Abstract
We tested whether the association between bone mineral density (BMD) and coronary artery calcification (CAC) varies according to dyslipidemia in community-living individuals. Between 2002 and 2005, 305 women and 631 men (mean age of 64 years) and naïve to lipid-modifying medications and estrogen use were assessed for spine BMD, CAC, and total (TC), HDL- and LDL-cholesterol and triglycerides.
Participants
Random sample of participants from the Multi-Ethnic Study of Atherosclerosis (MESA) without clinical cardiovascular disease.
Predictor variable
Spine BMD at the L3 vertebrate by computer tomography (CT).
Main outcome
CAC prevalence by CT.
Effect Modifier
Total cholesterol to HDL ratio (TC:HDL) ≥ 5.0.
Results
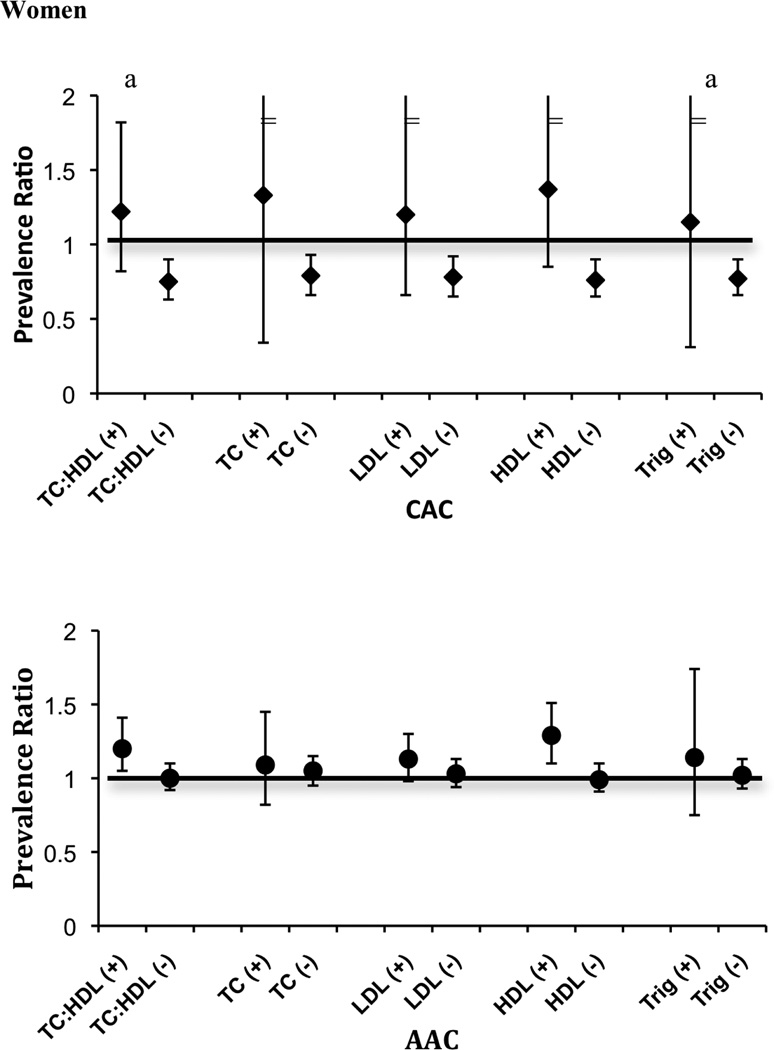
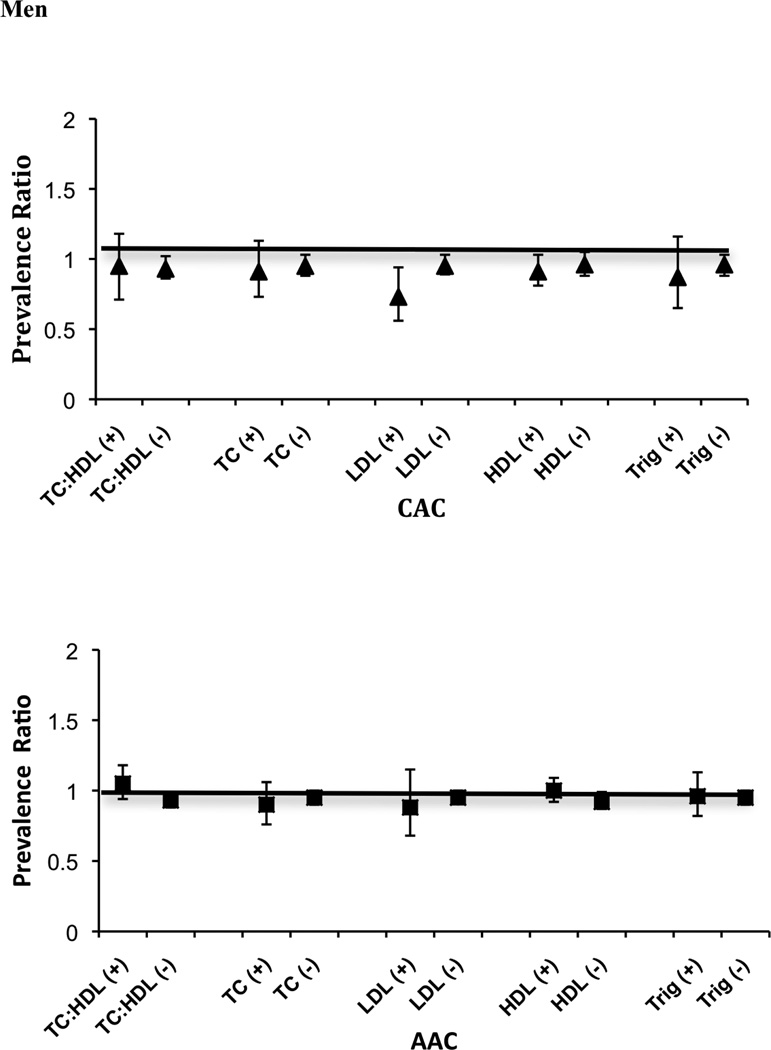
The association of BMD with CAC differed in women with TC:HDL < 5.0 vs. higher (p-interaction =0.01). In age and race adjusted models, among women with TC:HDL < 5.0, each SD (43.4 mg/cc) greater BMD was associated with a 25% lower prevalence of CAC (Prevalence Ratio [PR] 0.75, 95% confidence interval [CI] 0.63–0.89), whereas among women with higher TC:HDL, higher BMD was not significantly associated with CAC (PR 1.22, 95% CI 0.82–1.82). Results were similar using other definitions of hyperlipidemia. In contrast, no consistent association was observed between BMD and CAC in men irrespective of the TC:HDL ratio (p interaction 0.54).
Conclusion
The inverse association of BMD with CAC is stronger in women without dyslipidemia. These data argue against the hypothesis that dyslipidemia is the key factor responsible for the inverse association of BMD with atherosclerosis.
INTRODUCTION
Previous studies indicate that calcium deposition is associated with atherosclerosis (1, 2) and calcified atherosclerosis is a useful marker of cardiovascular disease risk.(3) Epidemiologic reports have reported inverse associations between bone mineral density (BMD) and calcified atherosclerosis, independent of their shared risk factors including age, sex, race/ethnicity, cigarette smoking, and physical activity.(4–8) Potential confounding factors such as sex hormones, interleukin-6, and parathyroid hormone have not accounted for this association.(5–7, 9) A leading explanation for the inverse association of BMD with calcified atherosclerosis implicates dyslipidemia.(10–13) Experimental studies show the common monocytic origin of multi-nucleated macrophages and osteoclasts and their dependence on exogenous LDL cholesterol.(14) Parhami and colleagues demonstrated that oxidized low density lipoprotein (LDL) inhibits osteoblastic differentiation of bone marrow stromal cells,(15) and also induces the calcification of vascular cells.(16) Atherosclerosis-susceptible mice fed with high-fat diets develop greater atherosclerosis and reduced bone mineralization when compared to control mice.(17) Translating these findings to humans would suggest that the inverse association of BMD with calcified atherosclerosis might be exaggerated in persons with dyslipidemia.
We previously reported that an association between BMD and calcified atherosclerosis was not attenuated after adjusting for dyslipidemia. We did not investigate the potential for effect-modification.(8) To our knowledge, whether or not the association between BMD and coronary artery calcification (CAC) differs according to dyslipidemia in community-living individuals has not been investigated in humans. To test this hypothesis, we used volumetric lumbar trabecular bone mineral density (vBMD), CAC, and abdominal aortic calcium (AAC), all measured by computed tomography (CT), in an ethnically diverse sample of community-living men and women who were free of clinical cardiovascular disease and not taking lipid-lowering or hormone replacement medications.
METHODS
Details of the Multi-Ethnic Study of Atherosclerosis (MESA) have been published.(18) In brief, the MESA is an observational cohort of volunteers recruited between July 2000 and August 2002 from six field centers in the United States. The study population consists of 6,814 men and women who were aged 45 to 84 years and identified themselves as Non-Hispanic White, Chinese American, African-American or Hispanic, who were free of clinical cardiovascular disease.
This report is based on a random sample of MESA participants who were also participants in MESA Abdominal Aortic Calcium Study (MESA-AACS). MESA-AACS participants were recruited during one of two follow-up visits between August 2002 and September 2005 from five of the six MESA field centers: Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. Of 2202 MESA potential participants, 2172 agreed to participate, and 1974 satisfied eligibility criteria, including no recent abdominal CT, known post-menopausal status, and completed scanning. Among these, 1926 provided scans that allowed measurement of lumbar spine vBMD. To avoid pharmacologic effects, 255 women and 256 men were excluded based on reported prior or current use of lipid-lowering medications (statins, niacin, fibrates, and/or cholestyramines). An additional 365 women were excluded due to use of postmenopausal hormone therapy in the past 2 years, 16 were excluded due to missing medication data, and 98 were excluded for missing lipid or covariate data resulting in a final analytic sample included 936 participants (305 women and 631 men). Written informed consent was given by each participant, and institutional review board approval was obtained from participating academic centers.
Computed Tomography Scanning
Participants were randomly selected for CT scanning of the chest at one of two clinical visits between August 2002 and September 2005. Scans were performed either with an ECG-triggered (at 80% of the RR interval) electron-beam CT scanner (Chicago and Los Angeles; Imatron C-150, Imatron)(19) or with prospectively ECG-triggered scan acquisition at 50% of the RR interval with a multidetector CT system(20) that acquired 4 simultaneous 2.5-mm slices for each cardiac cycle in a sequential or axial scan mode (New York, Forsyth County, and St. Paul field centers; Imatron C-150 and Sensation 64, GE Lightspeed, Siemens S4+ Volume Zoom and Siemens Sensation 16). For accuracy, two chest scans were performed for each individual.
CT of the abdominal aorta was performed a single time for each individual. For electron-beam CT, scanners were set as follows: scan collimation of 3mm; slice thickness of 6mm; reconstruction using 25 6mm slices with 35 cm field of view and normal kernel. For multi-detector CT, images were reconstructed in a 35 cm field of view with 5mm slice thickness. Participants were scanned along with phantoms of known physical calcium concentration to convert CT numbers directly to equivalent vBMD in mg/cc.
Calcium Scoring
Scans were read centrally by the MESA CT Reading Center at Harbor-University of California, Los Angeles Research and Education Institute. Calcium scores among scanning centers and between participants were adjusted with a standard calcium phantom scanned simultaneously for each participant. At least two adjacent pixels with an attenuation coefficient >130 Hounsfield units (modified to adjust for section thickness) defined a calcified lesion, and the average coronary calcium scores across the two scans taken concurrently was calculated using the method of Agatston.(21) Calcium was considered present given an Agatston score greater than 0. Rescan agreement was found to be high with both electron beam tomography and multi-detector CT scanners.(22) Interobserver agreement and intraobserver agreement were very high (κ=0.93 and 0.90, respectively).(23)
Bone density measurement
CT data were collected using the Image Analysis QCT 3D PLUS software program (Image Analysis, Columbia, Kentucky) during follow-up visits. Measurements of vBMD in a virtual 10mm-thick slice of trabecular bone from each vertebra (L2 to L4) used software directed, automated placement of the region of interest (ROI) in the anterior one-half to one-third of the vertebral body where it 1) encompassed a large area exclusively of trabecular, or cancellous bone, 2) excluded cortical bone, and 3) excluded the basivertebral plexus. Scans were read centrally at the MESA CT Reading Center at Harbor-University of California Medical Center (Los Angeles, California) by a trained reader blinded to arterial calcium scoring. The reader examined each ROI and changed its placement to exclude vertebral abnormalities such as bone islands and diffuse density variations or to exclude an entire vertebra from measurement if the following abnormalities were noted: fractures, metastatic lesions, osteophytes, benign focal lesions within the vertebra, any other vertebral pathology. The present analyses use vBMD from the third lumbar vertebra, the most commonly readable vertebra for all participants.
In a random sample of 25 scans re-read on three occasions by the blinded scan reader, there was 100 percent agreement in inclusion or exclusion for each vertebra (L2-L4) and no evidence of systematic differences between reads or a time effect in the data.
Clinical measurements
Participants completed a clinical examination and detailed questionnaire. Age, sex, race/ethnicity, current medications (based on self report and examination of pill bottles), physical activity patterns (mets × min/week), cigarette smoking history (ever/never/former), and previous medical diagnoses were recorded. In addition, alcohol consumption (never/former/current and average drinks per week) were calculated from a self-administered food frequency questionnaire and dietary supplement form. Body mass index was calculated as mass in kilograms divided by height in meters squared. Blood pressure was measured three times with participants at rest in the seated position. The average of the last two measurements was used to define hypertension as systolic pressure ≥140 mm Hg or diastolic pressure ≥90 mm Hg or current use of antihypertensive medication.
Laboratory measurements
Fasting (8-hour) morning plasma glucose was measured upon enrollment between July 2000 and August 2002, using standard laboratory methods previously described.(18) Diabetes was defined by a baseline fasting plasma glucose ≥126 mg/dL or the use of hypoglycemic medications.
Total cholesterol, high density lipoprotein cholesterol (HDL-C), and triglycerides were measured from morning fasting blood samples (Roche Diagnostics, Indianapolis, IN 46250). Analyses reported here use measurements ascertained between 2002 and 2004, that were concurrent with the time of the CT scans for vBMD, CAC, and AAC. Low density lipoprotein (LDL) cholesterol was calculated by the Friedewald equation.(24)
The total cholesterol:HDL cholesterol (TC:HDL) ratio was chosen because of its robust associations with CVD, and because no other lipid measure has been shown to improve power for CVD outcomes in epidemiologic studies and randomized clinical trials after first considering TC:HDL.(25) For interpretability of interactions and comparison, a cut point of less than 5 was chosen for TC:HDL.(26) For secondary dyslipidemia measures, cut point were based on NCEP ATPIII guidelines as follows: total cholesterol greater than 240 mg/dL; LDL cholesterol greater than 160 mg/dL; HDL cholesterol less than 40 mg/dL; triglycerides greater than 200 mg/dL.(27)
Statistical Analyses
All analyses were stratified by sex based on the well-known sex differences of CAC (28) and vBMD in men and women.(29) Chi-square tests and generalized linear models were used to compare distributions of categorical and continuous variables, respectively. Because the odds ratio requires the rare disease assumption to accurately estimate the relative risk, and the prevalence of CAC and AAC were each > 50% in the study sample,(30) the association between vBMD and presence of calcified atherosclerosis was evaluated using relative risk estimates using a generalized linear regression model with log link, Gaussian error, and robust estimates of variance. The relative risk estimates were reported here as a prevalence ratios given the cross-sectional study design. Tests of interaction of vBMD by dyslipidemia status were performed first after adjustment for age and race/ethnicity, and then after adjustment for other covariates including potential CVD and osteoporosis risk factors as well as other covariates determined a priori from literature review. Covariates were added individually to models to inspect their potential effects on the interaction. Changes in direction of prevalence ratio for either lipid stratum or changes from statistically significant interactions were reported as material changes in the results
RESULTS
The sex-specific characteristics of 936 study participants by TC:HDL ratio are shown in Table 1. The average age was 64 years in woman and 63 years in men. Non-Hispanic white participants were 20% of women and 37% of men. Prevalence of CAC, AAC and TC:HDL-defined dyslipidemia was 34%, 64% and 15% in women and 55%, 67% and 24% in men, respectively. Using ATPIII criteria based definitions for dyslipidemia, hyperlipidemia by each measure was more common among participants with TC:HDL≥5 (p<0.001 for each).
Table 1.
Distribution of clinical characteristics according to sex and total cholesterol to high density cholesterol ratio (TC:HDL)
| WOMEN | MEN | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TC:HDL ≥ 5.0 | TC:HDL ≥ 5.0 | ||||||||
| ALL | No | Yes | P | ALL | No | Yes | P | ||
| Sample % (n) | § | 305 | 85 (259 | 15 (46) | 631 | 76 (480) | 24 (151) | ||
| Age, years (SD) | ∞ | 64 (10) | 64 (11) | 64 (9) | 63 (10) | 64 (10) | 61 (9) | c | |
| Ethnicity | § | c | |||||||
| Non-Hispanic White | 20 (61) | 20 | 17 | 37 (231) | 38 | 33 | |||
| Chinese American | 20 (60) | 20 | 17 | 16 (98) | 16 | 13 | |||
| African American | 28 (84) | 29 | 17 | 20 (127) | 23 | 13 | |||
| Hispanic | 33 (100) | 30 | 48 | 28 (175) | 23 | 42 | |||
| Coronary artery calcium | § | 34 (104) | 32 | 46 | 55 (348) | 55 | 54 | ||
| Abdominal aortic calcium | § | 64 (194) | 60 | 83 | b | 67 (424) | 66 | 72 | |
| Total cholesterol > 240 mg/dL | § | 11 (35) | 8 | 33 | c | 5 (33) | 3 | 14 | c |
| LDL-C > 160 md/dL | § | 9 (28) | 6 | 28 | c | 6 (40) | 3 | 18 | c |
| HDL-C <40 mg/dL | § | 9 (27) | 3 | 39 | c | 32 (202) | 17 | 79 | c |
| Triglycerides > 200 mg/dL | § | 14 (42) | 10 | 37 | c | 12 (76) | 5 | 36 | c |
| Hypertension | § | 45 (137) | 44 | 48 | 38 (240) | 39 | 34 | ||
| Diabetes | § | 12 (36) | 12 | 11 | 13 (79) | 12 | 15 | ||
| Ever smoke | § | 36 (111) | 36 | 41 | 64 (401) | 64 | 63 | ||
| Current alcohol consumption | § | 36 (109) | 36 | 33 | 60 (374) | 62 | 51 | a | |
| Bone density, mg/cc | ∞ | 112±43 | 112±44 | 113±41 | 123±39 | 121±41 | 124±34 | ||
| BMI, kg/m2 | 28.7±5.9 | 28.5±5.9 | 29.9±5.9 | 27.5±4.3 | 28.5±4.2 | 27.2±4.3 | b | ||
| Physical activity, met-min/week | # | 563 (0–1515) | 630 (0–1620) | 191 (0–1155) | 840 (158–1995) | 840 (201–2096) | 840 (0–1883) | ||
Displays percentage (n) for discrete variables
Displays mean±standard deviation
Displays median (interquartile range)
LDL-C is low density lipoprotein cholesterol; HDL-C is high density lipoprotein cholesterol.
=p<0.05
=p<0.01
=p<0.001
Table 2 presents age- and race/ethnicity- adjusted characteristics for each sex according to TC:HDL ratio. In these analyses, high TC:HDL ratio was associated with AAC prevalence in both sexes, and also with CAC in women. Men with high TC;HDL ratio consumed less alcohol and had higher BMI; results not observed in women.
Table 2.
Age- and race/ethnicity-adjusted distribution of clinical characteristics according to sex and total cholesterol to high density cholesterol ratio (TC:HDL)
| WOMEN | MEN | ||||||
|---|---|---|---|---|---|---|---|
| TC:HDL ≥ 5.0 | TC:HDL ≥ 5.0 | ||||||
| No | Yes | No | yes | ||||
| Sample % (n) | 85 (259) | 15 (46) | a | 76 (480) | 24 (151) | a | |
| Coronary artery calcium | § | 27 (21, 34) | 45 (29, 61) | a | 56 (51, 61) | 60 (50, 68) | |
| Abdominal aortic calcium | § | 65 (58, 72) | 90 (79, 96) | c | 70 (64, 75) | 82 (75, 88) | b |
| Hypertension | § | 43 (37, 50) | 50 (35, 66) | 37 (33, 42) | 36 (28, 44) | ||
| Diabetes | § | 11 (7, 15) | 10 (4, 23) | 11 (8, 14) | 13 (8, 19) | ||
| Ever smoked | § | 26 (19, 36) | 34 (20, 51) | 64 (59, 68) | 65 (57, 72) | ||
| Current alcohol consumption | § | 35 (29, 41) | 32 (20, 47) | 63 (58, 67) | 51 (43, 60) | a | |
| Bone density, mg/cc | ∞ | 112 (108, 116) | 114 (106, 123) | 123 (120, 126) | 122 (117, 127) | ||
| BMI, kg/m2 | ∞ | 28 (28, 29) | 30 (28, 31) | 27.3 (27.0, 27.7) | 28.2 (27.6, 28.9) | a | |
| Physical activity, met-min/week | ∞ | 1120 (937, 1304) | 840 (401, 1279) | 1514 (1344, 1683) | 1295 (989, 1601) | ||
Displays percentages (95%CI)
Displays mean (95%CI)
=p<0.05
=p<0.01
=p<0.001
Figure 1 displays the age- and race/ethnicity- adjusted prevalence ratio for CAC or AAC associated with a one standard deviation greater vBMD (43.4 mg/cc in women and 39.1 mg/cc in men) according to each defined category of dyslipidemia. Greater vBMD was consistently associated with lower prevalence of CAC in women without dyslipidemias, whereas among women with dyslipidemia, the point estimates for greater vBMD trended towards greater prevalence of CAC although the associations were not significant within this strata. P-values for interaction were statistically significant for TC:HDL ratio and triglycerides in women (P interaction = 0.002 and 0.01, respectively). In women, the association of vBMD with AAC showed a similar pattern to CAC, but no statistically significant interactions were detected. In men, the associations of vBMD with AAC and CAC were much more modest in strength, and did not show significant differences according to lipid levels (all p-interactions > 0.15). We additionally evaluated whether the association of vBMD with CAC differed by diabetes status, and observed no evidence of effect modification in either sex (P interaction = 0.92 in women and 0.78 in men). We also conducted a sensitivity analysis excluding 11 women and 2 men that were taking bisphosphonates at the time of the baseline study visit. Results were essentially unchanged in the remaining subjects (data not shown).
Figure 1.
a=p<0.05 for bone density by dyslipidemia interaction.
“−” or “+” indicates absence or presence of dyslipidemia based on the following criteria: total cholesterol to HDL ratio (TC:HDL) ≥ 5.0; total cholesterol (TC) ≥ 240 mg/dL; LDL-cholesterol ≥ 160 mg/dL; HDL-cholesterol <40 mg/dL; triglycerides (TRIG) ≥ 200.
“=” error bar continue.
Table 3 displays associations between one SD greater vBMD with CAC and AAC by dyslipidemia groups, after adjustment for age, race/ethnicity, body mass index, hypertension, diabetes, alcohol consumption, cigarette smoking, and physical activity. Patterns were similar to those in Figure 1, and were not materially attenuated with addition adjustment.
Table. 3.
Multiply-adjusted prevalence ratio (PR) of coronary artery calcium (CAC) or abdominal aortic calcium (AAC) associated with a positive single standard deviation increment of lumber of bone density for different lipid profiles
| WOMEN | MEN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAC | AAC | CAC | AAC | ||||||||
| N | PR | 95% CI | PR | 95% CI | N | PR | 95% CI | PR | 95% CI | ||
| 305 | 631 | ||||||||||
| TC:HDL ratio | p=0.01 | p=0.24 | p=0.54 | p=0.15 | |||||||
| < 5.0 | 0.75 | 0.64, 0.89 | 0.99 | 0.91, 1.09 | 0.94 | 0.88, 1.01 | 0.94 | 0.89, 0.99 | |||
| ≥ 5.0 | 0.96 | 0.46, 1.98 | 1.13 | 0.97, 1.31 | 0.97 | 0.79, 1.19 | 1.05 | 0.93, 1.19 | |||
| Total cholesterol | p=0.24 | p=0.75 | p=0.75 | p=0.72 | |||||||
| < 240 mg/dL | 0.76 | 0.63, 0.92 | 1.03 | 0.94, 1.12 | 0.95 | 0.89, 1.02 | 0.96 | 0.91, 1.01 | |||
| ≥ 240 mg/dL | 1.52 | 0.61, 3.75 | 1.03 | 0.74, 1.43 | 0.95 | 0.34, 2.67 | 0.88 | 0.72, 1.05 | |||
| LDL cholesterol | p=0.61 | p=0.66 | p=0.65 | p=0.99 | |||||||
| < 160 mg/dL | 0.76 | 0.62, 0.92 | 1.01 | 0.92, 1.11 | 0.96 | 0.90, 1.03 | 0.96 | 0.91, 1.01 | |||
| ≥160 mg/dL | 1.57 | 0.89, 2.78 | 0.94 | 0.69, 1.28 | 0.75 | 0.54, 1.05 | 0.92 | 0.62, 1.35 | |||
| HDL cholesterol | p=0.14 | p=0.08 | p=0.24 | p=0.54 | |||||||
| > 40 mg/dL | 0.51 | 0.32, 0.81 | 0.99 | 0.90, 1.09 | 0.97 | 0.90, 1.04 | 0.93 | 0.88, 0.99 | |||
| ≤ 40 mg/dL | 1.09 | 0.42, 2.81 | 1.08 | 0.92, 1.27 | 0.91 | 0.80, 1.03 | 1.00 | 0.92, 1.10 | |||
| Triglycerides | p=0.02 | p=0.38 | p=0.65 | p=0.34 | |||||||
| < 200 mg/dL | 0.75 | 0.65, 0.87 | 1.01 | 0.93, 1.11 | 0.96 | 0.90, 1.03 | 0.95 | 0.91, 1.01 | |||
| ≥ 200 mg/dL | 0.87 | 0.53, 1.41 | 1.00 | 0.68, 1.48 | 0.86 | 0.68, 1.11 | 1.00 | 0.85, 1.17 | |||
p-values are for interaction.
TC:HDL ratio= total cholesterol to HDL-cholesterol ratio.
Models are adjusted for age, ethnicity, body mass index, hypertension, diabetes, alcohol consumption, cigarette smoking, physical activity
DISCUSSION
We tested the hypothesis that serum lipid levels may modify the association between vBMD and calcified atherosclerosis in a sample of community-living participants without clinical cardiovascular disease and naïve to lipid-modifying medications and hormone replacement therapy. We found that the inverse associations between vBMD and calcified atherosclerosis were limited to women without dyslipidemia and to the CAC endpoint. In men, the inverse association of vBMD with CAC and AAC were more modest and often altogether absent, and we observed no evidence of effect modification by lipid status.
An atherosclerosis-lipid-bone model has been proposed to explain relationships between low vBMD and atherosclerosis.(11, 13) In this construct, LDL and oxidative stress result in oxidized LDL, a strongly atherogenic class of compounds that promote osteoblastic differentiation of resident vascular cells.(16) Plaque formation takes place in arterial sites within the highly-vascularized bone tissue.(10) Here, oxidized LDL stimulates bone marrow stromal cells, which are located adjacent to the subendothelial matrix of bone vessels, to favor adipogenic rather than osteoblastic differentiation.(31) The result is simultaneous arterial calcification and skeletal bone loss. These elegant hypothesis supported laboratory studies would suggest that individuals with dyslipidemia might be predisposed to simultaneous bone loss and vascularization. The present study is the first epidemiologic study to our knowledge to investigate the atherosclerosis-lipid-bone model in humans. In women, we observed that the inverse association of vBMD with calcified atherosclerosis was confined to women without dyslipidemia. Conversely, the atherosclerosis-lipid-bone model suggests that dyslipidemia is the key factor responsible for the inverse association of vBMD with calcified atherosclerosis. Thus, the present study does not support the atherosclerosis-lipid-bone model in community-living individuals naïve to lipid lowering and hormone replacement medications.
Other mechanisms may explain the inverse association of vBMD with calcified atherosclerosis. Oxysterols, active components of oxidized LDL,(32) include numerous oxygenated derivatives of cholesterol and can be found in the circulation and body tissues as a result of dietary intake, auto-oxidation of cholesterol, and the action of mono-oxygenases.(33) In a recent series of experiments, various oxysterols demonstrated strong osteogenic effects including inhibition of the adipogenic effects of oxidized LDL on marrow stromal cells and promotion of osteoblastic differentiation of these cells through multiple effects.(34) In related experiments, oxysterols stimulated the calcification of osteoblast-like vascular smooth muscle cells.(35) The actions of oxysterols vary according to their combinations and concentrations, which also vary widely in human tissues.(32) These findings also raise the testable hypothesis that osteoblastic combinations of oxysterols predominate in dyslipidemic states but not normal lipid states. Future studies will be required to test this hypothesis.
Alternatively, abnormal regulation of mineral metabolism may account for the inverse association. For example, higher serum phosphorus levels have recently been associated with calcified atherosclerosis independent of kidney function, other markers of mineral metabolism (calcium, vitamin D, and parathyroid hormone), and traditional CVD risk factors (36), and studies in animal models have provided insight into putative mechanisms. (37) Estradiol has phosphaturic properties (38, 39), and as a consequence, post-menopausal women consistently have higher serum phosphorus levels than similarly aged men (40–42). Future studies should evaluate whether regulation of phosphorus may account for sex differences in the inverse association of vBMD with calcified atherosclerosis.
This study benefited from a population-based sample of both men and women, and state-of-the-art measurements of calcified atherosclerosis and vBMD. (43) Participants taking the most common pharmacologic therapies for dyslipidemia and bone loss were excluded, thus minimizing confounding by a pharmacologic effect. Conclusions from the study are bolstered by investigation of multiple definitions of dyslipidemia. The study also has limitations. Accordingly, the presence of a multi-ethnic population sample is a study limitation. Further, this study used measures of vBMD and calcified atherosclerosis taken at one-point in time, so temporal relationships of associations could not be addressed. The study sample was limited to persons without prevalent CVD, and not taking lipid lowering or hormone replacement therapies. Results may differ in other settings. We lacked data on glucocorticoid use. Lastly, additional markers of osteoporosis and skeletal health (vitamin D, parathyroid hormone, thyroid hormone, sex hormones, and markers of bone turnover) were not available.
In conclusion, the inverse association of vBMD with calcified atherosclerosis is stronger in women without dyslipidemia. These data argue against the hypothesis that dyslipidemia alone is responsible for the inverse association of vBMD with atherosclerosis. Further laboratory and population-based investigations are required to investigate other factors that may explain the inverse association of vBMD with atherosclerosis.
Acknowledgements
This research was supported by NIH Grants R01HL72403 (JAH, MHC) and R21HL091217 (NEJ, JHI) and by contracts N01-HC-95159 through N01-HC-95169 with the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The authors wish to acknowledge ‘marc veron ag informatik information internet’ (Allschwil/Switzerland) for donating technical expertise and software for data transfer.
Footnotes
Disclosures: None
Greater bone density is associated with less atherosclerosis in women with dyslipidemia but not in women without dyslipidemia.
Contributor Information
Nicole E. Jensky, Email: njensky@ucsd.edu.
Joseph A Hyder, Email: joseph.a.hyder@gmail.com.
Matthew A. Allison, Email: mallison@ucsd.edu.
Nathan Wong, Email: ndwong@uci.edu.
Victor Aboyans, Email: victor.aboyans@unilim.fr.
Roger S. Blumenthal, Email: rblument@jhmi.edu.
Pamela Schreiner, Email: schre012@umn.edu.
J Jeffrey Carr, Email: jcarr@wfubmc.edu.
Christina L. Wassel, Email: cwassel@ucsd.edu.
Joachim H. Ix, Email: joix@ucsd.edu.
Michael H. Criqui, Email: mcriqui@ucsd.edu.
References
- 1.Demer LL, Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–1743. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 2.McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg's sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 4.Hyder JA, Allison MA, Barrett-Connor E, Detrano R, Wong ND, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Bone mineral density and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis. 209:283–289. doi: 10.1016/j.atherosclerosis.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. Am J Epidemiol. 2004;160:549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- 6.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 7.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 8.Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–194. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arlt W, Hewison M. Hormones and immune function: implications of aging. Aging Cell. 2004;3:209–216. doi: 10.1111/j.1474-9728.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- 10.Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. 2000;20:2346–2348. doi: 10.1161/01.atv.20.11.2346. [DOI] [PubMed] [Google Scholar]
- 11.Parhami F, Demer LL. Arterial calcification in face of osteoporosis in ageing: can we blame oxidized lipids? Curr Opin Lipidol. 1997;8:312–314. doi: 10.1097/00041433-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids. 2003;68:373–378. doi: 10.1016/s0952-3278(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 13.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 14.Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, Reszka AA. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ. 2004;11(Suppl 1):S108–S118. doi: 10.1038/sj.cdd.4401399. [DOI] [PubMed] [Google Scholar]
- 15.Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, Demer LL. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- 16.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 17.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Breen JF, Sheedy PF, 2nd, Schwartz RS, Stanson AW, Kaufmann RB, Moll PP, Rumberger JA. Coronary artery calcification detected with ultrafast CT as an indication of coronary artery disease. Radiology. 1992;185:435–439. doi: 10.1148/radiology.185.2.1410350. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Danitschek JA, Goff DC, Crouse JR, 3rd, D'Agostino R, Chen MY, Burke GL. Coronary artery calcium quantification with retrospectively gated helical CT: protocols and techniques. Int J Cardiovasc Imaging. 2001;17:213–220. doi: 10.1023/a:1010604724001. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary Calcium Measure-ments: Effect of CT Scanner Type and Calcium Measure on Rescan Reproducibility--MESA Study. Radiology. 2005 doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 23.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Natarajan S, Glick H, Criqui M, Horowitz D, Lipsitz SR, Kinosian B. Cholesterol measures to identify and treat individuals at risk for coronary heart disease. Am J Prev Med. 2003;25:50–57. doi: 10.1016/s0749-3797(03)00092-8. [DOI] [PubMed] [Google Scholar]
- 26.American Heart Association. http://www.americanheart.org/. In: [Google Scholar]
- 27.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Allison MA, Criqui MH, Wright CM. Patterns and Risk Factors for Systemic Calcified Atherosclerosis. Arterioscler Thromb Vasc Biol. 2003 doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 29.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160:301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 31.Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- 32.Colles SM, Maxson JM, Carlson SG, Chisolm GM. Oxidized LDL-induced injury and apoptosis in atherosclerosis. Potential roles for oxysterols. Trends Cardiovasc Med. 2001;11:131–138. doi: 10.1016/s1050-1738(01)00106-2. [DOI] [PubMed] [Google Scholar]
- 33.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 34.Shouhed D, Kha HT, Richardson JA, Amantea CM, Hahn TJ, Parhami F. Osteogenic oxysterols inhibit the adverse effects of oxidative stress on osteogenic differentiation of marrow stromal cells. J Cell Biochem. 2005;95:1276–1283. doi: 10.1002/jcb.20497. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Yuan L, Xu S, Zhang T, Wang K. Cholestane-3beta, 5alpha, 6beta-triol promotes vascular smooth muscle cells calcification. Life Sci. 2004;76:533–543. doi: 10.1016/j.lfs.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, Detrano R, Post W, Wong ND. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 30:2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faroqui S, Levi M, Soleimani M, Amlal H. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int. 2008;73:1141–1150. doi: 10.1038/ki.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng J, Ohlsson C, Laughlin GA, Chonchol M, Wassel CL, Ljunggren O, Karlsson MK, Mellstrom D, Orwoll ES, Barrett-Connor E, Ix JH. Associations of estradiol and testosterone with serum phosphorus in older men: the Osteoporotic Fractures in Men study. Kidney Int. 78:415–422. doi: 10.1038/ki.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirillo M, Ciacci C, De Santo NG. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359:864–866. doi: 10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 41.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang TF, Guglielmi G, van Kuijk C, De Serio A, Cammisa M, Genant HK. Measurement of bone mineral density at the spine and proximal femur by volumetric quantitative computed tomography and dual-energy X-ray absorptiometry in elderly women with and without vertebral fractures. Bone. 2002;30:247–250. doi: 10.1016/s8756-3282(01)00647-0. [DOI] [PubMed] [Google Scholar]