Abstract
Extraction of relevant information from highly complex environments is a prerequisite to survival. Within odour mixtures, such information is contained in the odours of specific elements or in the mixture configuration perceived as a whole unique odour. For instance, an AB mixture of the element A (ethyl isobutyrate) and the element B (ethyl maltol) generates a configural AB percept in humans and apparently in another species, the rabbit. Here, we examined whether the memory of such a configuration is distinct from the memory of the individual odorants. Taking advantage of the newborn rabbit's ability to learn odour mixtures, we combined behavioural and pharmacological tools to specifically eliminate elemental memory of A and B after conditioning to the AB mixture and evaluate consequences on configural memory of AB. The amnesic treatment suppressed responsiveness to A and B but not to AB. Two other experiments confirmed the specific perception and particular memory of the AB mixture. These data demonstrate the existence of configurations in certain odour mixtures and their representation as unique objects: after learning, animals form a configural memory of these mixtures, which coexists with, but is relatively dissociated from, memory of their elements. This capability emerges very early in life.
Keywords: Oryctolagus cuniculus, newborn, odour mixture, configural perception, memory, representation
1. Introduction
Animals constantly interact with the environment. Specifically, they must discriminate the sensory information available in the surroundings and extract that which is the most relevant for survival and development. Indeed, the environment is highly complex in terms of the number and diversity of stimuli. For instance, mammals must process composite visual and auditory stimuli, or mixtures of chemical cues, to, respectively, recognize the face, the voice or the odour of conspecifics [1–4]. However, this processing raises a question currently debated in the scientific literature, namely do the face, the voice and the odour each constitute for the receiver a sum of elements or individual unique cues? Here, we consider this problem within olfaction.
In some cases, mixtures of odorants are perceived as a collection of independent, identifiable elements; the perception is then elemental (e.g. [5–7]). However, some mixtures induce a configural processing, meaning that the mixture gives rise either to a unique and novel perceptual odour quality, different from the odour qualities of the elements, or to a novel quality perceived in addition to the qualities of the odorants [8]. Configural odour processing has been described in a variety of species (e.g. bee, catfish, human, spiny lobster, moth and rat), with different approaches (e.g. [9–16]). For instance, data in human adults revealed that a mixture of two odorants (AB), one smelling like strawberry (A: ethyl isobutyrate) and the other like caramel (B: ethyl maltol), generates the configural perception of a pineapple odour at a specific ratio of A/B [17,18].
Interestingly, recent results in a young mammal, the newborn rabbit, showed similar configural processing abilities with the same AB mixture. After the learning of one element (A or B), rabbit pups do not respond to the AB mixture (while they respond very well to an AC mixture; odorant C: guaïacol), suggesting that they perceive in AB something more than the odours of A and B, i.e. an AB configuration. Conversely, after the learning of the AB mixture, they respond both to A, B and to AB, suggesting that they acquire both the elements and the configuration during the conditioning [19–21]. Therefore, newborn rabbits are strongly suspected to perceive the AB mixture in a weak configural way, i.e. to perceive three distinct odours in the mixture: the odour of the AB configuration in addition to the odours of the elements A and B. When the ratio of A/B is modified, the pups strongly respond to this A′B′ mixture after the learning of one element (A or B), suggesting a shift from the configural representation to the elemental one [22]. However, a clear demonstration that configural representation is distinct from representation of each element was still lacking in the literature for either young or adult organisms. Here, we combined a behavioural approach with pharmacological tools to assess the perception and retention of complex odour stimuli (mixtures) in the form of entities (configurations) or through their constituting elements.
After learning, memories become stabilized within hours following a consolidation phase involving protein synthesis [23,24]. The use of protein synthesis inhibitors, such as anisomycin (AN), during this time-limited period disrupts memory consolidation and consequently erases memory. Retrieval can return memories to a labile, protein synthesis-dependent state, a process referred to as memory reconsolidation [25,26]. Again, disruption of memory reconsolidation, by the use of protein synthesis inhibitors, erases the reactivated memory. This process of reconsolidation has been demonstrated across species, memory paradigms and, in particular, in rabbit pups after odour conditioning [27]. Moreover, this process is selective to the reactivated memory. For instance, in newborn rabbits initially conditioned to two odorants, injection of AN after reactivating only one of the elements abolishes the response to this odorant without interfering with the response to the other non-reactivated odorant [28,29].
Here, in three successive experiments, we systematically used a similar three step procedure including (a) a conditioning phase inducing olfactory learning of a mixture (AB or A′B′), (b) a reactivation phase to reactivate some (A and B) or all (A, B and AB) of the previously conditioned information in order to induce amnesia of the reactivated information using pharmacological treatment [28,29] and (c) a testing phase to assess behavioural responsiveness towards the mixture and its elements. First, in rabbit neonates previously conditioned to the configural AB mixture, we injected AN after separate reactivation of its elements (odorants A and B) in order to specifically erase the memory of A and the memory of B; then, we evaluated the consequences on the response to the AB mixture (Experiment 1). An absence of response to AB will indicate that responsiveness to the mixture critically depends on the representation of the elements. Conversely, response to AB after amnesia of A and B will indicate that the configural memory of the mixture is clearly dissociated from the memories of its elements. As a second step, we made a control experiment with the A′B′ mixture (Experiment 2). As this mixture is perceived in an elemental way by rabbit pups, i.e. without a configural odour but as the sum of the element odours, pharmacological disruption of A and B memories after reactivation should be followed by amnesia of A′B′. Finally, after neonatal conditioning to the configural AB mixture, we determined the effect of preventing AB memory reconsolidation on the memory of the AB mixture and of its components. Based on previous work, rabbit pups were strongly suspected to perceive both the elements (A and B) and the AB configuration during exposure to the AB mixture. As a consequence, the reactivation of the whole mixture should reactivate the memory of its three representations (A, B and AB) and amnesic treatment should induce forgetting of all these memories (Experiment 3). Taken together, these experiments aimed to uncover chemosensory perceptual and memory mechanisms available to promote initial decisions and actions critical for social relationships and feeding behaviour in mammals.
2. Material and methods
(a). Animals and housing conditions
Male and female New-Zealand rabbits, Oryctolagus cuniculus (Charles River strain, L'Arbresle, France), from the Centre de Zootechnie (University of Burgundy, Dijon) were kept in individual cages. A nest-box (0.39 × 0.25 × 0.32 m) was added on the outside of the pregnant females' cages 2 days before delivery (day of delivery was day 0: d0). To equalize pups' nursing experience, all females had access to their nest between 11.30 and 11.45. This procedure allowed females to follow the brief (3–4 min) daily nursing of the species [30]. Animals were kept under a constant 12 L : 12 D cycle (light on at 7.00) with ambient air temperature maintained at 21–22°C. Water and pelleted food (Lapin Elevage 110, Safe, France) were provided ad libitum. In the study, 68 newborns (from 18 l) were used.
(b). Odorants
The odorants consisted of 2-methylbut-2-enal (the mammary pheromone, MP, CAS 497-03-0) [27,31], ethyl isobutyrate (odorant A, CAS# 97-62-1), ethyl maltol (odorant B, CAS 4940-11-8) for pure components and of AB and A′B′ mixtures. The AB mixture included 0.3 × 10−5 and 0.7 × 10−5 g ml−1 of components A/B; this 30/70 v/v ratio elicits configural perception of a pineapple odour in human adults due to blending properties [17,32] and seems to induce weak configural perception in newborn rabbits (i.e. perception of three distinct odours in the mixture, the odours of A, B and AB) [19–22]. The A′B′ mixture included 1.5 × 10−5 and 0.7 × 10−5 g ml−1 of components A/B; this 68/32 v/v ratio elicits elemental perception of the mixture in newborn rabbits (i.e. perception of two odours only, those of the element A and the element B) [22]. In contrast with MP, none of the A, B, AB and A′B′ stimuli triggered spontaneous sucking behaviour of rabbit pups; they were therefore considered as initially neutral [19–22].
The MP allowed us to induce the learning of the AB or A′B′ mixtures through associative conditioning (see section (c)). MP served as the unconditioned stimulus and was used at 10−5 g ml−1, a concentration known to be highly efficient in promoting conditioning [31], whereas the AB or A′B′ mixtures served as the conditioned stimuli. Thus, the AB–MP and A′B′–MP blends included 1 × 10−5 g ml−1 of MP and, respectively, 0.3 and 0.7 × 10−5 g ml−1, or 1.5 and 0.7 × 10−5 g ml−1 of A and B.
Single odorants A and B (10−5 g ml−1), or the AB mixture were also used in the reactivation procedure, and the same stimuli plus the A′B′ mixture were used during behavioural testing.
All the odorants were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France) and all the final solutions were prepared in a solvent composed of 0.1% of ethanol (anhydrous, Carlo Erba, Val de Reuil, France) and 99.9% of MilliQ water (Millipore, Molsheim, France).
(c). Phase 1: odour conditioning
Conditioning sessions were run on day 1 in an experimental room close to the breeding room. The pups were transferred by groups of 4 (2 per litter) into a box lined with nest materials and maintained at room temperature. The MP-induced conditioning was run following a procedure previously described, which consisted in a single, brief and simultaneous exposure to both the unconditioned MP and the conditioned stimulus. Thus, for the conditioning session, 4 ml of the MP–AB mixture (Experiments 1 and 3) or MP–A′B′ mixture (Experiment 2) were pipetted on to a pad (19 × 14 cm, 100% cotton) then held 2 cm above the pups for 5 min. This exposure is known to induce very rapid learning of the stimulus paired with MP, here an odour mixture (e.g. [19,27,28,31]). The conditioning session occurred 1 h before the daily nursing (10.30), to equalize the pups' motivational state and limit the impact of satiation on responses [33]. Two minutes after the end of the conditioning, the pups were individually marked with weakly odorous ink and returned to their nest. The box containing the pups was rinsed with alcohol and distilled water after each conditioning session.
(d). Phase 2: reactivation and pharmacological treatment
On day 2, 24 h after the conditioning, the memory of pups was reactivated in Experiment 1 and 2 by successive exposure to each element A and B (the order of presentation of A and B was counterbalanced between pups from a same group). The exposure consisted in stimulation with each odorant A and B during 2.5 min (inter-stimulation delay: 1 min) following the same procedure as for conditioning (odorized cotton pad held above the litter). In Experiment 3, the AB mixture itself was used during a 5 min long reactivating exposure.
In each experiment, immediately after reactivation, AN (Aldrich) was injected to half of the pups (42 mg kg−1, i.p.) after dilution in 0.9% NaCl solution and adjustment of pH 7.2 with 1 N HCl [27,28]. Control for the effect of AN injection was realized with the other half of animals receiving saline 0.9%. As in other studies with other newborn or adult mammals (e.g. [23,34–36]), we considered that AN in newborn rabbits may induce a real amnesia and not a perturbation in responsiveness due to an aversive effect [27,28]. Pups were returned to the nest immediately after AN or saline injection.
(e). Phase 3: behavioural assay
The behavioural assay occurred on day 3 (i.e. 24 h after Phase 2 of reactivation) in the experimental room previously used for conditioning and reactivation. It also happened 1 h before the daily nursing to limit the impact of satiation on motivation and behavioural responsiveness [33]. The assay consisted of an oral activation test during which a pup was immobilized in one gloved hand of the experimenter, its head being left free. Each odour stimulus (odorant A, odorant B and the AB or A′B′ mixture) was presented for 10 s with a glass rod 0.5 cm in front of the nares (e.g. [19,27,28,31]). A test was positive when the conditioned stimulus elicited (on/off response) head-searching movements (vigorous, low-amplitude horizontal and vertical scanning movements displayed after stretching towards the rod) usually followed by grasping movements (labial seizing of the rod extremity). Non-responding pups displayed no response except sniffing. Pups were tested in groups of 4 or 6, mixing AN- and saline-treated newborns. The experimenters did not know whether the currently tested pups belonged to one or the other treatment group. Each pup participated in only one experiment but was successively tested with three stimuli, i.e. odorant A, odorant B and the AB mixture in Experiments 1 and 3, and odorant A, odorant B and the A′B′ mixture in Experiment 2. Successive testing involved the presentation of a first stimulus to a pup, then a second stimulus to another pup, and so on with an inter-trial interval of 60 s. The order of stimulus presentation was systematically counterbalanced from one pup to another. If a pup responded to a stimulus, its nose was softly dried before the next stimulation. The pups were immediately reintroduced into their nest after testing.
(f). Statistics
Owing to the death of four pups (two AN, two saline), the analyses focused on 64 newborns. The frequencies of responding pups were compared using Pearson's χ2 test when the groups were independent (i.e. distinct groups tested for their response to a same stimulus) or Cochran's Q test when the groups were dependent (i.e. pups from a same group tested for their response to three stimuli). When Cochran's Q test was significant, proportions of responding pups were compared 2 × 2 by McNemar's χ2 test. Degrees of freedom are indicated when more than 1. Data were considered as significant when the two-tailed test ended with p < 0.05.
3. Results
(a). Learning of the AB configural mixture, amnesia of A and B and resulting memory of AB
The AB mixture is hypothesized to be weakly configurally perceived by rabbit neonates, meaning that pups should perceive the odour of AB in addition to the odours of the element A and the element B in the mixture. If this is true, after conditioning to the whole mixture, the memory of the AB configural odour might be distinct from the memory of the element odours. To assess whether conditioning to the AB mixture induced separate memories of the suspected AB configural odour compared with the odours of the A and B elements, 24 rabbit pups were conditioned to AB by pairing with MP on day 1, reactivated by successive exposure to the A and B elements on day 2 (without MP) then immediately injected with saline (n = 10, control group) or AN (n = 14, experimental group) and tested for their behavioural responsiveness to A, B and AB on day 3. The AN treatment should induce amnesia of the element odours, but not necessarily of the AB configuration. In the control group, which did not receive AN treatment, the pups should respond strongly and equally to the odours of A, B and AB.
One AN-treated pup died between days 2 and 3; the results therefore concerned 10 versus 13 neonates. On day 3, the saline-treated pups responded highly both to the AB mixture and to the components (more than 80%, Q = 2, ddl = 2, p > 0.38). Conversely, AN-treated pups displayed distinct responsiveness to the stimuli (Q = 12.3, ddl = 2, p = 0.006); they did not respond to the odorants presented separately (less than 8%), but were still robustly responsive to the AB mixture (more than 60%, AB versus A or B: χ2 > 5.6, p < 0.018). While AN-treated pups responded clearly less to the A and B odorants than saline-treated neonates (χ2 > 9.5, p < 0.01 for each odorant), they maintained a similar level of responsiveness to AB (χ2 = 1.12, p = 0.29) (figure 1).
Figure 1.
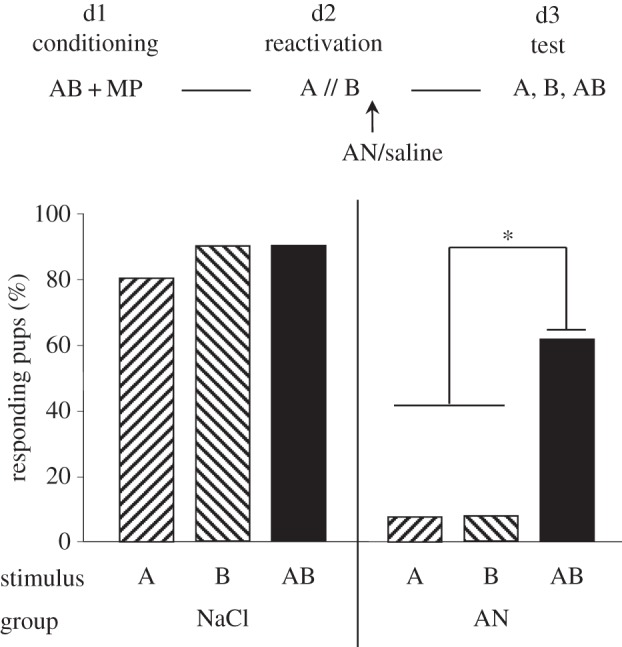
Proportions of 3-day-old rabbit pups responding in an oral activation test to odorant A (ethyl isobutyrate), odorant B (ethyl maltol) and the AB mixture (30/70 ratio of A/B), after conditioning to AB on day 1 by pairing with MP, and reactivation on day 2 by successive exposure to A and to B followed by immediate injection of AN or NaCl (saline). *p < 0.05.
Thus, after conditioning to the AB mixture and separated reactivation of odorants A and B, AN-injection was followed by retrograde amnesia of the odours of the two components. This amnesia was, however, not sufficient to prevent responsiveness to the whole mixture; the memory of the mixture, and only of the mixture, remained intact.
(b). Learning of the A′B′ elemental mixture, amnesia of A and B and resulting memory of A′B′
Previous evidence suggests that, in contrast to the AB mixture, the A′B′ mixture is olfactorily perceived by rabbit pups as the sum of its elements but not as a configuration, i.e. that pups perceived the odours of A and of B but not of an A′B′ configuration in the A′B′ mixture [22]. Therefore, we hypothesized that, after conditioning to A′B′, reactivation and amnesia of the elements A and B should dramatically impair responsiveness to the A′B′ mixture, in contrast to what we observed in Experiment 1 with the AB mixture. To run this control experiment, 24 new rabbit pups were conditioned to A′B′ by association with MP on day 1, exposed successively to A and to B and immediately injected with saline (n = 12, control group) or AN (n = 12, experimental group) on day 2 and tested for their behavioural responsiveness to A, B and A′B′ on day 3. Pups from the AN group were hypothesized to neither respond to A or to B nor to A′B′, whereas saline-injected control pups should respond both to the elements and to the mixture.
Three pups died between days 2 and 3 (respectively, saline, n = 2 and AN, n = 1); thus analysis concerned 10 versus 11 pups. On day 3, the saline-treated pups fully responded to A, B and AB (100%). Comparatively, AN-treated neonates responded extremely weakly to the stimuli, not only to the odorants but also to the A′B′ mixture (less than 10%). For each stimulus, the responsiveness was therefore higher in saline- than in AN-treated pups (χ2 > 13.9, p < 0.001 in all 2 × 2 comparisons) (figure 2).
Figure 2.
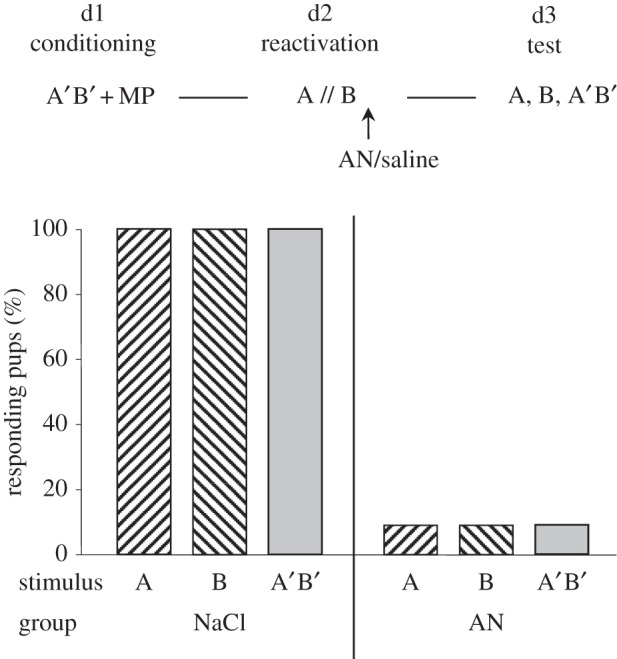
Proportions of 3-day-old rabbit pups responding in an oral activation test to odorant A (ethyl isobutyrate), odorant B (ethyl maltol) and the A′B′ mixture (68/32 ratio of A/B), after conditioning to A′B′ on day 1 by pairing with MP, and reactivation on day 2 by successive exposure to A and to B followed by immediate injection of AN or NaCl (saline).
Thus, in rabbit pups initially conditioned to the A′B′ mixture, reactivation with odorants A and B followed by blockade of reconsolidation impaired the memory both of the elements and of the A′B′ mixture. Responsiveness to the components seemed essential for response to their mixture at this ratio.
(c). Learning then amnesia of the AB configural mixture and resulting memory of AB, A and B
Results of Experiment 1 showed that after MP-induced conditioning to the AB mixture, rabbit pups responded to the mixture even after forgetting its elements A and B. This suggested distinct memory of the AB configural odour versus memory of the A and B element odours and accounted for the weak configural perception of the AB mixture, i.e. the perception during conditioning of a specific AB odour in addition to the odour of each element. To finally confirm the weak configural perception of the AB mixture, we induced its conditioning, reactivated the memory of the whole mixture (not of its elements only as in Experiment 1) before inducing amnesia and tested the responsiveness of the pups to AB, A and B. In this particular situation, if AB was perceived as the sum of two elements plus one configuration, the presentation of AB during the reactivation phase should reactivate the memory both of the configuration and of the elements which then should both be erased by the pharmacological treatment. This should consequently impede the pups' responsiveness to each of the three odours perceived in the mixture, A, B and AB. Thus, 20 pups were conditioned to AB by association with MP on day 1, reactivated with AB before injection of saline (n = 10, control group) or AN (n = 10, experimental group) on day 2 and tested for their responsiveness to A, B and AB on day 3. Pups from the AN group should not respond to any of the stimuli, while those from the saline group should respond to all.
All saline-treated pups responded to the odorants A and B, and to the AB mixture. Conversely, AN-treated pups displayed no or extremely weak responsiveness to any of the stimuli (less than 10%; Q = 2, ddl = 2, p = 0.38). As in Experiment 1, saline-treated pups responded therefore more to the odorants than AN-treated neonates (χ2 = 12.9, p < 0.001 for each odorant). However, here, injection of AN was followed by a major drop in responsiveness to AB compared with injection of saline (χ2 = 16.2, p < 0.001) (figure 3).
Figure 3.
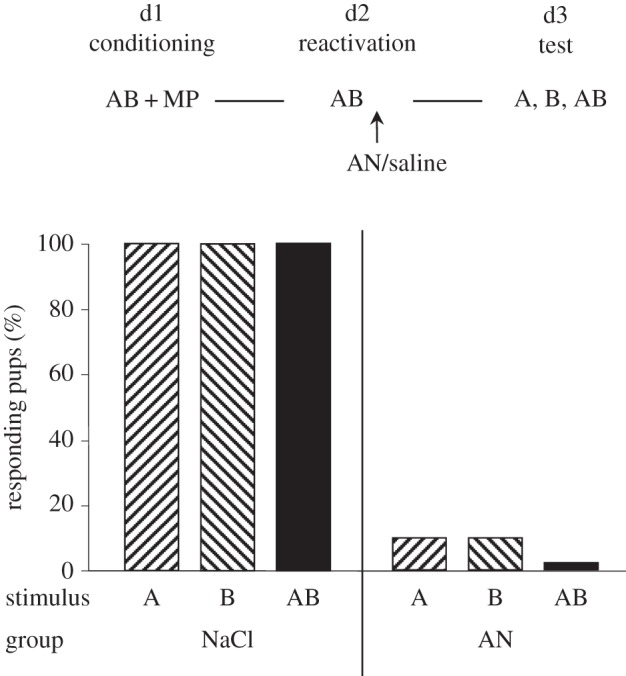
Proportions of 3-day-old rabbit pups responding in an oral activation test to odorant A (ethyl isobutyrate), odorant B (ethyl maltol) and the AB mixture (30/70 ratio of A/B), after conditioning to AB on day 1 by pairing with MP, and reactivation on day 2 by exposure to AB followed by immediate injection of AN or NaCl (saline).
Thus, after AB conditioning, when reactivation concerned the whole AB mixture, amnesia of AB appeared suppressive of any response either to the mixture or to its elements.
4. Discussion
Natural odours are constituted by mixtures of distinct molecules which carry by themselves particular odours. In that context, it is often considered that configural processing, i.e. perception of some mixtures as odour objects, is an efficient way to reduce the complexity of the chemical surroundings and optimize the detection, identification and discrimination between stimuli carrying biological significance [4,37–39]. However, a clear demonstration of brain and cognitive processes that could selectively differentiate a complex odour as a whole—unique representation—from its odorant parts has been lacking. Here, we combined behavioural and pharmacological approaches to evaluate whether the representation of AB, an apparent configural mixture for rabbit pups at a specific ratio (30/70) [19–22], is relatively distinct from the representation of each individual odorant.
As main results, we provide evidence that after neonatal learning of the AB mixture, amnesia of A and B did not propagate to AB: pups that did not respond either to A or to B still responded to AB (Experiment 1). Thus, a particular memory of AB was created during conditioning to the mixture, in parallel to the memories of odorant A and odorant B, and all these memories created together become rapidly dissociated. However, after conditioning to the AB mixture, re-exposure to the whole mixture (Experiment 3) reactivated not only the AB configuration but also the elements A and B, which were all sensitive to the post-reactivation pharmacological treatment: the pups became amnesic to AB but also to A and to B. In terms of perception, this demonstrates something only previously suggested: the AB mixture evokes a configural odour perceived by newborn rabbits in addition to (not to the detriment of) the specific odours of A and B. In other words, the perception of the AB mixture by rabbit pups is weak configural and not robust configural (for previous suggestions of partial configural perception of AB, see [19–22]). The configural AB odour can be processed on its own and is sufficient to trigger the behavioural response to the AB mixture. Thus, in the context of neonatal odour perception, the results indicate that a complex stimulus can induce different percepts which are simultaneously memorized but form rapidly, relatively separated memory traces. The memories of the mixture and its components are not entirely independent, however. That is, while disruption of the component memory did not affect the configural AB memory, disruption of the configural AB memory did impair the memory of the elements. This asymmetrical relationship suggests complex interactions between these different, relatively distinct representations. As further evidence of interactions between the representations of A, B and AB, we have recently demonstrated competition between the elemental and configural long-term (several days) memories [40].
The memory treatment observed here could be also at play later in life, because in human adults the same AB mixture is known to evoke an odour (pineapple) different from those of its A and B elements (strawberry and caramel, respectively) [17,18]. Comparatively, when rabbit pups were conditioned to the elements A and B in a mixture, but at a ratio known to trigger the elemental perception of the mixture (A′B′, ratio 68/32) [22], amnesia of A and of B abolished the response to A′B′. This demonstrates that responsiveness to the A′B′ mixture is based exclusively on perception of elements A and B and that no mixture-specific memory is created at this ratio (see [41], for similar results with an elementally processed compound composed of a tone and a light).
The present findings, when combined with previous work [19,20,29], demonstrate that memory for odour mixtures may be encoded in a variety of ways, depending on the nature of the stimuli and post-training events. The evidence that memories of certain odour mixtures (as the AB mixture here) can be simultaneously, and relatively separately, configural and elemental suggests that even mixtures perceived configurally have traces of their components somewhere in the brain. Analysis of configural processing and the formation of odour objects suggest a strong role for the olfactory (piriform) cortex and plasticity of intracortical association fibre synapses which can link distributed, co-active cortical neurons [38]. Thus, while spatial coding of mixture-evoked activity within the olfactory bulb did not discriminate configural mixtures from their components [42–44], piriform cortical neural ensembles have been demonstrated to rapidly process co-occurring odorants into distinct representations, different from the representations of their component parts [45]. This cortical representation of configural odour objects is experience-dependent, promotes odour discrimination, and can be impaired by disrupting normal synaptic plasticity selectively within the piriform cortex [46–48].
It is unclear whether the simultaneous elemental representation of the components apparent here is also dependent on the piriform cortex. Processing of odours occurs in a variety of regions beyond the canonical olfactory pathway, including hippocampal formation, frontal areas and other limbic structures [49–51], even during early development [52]. In humans, it has been demonstrated that the brain can distinguish between single odorants and binary mixtures [53]. Moreover, combining odours that differ in hedonic quality (e.g. pleasant and unpleasant) can create a configurally pleasant odour percept [54]. However, when assessed with neural imaging, circuits normally selectively activated in response to the unpleasant component are still activated, even though the configural perception does not reflect this underlying component [54]. The present results suggest a similar distributed network process may occur in newborn rabbits, with memory for individual components occurring distinctively (at least in part) from the configural memory. Further work will be required to identify neural mechanisms and locations of elemental versus configural processing, including the roles of the olfactory bulb [7], olfactory cortex and elsewhere.
Finally and regarding adaptation, odour mixture perception has a major impact on animal behaviour, in both aquatic and terrestrial species, and contributes to decision-making related to food-searching, mate choice and spatial orientation and to interspecies interactions such as predator avoidance and plant pollination (e.g. [11,12,55–58]). However, knowledge about the way odour mixtures are precisely processed, retained and connected to behaviour remains scarce. Here, the findings demonstrate the existence of configurations in certain odour mixtures, depending on odorants' ratio, and their representation as unique objects. To date, these possibilities were only suggested at a perceptual level (in young as in adults). Here, they are evidenced even in an incompletely mature, neonatal organism and strengthened by straightforward results related to a more integrative level, memory; clearly, a specific memory of configural odour mixtures exists, and appears involved in rapid processing and responsiveness to behaviourally significant chemically complex stimuli. In newborn rabbits, odour learning occurs during the daily interaction with the mother devoted to nursing [30,59–62]. Memory of single odorants but also of configural information is certainly at work during this vital period of interaction. It could allow for neonates to acquire, represent and successfully retain complex odour information carried by the maternal body, which may help improve the relationships with the mother (attraction, sucking and recognition), as indirect information linked to the social, physical and feeding environment useful later in life. More generally, the reactivity to configural information contained in some complex sensory stimuli certainly allows an animal to categorize efficiently the diversity of stimuli which constitute its own world and to find familiarity in the most constant and crucial representations of this changing environment.
Acknowledgement
We sincerely thank Valérie Saint-Giorgio, Nicolas Malaty, Jérôme Antoine and all the Centre de Zootechnie from the University of Burgundy for their cooperation.
The study was carried out under the local, institutional and national rules (French Ministries of Agriculture, and of Research & Technology) regarding the care and experimental use of the animals. All experiments were conducted in accordance with ethical rules enforced by French law and were approved by the Ethical Committee for Animal Experimentation (Dijon, France; no. 2406).
Funding statement
The work was supported by French ANR-2010-JCJC-1410–1 MEMOLAP to G.C., T.T.D. and G.F., and by the Pôle VITAGORA.
References
- 1.Rhodes G, Ewing L, Hayward WG, Maurer D, Mondloch CJ, Tanaka JW. 2009. Contact and other-race effects in configural and component processing of faces. Br. J. Psychol. 100, 717–728. ( 10.1348/000712608X396503) [DOI] [PubMed] [Google Scholar]
- 2.Kazem AJ, Widdig A. 2012. Visual phenotype matching: cues to paternity are present in rhesus macaque faces. PLoS ONE 8, e55846 ( 10.1371/journal.pone.0055846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastein HB, Winter R, Vinoth Kumar AK, Kandula S, Schmidt S. 2013. Perception of individuality in bat vocal communication: discrimination between, or recognition of, interaction partners? Anim. Cogn. 16, 945–959. ( 10.1007/s10071-013-0628-9) [DOI] [PubMed] [Google Scholar]
- 4.Gottfried JA. 2010. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 11, 628–41. ( 10.1038/nrn2883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laing DG, Francis GW. 1989. The capacity of humans to identify odors in mixtures. Physiol. Behav. 46, 809–814. ( 10.1016/0031-9384(89)90041-3) [DOI] [PubMed] [Google Scholar]
- 6.Laska M, Hudson R. 1993. Discriminating parts from the whole: determinants of odor mixture perception in squirrel monkeys, Saimiri sciureus. J. Comp. Physiol. A 173, 249–256. ( 10.1007/BF00192984) [DOI] [PubMed] [Google Scholar]
- 7.Linster C, Cleland TA. 2004. Configurational and elemental odor mixture perception can arise from local inhibition. J. Comp. Neurosci. 16, 39–47. ( 10.1023/B:JCNS.0000004840.87570.2e) [DOI] [PubMed] [Google Scholar]
- 8.Kay LM, Crk T, Thorngate J. 2005. A redefinition of odor mixture quality. Behav. Neurosci. 119, 726–733. ( 10.1037/0735-7044.119.3.726) [DOI] [PubMed] [Google Scholar]
- 9.Derby C, Huston M, Livermore B, Lynn W. 1996. Generalization among related complex odorant mixtures and their components: analysis of olfactory perception in the spiny lobster. Physiol. Behav. 60, 87–95. ( 10.1016/0031-9384(95)02237-6) [DOI] [PubMed] [Google Scholar]
- 10.Jinks A, Laing DG. 1999. A limit in the processing of components in odour mixtures. Perception 28, 395–404. ( 10.1068/p2898) [DOI] [PubMed] [Google Scholar]
- 11.Valentincic T, Kralj J, Stenovec M, Koce A, Caprio J. 2000. The behavioral detection of binary mixtures of amino acids and their individual components by catfish. J. Exp. Biol. 203, 3307–3317. [DOI] [PubMed] [Google Scholar]
- 12.Wiltrout C, Dogras S, Linster C. 2003. Configurational and nonconfigurational interactions between odorants in binary mixtures. Behav. Neurosci. 117, 236–245. ( 10.1037/0735-7044.117.2.236) [DOI] [PubMed] [Google Scholar]
- 13.Gottfried JA. 2009. Function follows form: ecological constraints on odor codes and olfactory percepts. Curr. Opin. Neurobiol. 19, 422–429. ( 10.1016/j.conb.2009.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riffell JA, Lei H, Hildebrand JG. 2009. Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc. Natl Acad. Sci. USA 106, 19 219–19 226. ( 10.1073/pnas.0910592106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deisig N, Giurfa M, Sandoz JC. 2010. Antennal lobe processing increases separability of odor mixture representations in the honeybee. J. Neurophysiol. 103, 2185–2194. ( 10.1152/jn.00342.2009) [DOI] [PubMed] [Google Scholar]
- 16.Chapuis J, Wilson DA. 2011. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat. Neurosci. 15, 155–161. ( 10.1038/nn.2966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Berre E, Thomas-Danguin T, Beno N, Coureaud G, Etievant P, Prescott J. 2008. Perceptual processing strategy and exposure influence the perception of odor mixtures. Chem. Senses 33, 193–199. [DOI] [PubMed] [Google Scholar]
- 18.Barkat S, Le Berre E, Coureaud G, Sicard G, Thomas-Danguin T. 2012. Perceptual blending in odor mixtures depends on the nature of odorants and human olfactory expertise. Chem. Senses 37, 159–166. ( 10.1093/chemse/bjr086) [DOI] [PubMed] [Google Scholar]
- 19.Coureaud G, Thomas-Danguin T, Le Berre E, Schaal B. 2008. Perception of odor blending mixtures in the newborn rabbit. Physiol. Behav. 95, 194–199. ( 10.1016/j.physbeh.2008.05.018) [DOI] [PubMed] [Google Scholar]
- 20.Coureaud G, Hamdani Y, Schaal B, Thomas-Danguin T. 2009. Elemental and configural processing of odour mixtures in the newborn rabbit. J. Exp. Biol. 212, 2525–2531. ( 10.1242/jeb.032235) [DOI] [PubMed] [Google Scholar]
- 21.Sinding C, Thomas-Danguin T, Crepeaux G, Schaal B, Coureaud G. 2011. Experience influences elemental and configural perception of certain binary odour mixtures in newborn rabbits. J. Exp. Biol. 214, 4171–4178. ( 10.1242/jeb.063610) [DOI] [PubMed] [Google Scholar]
- 22.Coureaud G, Gibaud D, Le Berre E, Schaal B, Thomas-Danguin T. 2011. Proportion of odorants impacts the configural versus elemental perception of a blending mixture in newborn rabbits. Chem. Senses 36, 693–700. ( 10.1093/chemse/bjr049) [DOI] [PubMed] [Google Scholar]
- 23.Davis HP, Squire LR. 1984. Protein synthesis and memory: a review. Psychol. Bull. 96, 518–559. ( 10.1037/0033-2909.96.3.518) [DOI] [PubMed] [Google Scholar]
- 24.Dudai Y. 2004. The neurobiology of consolidations, or how stable is the engram? Annu. Rev. Psychol. 55, 51–86. ( 10.1146/annurev.psych.55.090902.142050) [DOI] [PubMed] [Google Scholar]
- 25.Nader K, Schafe GE, LeDoux JE. 2000. The labile nature of consolidation theory. Nat. Rev. Neurosci. 1, 216–219. ( 10.1038/35044580) [DOI] [PubMed] [Google Scholar]
- 26.Sara SJ. 2000. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 7, 73–84. ( 10.1101/lm.7.2.73) [DOI] [PubMed] [Google Scholar]
- 27.Coureaud G, Languille S, Schaal B, Hars B. 2009. Consolidation and reconsolidation processes support pheromone-induced memory in newborn rabbits. Learn. Mem. 16, 470–473. ( 10.1101/lm.1434009) [DOI] [PubMed] [Google Scholar]
- 28.Coureaud G, Languille S, Joly V, Schaal B, Hars B. 2011. Independence of first- and second-order memories in newborn rabbits. Learn. Mem. 18, 401–404. ( 10.1101/lm.2145111) [DOI] [PubMed] [Google Scholar]
- 29.Coureaud G, Tourat A, Ferreira G. 2013. Sensory preconditioning in newborn rabbits: from common to distinct odor memories. Learn. Mem. 20, 453–458. ( 10.1101/lm.030965.113) [DOI] [PubMed] [Google Scholar]
- 30.Zarrow MX, Denenberg VH, Anderson CO. 1965. Rabbit: frequency of suckling in the pup. Science 150, 1835–1836. ( 10.1126/science.150.3705.1835) [DOI] [PubMed] [Google Scholar]
- 31.Coureaud G, Moncomble A-S, Montigny D, Dewas M, Perrier G, Schaal B. 2006. A pheromone that rapidly promotes learning in the newborn. Curr. Biol. 16, 1956–1961. ( 10.1016/j.cub.2006.08.030) [DOI] [PubMed] [Google Scholar]
- 32.Le Berre E, Jarmuzek E, Béno N, Etiévant P, Prescott J, Thomas-Danguin T. 2010. Learning influences the perception of odor mixtures. Chem. Percept. 3, 156–166. ( 10.1007/s12078-010-9076-y) [DOI] [Google Scholar]
- 33.Montigny D, Coureaud G, Schaal B. 2006. Rabbit pup response to the mammary pheromone: from automatism to prandial control. Physiol. Behav. 89, 742–749. ( 10.1016/j.physbeh.2006.08.022) [DOI] [PubMed] [Google Scholar]
- 34.Gruest N, Richer P, Hars B. 2004. Memory consolidation and reconsolidation in the rat pup require protein synthesis. J. Neurosci. 24, 10 488–10 492. ( 10.1523/JNEUROSCI.2984-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desgranges B, Lévy F, Ferreira G. 2008. Anisomycin infusion in amygdala impairs consolidation of odor aversion memory. Brain Res. 1236, 166–175. ( 10.1016/j.brainres.2008.07.123) [DOI] [PubMed] [Google Scholar]
- 36.Merhav M, Rosenblum K. 2008. Facilitation of taste memory acquisition by experiencing previous novel taste is protein-synthesis dependent. Learn. Mem. 15, 501–507. ( 10.1101/lm.986008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson RJ, Wilson DA. 2007. Odour perception: an object-recognition approach. Perception 36, 1821–1833. ( 10.1068/p5563) [DOI] [PubMed] [Google Scholar]
- 38.Wilson DA, Sullivan RM. 2011. Cortical processing of odor objects. Neuron 72, 506–519. ( 10.1016/j.neuron.2011.10.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas-Danguin T, Sinding C, Romagny S, El Mountassir F, Barkat S, Atanasova B, Le Berre E, Le Bon AM, Coureaud G. 2014. The perception of odor objects in everyday life: a review on the processing of odor mixtures. Front. Psychol. 5, 504 ( 10.3389/fpsyg.2014.00504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coureaud G, Thomas-Danguin T, Datiche F, Wilson DA, Ferreira G. 2014. Differential memory persistence of odour mixture and components in newborn rabbits: competition between the whole and its parts. Front. Behav. Neurosci. 8, 211 ( 10.3389/fnbeh.2014.00211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones CE, Ringuet S, Monfils MH. 2013. Learned together, extinguished apart: reducing fear to complex stimuli. Learn. Mem. 20, 674–685. ( 10.1101/lm.031740.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin DY, Shea SD, Katz LC. 2006. Representation of natural stimuli in the rodent main olfactory bulb. Neuron 50, 937–949. ( 10.1016/j.neuron.2006.03.021) [DOI] [PubMed] [Google Scholar]
- 43.Grossman KJ, Mallik AK, Ross J, Kay LM, Issa NP. 2008. Glomerular activation patterns and the perception of odor mixtures. Eur. J. Neurosci. 27, 2676–2685. ( 10.1111/j.1460-9568.2008.06213.x) [DOI] [PubMed] [Google Scholar]
- 44.Johnson BA, Ong J, Leon M. 2010. Glomerular activity patterns evoked by natural odor objects in the rat olfactory bulb are related to patterns evoked by major odorant components. J. Comp. Neurol. 518, 1542–1555. ( 10.1002/cne.22289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson DA. 2003. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J. Neurophysiol. 90, 65–72. ( 10.1152/jn.00133.2003) [DOI] [PubMed] [Google Scholar]
- 46.Linster C, Menon AV, Singh CY, Wilson DA. 2009. Odor-specific habituation arises from interaction of afferent synaptic adaptation and intrinsic synaptic potentiation in olfactory cortex. Learn. Mem. 16, 452–459. ( 10.1101/lm.1403509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stettler DD, Axel R. 2009. Representations of odor in the piriform cortex. Neuron 63, 854–864. ( 10.1016/j.neuron.2009.09.005) [DOI] [PubMed] [Google Scholar]
- 48.Chapuis J, Wilson DA. 2013. Cholinergic modulation of olfactory pattern separation. Neurosci. Lett. 545, 50–53. ( 10.1016/j.neulet.2013.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenkranz JA, Grace AA. 2002. Dopamine-mediated modulation of odour-evoked amygdala potentials during Pavlovian conditioning. Nature 417, 282–287. ( 10.1038/417282a) [DOI] [PubMed] [Google Scholar]
- 50.Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, Ravel N. 2009. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J. Neurosci. 29, 10 287–10 298. ( 10.1523/JNEUROSCI.0505-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu W, Wilson DA. 2012. Odor-evoked activity in the mouse lateral entorhinal cortex. Neuroscience 223, 12–20. ( 10.1016/j.neuroscience.2012.07.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moriceau S, Wilson DA, Levine S, Sullivan RM. 2006. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 26, 6737–6748. ( 10.1523/JNEUROSCI.0499-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyle JA, Djordjevic J, Olsson MJ, Lundstrom JN, Jones-Gotman M. 2009. The human brain distinguishes between single odorants and binary mixtures. Cereb. Cortex 19, 66–71. ( 10.1093/cercor/bhn058) [DOI] [PubMed] [Google Scholar]
- 54.Grabenhorst F, Rolls ET, Margot C, da Silva MA, Velazco MI. 2007. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. J. Neurosci. 27, 13 532–13 540. ( 10.1523/JNEUROSCI.3337-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fine-Levy JB, Daniel PC, Girardot MN, Derby CD. 1989. Behavioral resolution of quality of odorant mixtures by spiny lobsters: differential aversive conditioning of olfactory responses. Chem. Senses 14, 503–524. ( 10.1093/chemse/14.4.503) [DOI] [Google Scholar]
- 56.Linster C, Smith BH. 1999. Generalization between binary odor mixtures and their components in the rat. Physiol. Behav. 66, 701–707. ( 10.1016/S0031-9384(99)00007-4) [DOI] [PubMed] [Google Scholar]
- 57.Deisig N, Lachnit H, Giurfa M, Hellstern F. 2001. Configural olfactory learning in honeybees: negative and positive patterning discrimination. Learn. Mem. 8, 70–78. ( 10.1101/lm.8.2.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riffell JA. 2012. Olfactory ecology and the processing of complex mixtures. Curr. Opin. Neurobiol. 22, 236–242. ( 10.1016/j.conb.2012.02.013) [DOI] [PubMed] [Google Scholar]
- 59.Kindermann U, Gervais R, Hudson R. 1991. Rapid odor conditioning in newborn rabbits: amnesic effect of hypothermia. Physiol. Behav. 50, 457–460. ( 10.1016/0031-9384(91)90094-5) [DOI] [PubMed] [Google Scholar]
- 60.Hudson R, Labra-Cardero D, Mendoza-Solovna A. 2002. Suckling, not milk, is important for the rapid learning of nipple-search odors in newborn rabbits. Dev. Psychobiol. 41, 226–235. ( 10.1002/dev.10073) [DOI] [PubMed] [Google Scholar]
- 61.Serra J, Ferreira G, Mirabito L, Lévy F, Nowak R. 2009. Post-oral and perioral stimulations during nursing enhance appetitive olfactory memory in neonatal rabbits. Chem. Senses 34, 405–413. ( 10.1093/chemse/bjp014) [DOI] [PubMed] [Google Scholar]
- 62.Coureaud G, Charra R, Datiche F, Sinding C, Thomas-Danguin T, Languille S, Hars B, Schaal B. 2010. A pheromone to behave, a pheromone to learn: the rabbit mammary pheromone. J. Comp. Physiol. A 196, 779–790. ( 10.1007/s00359-010-0548-y) [DOI] [PubMed] [Google Scholar]


