143. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2013;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MCH. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Beh Pharmacol. 2003;14:501–508. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-Y, Liu Z-C, Sun Y-G, Ross M, Kim S, Tsai F-F, Li Q-F, Jeffry J, Kim J-Y, Loh HH, Chen Z-F. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg L, Sundstrom E, Nordlund K, Verhofstad A, Johansson O. Serotonin in human allergic contact dermatitis. Ann N Y Acad Sci. 1999;885:422–426. doi: 10.1111/j.1749-6632.1999.tb08703.x. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci. 2013;33:6093–6101. doi: 10.1523/JNEUROSCI.0216-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol. 2014;111:1574–1589. doi: 10.1152/jn.00668.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. J Neurosci Meth. 2003;126:137–143. doi: 10.1016/s0165-0270(03)00074-8. [DOI] [PubMed] [Google Scholar]
- Onigbogi O, Ajayi AA, Ukponmwan E. Mechanisms of chloroquine-induced body-scratching behavior in rats: evidence of involvement of endogenous opioid peptides. Pharmacol Biochem & Behav. 2000;65:333–337. doi: 10.1016/s0091-3057(99)00221-x. [DOI] [PubMed] [Google Scholar]
- Phan NQ, Bernhard JD, Luger TA, Stander S. Antipruritic treatment with systemic μ-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63:680–688. doi: 10.1016/j.jaad.2009.08.052. [DOI] [PubMed] [Google Scholar]
- Purcell WM, Cohen DL, Hanahoe TH. Comparison of histamine and 5-hydroxytryptamine content and secretion in rat mast cells isolated from different anatomical locations. Int Arch Allergy Appl Immunol. 1989;90:382–386. doi: 10.1159/000235058. [DOI] [PubMed] [Google Scholar]
- Rasul A, Nordlund K, Wahlgren C-F. Pruritic and vascular responses induced by serotonin in patients with atopic dermatitis and in healthy controls. Acta Derm Venereol. 2012;93:277–280. doi: 10.2340/00015555-1473. [DOI] [PubMed] [Google Scholar]
- Scott PV, Bowen FE, Cartwright P, Mohan Rao BC, Deeley D, Wotherspoon HG, Sumrein IMA. Intrathecal morphine as sole analgesic during labour. Br Med J. 1980;281:351–353. doi: 10.1136/bmj.281.6236.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjörk HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Inv Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- Sourkes TL, Montine TJ, Missala K. α-Methylserotonin, a substitute transmitter for serotonergic neurons. Prog Neuropsychopharmacol Bio Psych. 1990;14:829–832. doi: 10.1016/0278-5846(90)90055-l. [DOI] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Carstens MI, Carstens E. Opioid modulation of facial itch-and pain-related responses and grooming behavior in rats. Acta Derm Venereol. 2012;92:515–520. doi: 10.2340/00015555-1364. [DOI] [PubMed] [Google Scholar]
- Su P-Y, Ko M-C. The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats. J Pharmacol Exp Ther. 2011;337:822–829. doi: 10.1124/jpet.111.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth. 2003;15:234–239. doi: 10.1016/s0952-8180(02)00501-9. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:856–872. [PubMed] [Google Scholar]
- Thomas DA, Hammond D. Microinjection of morphine into the rat medullary dorsal horn produces a dose-dependent increase in facial scratching. Brain Res. 1995;695:267–270. doi: 10.1016/0006-8993(95)00871-m. [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol. 2001;81:250–254. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res. 1997;46:412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- Yelton AR, Wildman BG, Erickson MT. A probability-based formula for calculating interobserver agreement. J App Beh Anal. 1977;10:127–131. doi: 10.1901/jaba.1977.10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]