Abstract
In cooperative breeding systems, dominant breeders sometimes tolerate unrelated individuals even if they inflict costs on the dominants. According to the ‘pay-to-stay’ hypothesis, (i) subordinates can outweigh these costs by providing help and (ii) dominants should be able to enforce help by punishing subordinates that provide insufficient help. This requires that dominants can monitor helping and can recognize group members individually. In a field experiment, we tested whether cooperatively breeding cichlid Neolamprologus pulcher subordinates increase their help after a forced ‘idle’ period, how other group members respond to a previously idle helper, and how helper behaviour and group responses depend on group size. Previously, idle helpers increased their submissiveness and received more aggression than control helpers, suggesting that punishment occurred to enforce help. Subordinates in small groups increased their help more than those in large groups, despite receiving less aggression. When subordinates were temporarily removed, dominants in small groups were more likely to evict returning subordinates. Our results suggest that only in small groups do helpers face a latent threat of punishment by breeders as predicted by the pay-to-stay hypothesis. In large groups, cognitive constraints may prevent breeders from tracking the behaviour of a large number of helpers.
Keywords: conflict, cooperation, helping, territory defence, eviction, cichlids
1. Introduction
Cooperative breeding, where subordinate individuals help to rear a brood produced by dominant breeders, occurs in several species of invertebrates and vertebrates, including mammals, birds and fish [1]. Several mechanisms have been proposed to explain how selection can favour cooperative behaviours that directly benefit other group members at the expense of the direct fitness of the actor. Evolutionary explanations distinguish between indirect and direct benefits of helping [2]. Kin selection explains altruistic behaviour of individuals helping relatives [3,4], whereas direct fitness benefits can be gained by helpers through three main routes: (i) by group augmentation through helping [5,6], which may increase the survival prospects of helpers; (ii) by increasing the chances of own future reproduction (for instance, through territory inheritance [7], acquisition of breeding skills [8] or an increase in ‘social prestige’, which may aid the acquisition of dominance or mating partners [9]); or (iii) the ‘pay-to-stay’ hypothesis [10] predicts that if subordinate group members inflict costs on dominant breeders due to competition for resources and reproduction, the ensuing conflict of interest can be resolved if helpers assume important duties such as territory defence or brood care to compensate for these costs [11,12].
Among the above-mentioned mechanisms, ‘pay-to-stay’ has received empirical [13–22] and theoretical support [11,12]. Empirical support to date stems mostly from a single species, however. In the cooperatively breeding cichlid Neolamprologus pulcher, evidence from several experimental studies in the laboratory and the field showed that cooperation is largely based on pay-to-stay in this species [15,17–19,21,23–25]. Evidence in other species is scarce [13,22], and reported indications of pay-to-stay may also be explained by alternative ultimate or proximate mechanisms [22]. Studies investigating pay-to-stay focused mainly on predictions regarding behavioural responses of group members towards helpers not paying in accordance with need [13–15,17]. Others investigated how the tolerance of subordinates depends on the need for help [18,26], how helpers adjust their cooperative effort to alternative options [19,27], how dominant individuals benefit from subordinates [16] and if the amount of helping increases tolerance in the group [28]. Most studies have supported predictions derived from the pay-to-stay hypothesis (but see [16,28]).
Among the mechanisms proposed to explain altruistic helping, only pay-to-stay predicts that dominant individuals enforce help by subordinates [11]. Therefore, several experimental studies attempted to test whether ‘idle’ helpers are punished by dominant individuals [13–15,17,29]. While some of these studies reported an increase in aggression on return of a temporarily removed helper [13,17], it has so far not been shown conclusively that punishment increases the helping effort of subordinates [30–32]. Furthermore, it has been argued that pay-to-stay may be unlikely to maintain cooperation because of the required cognitive skills [31]. Dominant individuals need to monitor helpfulness of individual subordinates and react accordingly by punishing idle helpers. Most experimental evidence for pay-to-stay has been established in small groups of cooperative breeders, sometimes well below the average group size [13,15,24], hence it remains unclear if cooperation in large groups can be maintained by this mechanism as well.
The cooperatively breeding cichlid N. pulcher lives in social groups containing 1 to more than 25 helpers varying in relatedness, size and sex [23,25,33–36]. In this species many important predictions derived from the pay-to-stay hypothesis have received experimental support. Neolamprologus pulcher helpers have been shown to increase their helping effort in the home territory both after being temporarily removed (field experiments [17]) and after being prevented from helping (laboratory experiments [14,15]). However, an important prediction of pay-to-stay models, namely that breeders increase their aggression to punish ‘lazy’ subordinates, has not yet been conclusively demonstrated in this species [14,15,17].
Punishment or elevated aggression by dominant individuals often leads to the eviction of subordinate group members in cooperative breeders [18,37–39]. Theory predicts that dominants should show an increased propensity to evict ‘lazy’ helpers and subordinates that directly compete with dominants for reproduction [40]. In N. pulcher, adult subordinates can steal fertilizations or bud off parts of the dominants' territory [18,41–45]. Eviction rates of subordinates are predicted to increase with decreasing group productivity [40], which is often associated with group size [36,46,47]. However, if dominants suffer cognitive constraints in large groups, eviction rates should be higher in small groups where dominants are able to monitor ‘lazy’ helpers.
Here, we exposed helpers from large and small groups to three experimental treatments in the field: (i) a temporary prevention of helping behaviour of a subordinate while being present in the territory; (ii) a temporary removal of a helper from the territory; and (iii) a treatment controlling for handling and manipulation procedures. We aim to answer the following questions: (1) Do N. pulcher helpers increase their submissiveness or helping effort, and/or do they receive more aggression from other group members after being prevented from helping while being present at the territory? (2) Do breeders and other helpers reduce their helping effort on return of the ‘idle’ helper, depending on group size? (3) Are small groups more efficient in executing punishment? (4) What is the functional context responsible for elevated aggression levels towards experimentally manipulated, ‘idle’ helpers? Two answers have been proposed for the fourth question: (i) punishment by dominant individuals [17,31] or (ii) competition over rank, where lower-ranked individuals try to step up the queue and inherit the position of the manipulated helper [17,48]. To distinguish between these possibilities, manipulating both the helping effort and helper presence is necessary. If punishment is the main driving force of aggression, helpers prevented from helping and helpers temporarily removed from the territory should receive similar amounts of aggression, and aggression should mainly be shown by dominant individuals. Alternatively, if a helper's absence is perceived as a higher level of cheating, aggression particularly of dominant individuals should be increased, which may result in a higher eviction probability when helpers are temporarily removed from the territory. If aggression reflects conflict over rank, helpers temporarily absent from the territory should receive aggression mainly from other helpers.
2. Material and methods
(a). Study species
Neolamprologus pulcher inhabits rocky habitats all along the shoreline of Lake Tanganyika, from 3 to 45 m depth [49]. Territories of N. pulcher cluster into colonies, where several social groups live in close proximity to each other [17,35,50]. The groups defend distinct patches of stones containing a central breeding shelter and often additional shelters for the other group members to hide from predator attacks [36,51]. Eggs are attached to the inner walls of the breeding shelters and are mainly cared for by the breeder females and small helpers [23]. Larger helpers engage in defence against con- and heterospecific territory intruders, and egg and fish predators, in digging out shelters for breeding and for protection from predators [21,23,33,52].
(b). General field methods
The study was conducted at the southern tip of Lake Tanganyika, at Kasakalawe Point, near Mpulungu, Zambia, from September to December 2011 and 2012, by scuba diving. Our experimental population consisted of five colonies of different sizes. From these colonies we haphazardly allocated 14 large (range: 16–25 members, including the breeder pair) and 21 small family groups (range: 5–7 members) to experimental groups. We haphazardly caught the experimental fish using a Plexiglas tube and two hand nets. Their standard lengths (SL; from the tip of the snout to the posterior end of the vertebral column) were measured using a measuring board with a 1 mm grid. Only helpers between 34 and 48 mm SL were used for the experiment. We took a small fin clip from one of the last dorsal fin rays, allowing us to recognize the focal helper during the following 3–4 days. As in previous studies, the removed fin rays, regrew within one to two months (e.g. [52]), and all marked fish resumed normal behaviour within a few minutes after handling (S. Fischer & F. Groenewoud 2011–2012, personal observation).
(c). Experimental treatments
(i). ‘Prevented help’ treatment (n = 20)
To prevent a focal fish from helping, it was placed in a clear plastic cylinder (height: 10 cm, diameter: 8.5 cm) equipped with a shelter built from two small PVC plates. Holes in the lid of the cylinder allowed for water exchange. The plastic cylinder holding the focal helper was placed close to its original shelter within the home territory. A few minutes after start of confinement, the focal helpers started to move freely in the cylinder and did not show obvious signs of stress. One individual even returned to the cylinder deliberately after the lid had been removed to release the fish back in its home territory. Throughout this treatment, the focal helper was present in the territory but did not participate in the typical helping duties such as territory defence and maintenance, and brood care. After 24 h, we released the focal helper from the cylinder and recorded its behaviour and the behaviours of all other group members for 10 min, which is sufficiently long to obtain a representative sample of the behavioural repertoire of N. pulcher [14,53].
(ii). Control treatment (n = 20)
A focal helper was placed in the plastic cylinder and, as in the prevented help treatment, it was individually marked and placed close to its original shelter at the territory. It was released after 5 min; the observer returned 24 h later and recorded the behaviour of the focal helper and the group members for 10 min. Where possible, we performed the prevented help and control treatments in the same group (only twice we had to use different groups), using similar-sized individuals (comparison of focal SL between controls and treatments: Welch two-sample t-test, t = 1.24, d.f. = 36.87, p = 0.22). This treatment served to control for potential effects of the catching and placement in the cylinder on the subsequent behaviour of the focal helper, and for the potential disturbance of other group members by these manipulations. It only partly controls for possible effects of the releasing procedure, because in the prevented help treatment the behavioural observations directly followed the release. To test for the potential influence of this temporal proximity, we performed a separate series of behavioural recordings on different family groups, which is presented in the electronic supplementary material.
(iii). Helper removal treatment (n = 20)
A focal fish was caught and transferred to a 0.8 × 0.8 × 0.6 m mesh cage that was installed outside of the colony area. After 24 h, the fish was relocated to its family. After releasing the fish, the behaviours of the focal helper and of all other group members were recorded for 10 min. After this observation, we visited each family after 24 h to confirm the acceptance status of the focal helper. For a graphical representation of all treatments, see the electronic supplementary material, figure A1.
(d). Behavioural recordings
During the 10 min behavioural observations, we recorded all submissive and aggressive behaviours of the focal fish (according to an established ethogram of N. pulcher [54–56]) shown towards other group members, predators and space competitors, and all aggressive behaviours of all other group members towards the focal helper, predators and space competitors. To score the behaviours, we used a handheld computer (Psion Teklogix Workabout Pro 7525) packed within a waterproof plastic bag and equipped with Noldus Pocket Observer v. 3.0. For our analysis, we distinguished between aggressive behaviours towards group members and towards non-group members (intruders). Aggression or ‘territory defence’ against intruding con- and heterospecific individuals was combined into a single ‘defence’ variable for the analysis.
We scored focal helpers as either evicted or accepted 24 h after the release. Accepted focal helpers immediately resumed their helping duties within the group. Evicted helpers received aggression after the release, subsequently left their group and did not return to the groups within 24 h.
(e). Statistical analyses
To compare the submissive behaviours, received aggression, frequency of defence behaviours of focal helpers, frequency of the total group defence behaviours and frequency of the defence behaviours of breeders and other helpers between the treatments and group sizes, generalized linear mixed models (GLMM) with log link function were used to account for a Poisson error structure. Treatment and group size of the family (large or small) were always fitted as fixed effects, the SL of the focal helper was included as a covariate and group identity as a random factor to account for repeated measurements within the same group. Full models always included the interaction between treatment and group size. To analyse the defence behaviour of breeders and other helpers, we included the class of group member (breeder, helper) as a factor in the model. Here, all two-way and three-way interactions between the treatment, group size and the class factor were included in the model. To simplify the models, we used stepwise backward elimination of non-significant interaction terms [57,58]. All models were checked for over-dispersion [57].
To analyse whether competition over rank or punishment is the main behavioural mechanism driving elevated aggression levels in the prevented help treatment, we calculated per capita aggression rates towards the focal helper from breeders and from helpers. Because N. pulcher groups have linear size-based hierarchies, potential competitors for a focal helper's rank can only be either of the same size or smaller than the focal helper [55]. As we could not catch and precisely measure all group members, we estimated the sizes of non-captured group members to the nearest 1 cm, resulting in a size distribution of 1 cm length classes. For the analysis of per capita aggression rates, we then divided helpers into two groups being either larger (all 1 cm classes larger than the focal helper) or ‘similar sized’ (i.e. same or a smaller 1 cm class than the focal helper). However, there was only one case where helpers 2 cm smaller than the focal participated in aggression towards the focal helper. The per capita received aggression rates were highly zero inflated, therefore we transformed the data into a binomial data structure, where focal helpers either received aggression or did not. To compare the probability of receiving aggression from breeders, larger or similar-sized helpers, we used a GLMM with a logit link to account for a binomial error structure. Here, the type of group members (breeder, larger and similar-sized helpers), as well as group size (large, small), were fitted as fixed effects. All interactions of the type of group members and group size were included in the model. To compare if helpers were treated differently in large or in small groups in the helper removal treatment, and after being removed versus after being prevented from helping while present, we used eviction rates rather than aggression frequencies. This was necessary because it was not possible to obtain the received aggression rates of evicted individuals, as the time span between release and eviction was very short. Eviction rates were statistically compared using Fisher's exact tests. For statistical analysis, we used R v. 3.0.1 [59] with the package ‘lme4’ [60].
3. Results
(a). Submission, helping effort and received aggression after experimental prevention of help
Subsequent to being prevented from helping by confinement in a clear cylinder (‘prevented help’ treatment) only focal helpers in small groups increased their amount of defence compared with helpers in the control treatment (figure 1a and table 1a).
Figure 1.
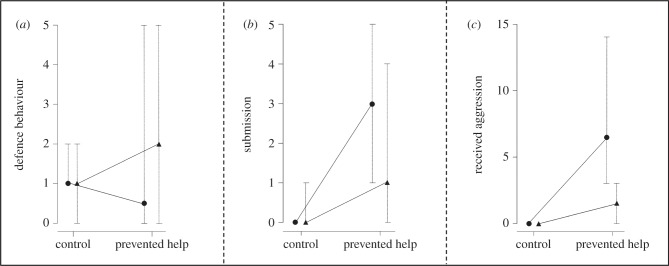
Comparisons between social behaviours per 10 min of observations in the control treatment and prevented help treatment (medians and interquartile ranges). (a) Defence behaviour of focal helpers, (b) submission of focal helpers, (c) aggression received by focal helpers from all other group members. Triangles represent small groups and circles large groups.
Table 1.
Comparison of the (a) absolute frequency of defence behaviours, (b) submissive behaviour and (c) received aggression of focal helpers in the prevented help and control treatments. Reference categories for estimates of factor ‘treatment’: control treatment; of factor ‘group size’: large groups. n = 21 groups; p-values of less than 0.05 are highlighted in bold, and 0.05 < p < 0.1 are italicized.
| factors | estimate ± s.e. | z-value | p-value |
|---|---|---|---|
| (a) focal defence | |||
| treatment | −0.116 ± 0.307 | −0.38 | 0.71 |
| group size | −0.977 ± 0.611 | −1.60 | 0.11 |
| SL | 0.094 ± 0.053 | 1.76 | 0.078 |
| treat × group size | 1.372 ± 0.525 | 2.61 | 0.009 |
| (b) submissive behaviour | |||
| treatment | 3.197 ± 0.729 | 4.37 | <0.0001 |
| group size | 1.633 ± 0.930 | 1.76 | 0.079 |
| SL | −0.102 ± 0.068 | −1.51 | 0.13 |
| treat × group size | −2.731 ± 0.854 | −3.2 | 0.001 |
| (c) received aggression | |||
| treatment | 3.47 ± 0.521 | 6.66 | <0.0001 |
| group size | −1.535 ± 0.539 | −2.85 | 0.004 |
| SL | 0.066 ± 0.065 | 1.01 | 0.31 |
After confinement in the prevented help treatment, focal helpers showed more submissive behaviour towards other group members and received more aggression from group members than after the control treatment (figure 1b,c and table 1b,c). In large groups, focal helpers showed a higher increase in submission after being confined in the cylinder compared with focal helpers in small groups (figure 1b; significant interaction term treatment × group size, table 1b). In larger groups, focal helpers generally tended to show more submission and they received more aggression (figure 1b,c and table 1b,c).
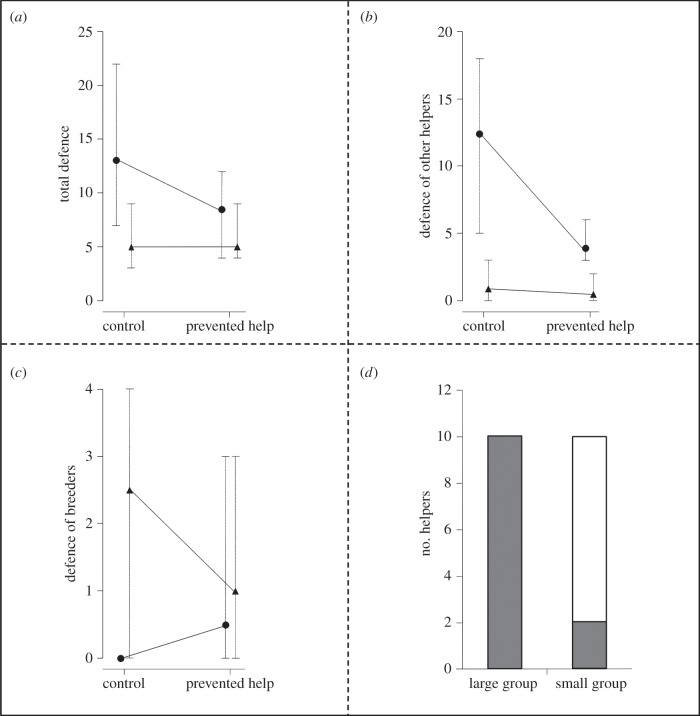
(b). Influence of group size on helping and eviction rates
After focal helpers were released in the prevented help treatment, the total group defence behaviours decreased in all groups compared with the control treatment (figure 2a and table 2a). Large groups reduced their total defence behaviours in this situation more than small groups (figure 2a and table 2a). When comparing the defence behaviours of breeders and of other helpers in small and large groups, we found that helpers decreased their defence significantly in large groups, whereas breeders did so in small groups (figure 2b,c; see three-way interaction in table 2b).
Figure 2.
Influence of group size on (a–c) defence behaviours per 10 min of observation in the control and the prevented help treatment and (d) eviction rates in the removal treatment. (a) Total group defence, (b) defence behaviour of other helpers, (c) defence behaviour of breeders, (d) comparison of the number of accepted and evicted focal helpers between small and large groups in the helper removal treatment. (a–c) Triangles represent small groups and circles large groups; medians and interquartile ranges are shown. (d) Grey bars represent accepted focal helpers and white bars evicted focal helpers.
Table 2.
Comparisons of (a) the total group defence and (b) the defence of all other group members against conspecifics and predators in the prevented help and control treatments. Reference categories for estimates of factor ‘treatment’: control treatment; of factor ‘group size’: large groups; and of factor ‘class’: breeders. n = 21 groups; p-values of less than 0.05 are highlighted in bold.
| factors | estimate ± s.e. | z-value | p-value |
|---|---|---|---|
| (a) total defence | |||
| treatment | −0.621 ± 0.14 | −4.44 | <0.0001 |
| group size | −0.8 ± 0.234 | −3.84 | 0.0001 |
| treat × group size | 0.625 ± 0.238 | 2.62 | 0.009 |
| (b) defence of other group members | |||
| treatment | 1.18 ± 0.573 | 2.06 | 0.04 |
| group size | 1.0 ± 0.573 | 3.49 | 0.0005 |
| class | 3.385 ± 0.51 | 6.64 | 0.0001 |
| treat × group size | −1.53 ± 0.65 | −2.36 | 0.02 |
| treat × class | −2.166 ± 0.5 | −3.61 | 0.0003 |
| group size × class | −3.847 ± 0.598 | −6.44 | <0.0001 |
| treat × group size × class | 1.734 ± 0.786 | 2.21 | 0.027 |
In the helper removal treatment, focal helpers faced a significantly higher eviction probability in small groups compared with helpers in large groups (Fisher's exact test, n = 20, p = 0.003; figure 2d). In the prevented help treatment all helpers were re-integrated after the release.
(c). Causes of change in aggression
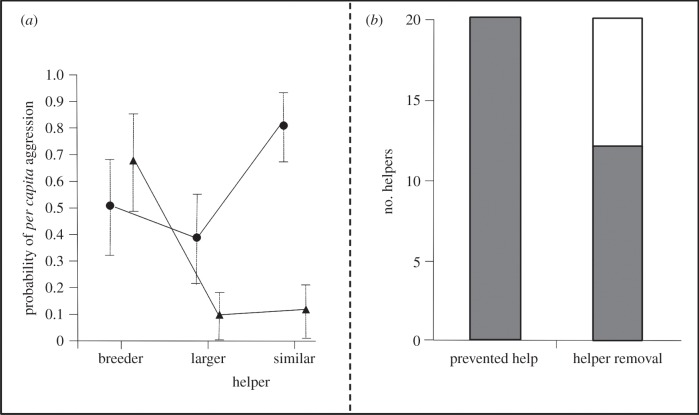
To disentangle whether changes in the other group members' aggression reflect punishment or rather conflicts over rank between helpers, we analysed per capita aggression rates and compared the eviction probabilities of focal helpers after being prevented from helping and after being temporarily removed from the territory. After prevention from helping, focal helpers in small groups received aggression mainly from breeders, whereas in large groups mainly similar-sized helpers displayed aggression towards the focal helper (figure 3a and table 3).
Figure 3.
(a) Comparison of the probability of the per capita received aggression from breeders, larger and similar-sized helpers between small and large groups in the prevented help treatment (medians and interquartile ranges). Triangles represent small groups and circles large groups. (b) Comparison of the number of accepted and evicted focal helpers between the prevented help and the helper removal treatments; grey bars represent accepted focal helpers and white bars evicted focal helpers.
Table 3.
Comparison of the per capita received aggression of focal helpers in the prevented help treatment. Reference categories for estimates of factors ‘larger helper’ and ‘similar helpers’: breeder; and of factor ‘group size’: large groups. n = 20 groups; p-values of less than 0.05 are highlighted in bold, and 0.05 < p < 0.1 are italicized.
| factors | estimate ± s.e. | z-value | p-value |
|---|---|---|---|
| per capita received aggression | |||
| larger helper | −0.421 ± 0.906 | −0.47 | 0.64 |
| similar helper | 1.433 ± 1.022 | 1.40 | 0.16 |
| group size | 0.879 ± 0.957 | 0.92 | 0.36 |
| larger helper × group size | −2.718 ± 1.571 | −1.730 | 0.084 |
| similar helper × group size | −4.572 ± 1.641 | −2.79 | 0.005 |
Helpers that had been temporarily removed from the territory were significantly more often evicted from their home territory than helpers that had been only prevented from helping but had been present in the territory (Fisher's exact test, n = 20, p = 0.001; figure 3b).
4. Discussion
In this study, we experimentally manipulated the helping behaviour of subordinates in a cooperative breeder living under natural conditions. In our prevented help treatment, we managed for the first time to reduce helping behaviour in a field experiment without removing the helper from the territory, thus allowing us to test for responses to ‘idle’ helpers. Other studies experimentally manipulating behaviours of subordinates have either varied the need for help (e.g. [61,62]) or increased helping behaviours by supplementary feeding experiments [63].
Our results suggest that helpers in small groups compensated for an ‘idle’ period by increased investment in territory defence, and that they faced a higher eviction probability after temporary removal from the territory. Furthermore, all helpers increased their submissiveness towards other group members, which confirms results of a previous laboratory experiment [15]. After being prevented from helping, helpers in large groups received more aggression, probably because more group members were present, and they increased submission more than helpers in small groups, which may indicate an appeasement strategy [15]. However, in contrast to previous experiments, where the breeders' aggression was not found to increase significantly after the helpers' resumption of their normal duties [15,17], here we found that breeders and other helpers increased their aggression towards focal helpers after being experimentally prevented from helping. Focal helpers increased their defence behaviours after being prevented from helping only in small groups. A closer inspection of the elevated aggression rates in the prevented help treatment revealed that mainly breeders directed their aggression towards ‘idle’ focal helpers in small, but not in large groups. This supports the hypothesis that punishment may be the main function of elevated aggression levels in small groups, where breeders may be better able to control helpers and punish them when the breeders' expectation to receive help in territory defence has been violated. In large groups, mainly helpers similar in size to the focal helper showed elevated aggression levels. This did not result in higher cooperative effort of the focal helper, suggesting that the ultimate function of increased aggression towards the focal helper in large groups might be competition over rank among group members. This is consistent with previous experimental work suggesting that competition over rank rather than coercion might be responsible for increased aggression after helper removal [17,32].
Punishment, where dominant individuals penalize ‘idle’ subordinates, is a crucial component of cooperation based on the pay-to-stay mechanism [11]. It has been argued that unequivocal evidence for punishment is lacking in cooperatively breeding animals [31]. In two other studies where helpers were experimentally removed, both breeders and other helpers increased their aggression towards returning helpers [13,29]. However, an increase in aggression by group members in ‘idle’ helpers has not been documented when the behaviour of helpers was manipulated while they remained in the territory. Instead, in a previous experiment, such treatment caused N. pulcher helpers of small groups to pre-emptively compensate for experimentally induced idleness by increasing helping and submissive behaviour [15]. Also, it has not been shown that whether dominant group members increase their aggression towards ‘idle’ helpers is dependent on group size.
Our results suggest that in large groups factors other than the breeders' demand for help [18,40,64,65] might determine aggression rates. Our results are consistent with the idea that members of large groups may be limited in their ability to reliably track the behaviours and presence of all other group members. Therefore, an ‘idle’ or absent helper may more easily remain undetected by other group members in large groups, whereas it might be possible to monitor activities of each helper in small groups. Neolamprologus pulcher breeders are able to individually recognize subordinate group members [66,67]. It may be more difficult in larger groups to remember all one's own helpers beyond a certain time of separation. Based on a theoretical model and a simulation study, similar arguments have been put forward for banded mongooses (Mungos mungo) and for humans, where either reproductive restraint or monitoring of other group members does not work in large groups [37,68]. Thus, proximate constraints may influence the mutual exchange mechanism maintaining cooperation in N. pulcher. Alternatively, large group size may erode the benefits of punishing ‘idle’ subordinates for the breeders, and hence they refrain from punishing despite having the cognitive ability to do so. Such an adaptive explanation has been suggested by models of the evolution of reciprocity and altruistic punishment [69]. Our data do not allow us to differentiate whether proximate constraints or adaptive causes explain the lack of punishment in large groups.
Remarkably, all conclusive experimental evidence for pay-to-stay results from experimental laboratory studies using small group size (e.g. group size 4 [15]; group size 3 [24]). If the pay-to-stay mechanism is limited by the ability to enforce help and to coerce subordinates also in large groups, with increasing group size the function of helping might gradually switch from pay-to-stay towards group augmentation benefits; that is, a mutualism without coercion. This idea is consistent with data suggesting high fitness pay-offs for breeders and helpers in large groups [35,36].
With the notable exception of N. pulcher [15,17–19,21,23–25], among cooperative breeders there seems to be little conclusive evidence showing that dominants use aggression to elevate the work rates of helpers [70]. This raises the question of how social and ecological conditions in N. pulcher differ from other known cooperative systems and which ecological factors enable dominant breeders to enforce help from subordinates. First, N. pulcher groups have a low average relatedness among group members [25,34] due to a frequent exchange of the dominant breeders of groups by predation, and therefore kin-selected benefits of helping are small, particularly for older helpers in their late juvenile and early adult stage. Second, outside options for helpers are strongly constrained because of an extremely high predation risk outside of groups [51]. Founding of new groups appears to be difficult, as it was observed only rarely, and small groups have a low chance of persistence [35]. Therefore, voluntary dispersal occurs only if vacancies arise due to predation in existing groups [19]. Thus, the particular relatedness structure and ecology of N. pulcher combined with the unique possibility to experimentally manipulate this system such that alternative mechanisms can be excluded may explain why the pay-to-stay mechanism has received its strongest support to date in this cichlid fish. We would like to propose that the potential for helper coercion should be studied in cooperative breeders, which share the prerequisites of low within-group relatedness and constrained outside options. Potential candidates may be found among cooperative breeding bird species such as the pied kingfisher or white-winged trumpeter, where unrelated individuals can join in alloparental care and single pairs are unable to defend a territory to raise young (for a review, see [71]).
In conclusion, we found that punishment of lazy helpers can explain why subordinates pay to stay in N. pulcher, at least in small groups. Our study shows that punishment of ‘idle’ helpers is not dependent on the physical absence of the subordinate (e.g. [13,17]). Instead, dominant individuals seem to directly evaluate the helping effort of subordinates in this species, in which help is traded for acceptance in the territory. Furthermore, our results suggest that group size may influence the regulation of conflicts between group members by changing the relative importance of breeders and helpers in penalizing ‘idle’ subordinates. We propose that preventing subordinates from helping duties without removing them from the territory should be generally adopted in studies focusing on the compensation ability of ‘idle’ helpers (e.g. [13,17,72]). Our results have important general implications for the understanding of cooperation in highly social species, where unrelated individuals help dominant breeders to raise their offspring [24,73–75].
Supplementary Material
Acknowledgements
We thank the whole Tanganyika field crews 2011 and 2012, in particular, Arne Jungwirth, Joachim G. Frommen and Mathilde Bessert-Nettelbeck, for helping us to collect the data. We are grateful to Redouan Bshary and Michael Taborsky for discussion and comments on an earlier version of the manuscript. The manuscript was greatly improved by the comments of two anonymous reviewers. Ruben Shapola and Danny Sinyinza supported this study by logistical help.
Our study adheres to the legal requirements of Zambia and Switzerland, and was conducted under the Memorandum of Understanding of 2008 between the Ministry of Agriculture and Cooperatives, Zambia, the University of Zambia, the Universities of Basel and Bern, Switzerland and the University of Graz, Austria. All experiments were conducted according to the guidelines of the Association for the Study of Animal Behaviour.
Data accessibility
Data are deposited in the Dryad digital depository [76].
Funding statement
This project was financially supported by the Swiss National Science Foundation (SNSF).
References
- 1.Bergmüller R, Johnstone RA, Russell AF, Bshary R. 2007. Integrating cooperative breeding into theoretical concepts of cooperation. Behav. Process. 76, 61–72. ( 10.1016/j.beproc.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 2.Lehmann L, Keller L. 2006. The evolution of cooperation and altruism—a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376. ( 10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- 3.Hamilton WD. 1964. The genetical evolution of social behaviour I. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 4.Hamilton WD. 1964. The genetical evolution of social behaviour II. J. Theor. Biol. 7, 17–52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 5.Woolfenden GE. 1975. Florida scrub jay helpers at nest. Auk 92, 1–15. ( 10.2307/4084414) [DOI] [Google Scholar]
- 6.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196. ( 10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JL, Brown ER. 1984. Parental facilitation: parent–offspring relations in communally breeding birds. Behav. Ecol. Sociobiol. 14, 203–209. ( 10.1007/bf00299620) [DOI] [Google Scholar]
- 8.Komdeur J. 1996. Influence of helping and breeding experience on reproductive performance in the Seychelles warbler: a translocation experiment. Behav. Ecol. 7, 326–333. ( 10.1093/beheco/7.4.417) [DOI] [Google Scholar]
- 9.Zahavi A. 1974. Communal nesting by Arabian babbler: a case of individual selection. Ibis 116, 84–87. ( 10.1111/j.1474-919X.1974.tb00225.x) [DOI] [Google Scholar]
- 10.Gaston AJ. 1978. The evolution of group territorial behavior and cooperative breeding. Am. Nat. 112, 1091–1100. ( 10.1086/283348) [DOI] [Google Scholar]
- 11.Kokko H, Johnstone RA, Wright J. 2002. The evolution of parental and alloparental effort in cooperatively breeding groups: when should helpers pay to stay? Behav. Ecol. 13, 291–300. ( 10.1093/beheco/13.3.291) [DOI] [Google Scholar]
- 12.Hamilton IM, Taborsky M. 2005. Unrelated helpers will not fully compensate for costs imposed on breeders when they pay to stay. Proc. R. Soc. B 272, 445–454. ( 10.1098/rspb.2004.2961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulder RA, Langmore NE. 1993. Dominant males punish helpers for temporary defection in superb fairy-wrens. Anim. Behav. 45, 830–833. ( 10.1006/anbe.1993.1100) [DOI] [Google Scholar]
- 14.Zöttl M, Fischer S, Taborsky M. 2013. Partial brood care compensation by female breeders in response to experimental manipulation of alloparental care. Anim. Behav. 85, 1471–1478. ( 10.1016/j.anbehav.2013.03.045) [DOI] [Google Scholar]
- 15.Bergmüller R, Taborsky M. 2005. Experimental manipulation of helping in a cooperative breeder: helpers ‘pay to stay’ by pre-emptive appeasement. Anim. Behav. 69, 19–28. ( 10.1016/j.anbehav.2004.05.009) [DOI] [Google Scholar]
- 16.Mitchell JS. 2003. Social correlates of reproductive success in false clown anemonefish: subordinate group members do not pay-to-stay. Evol. Ecol. Res. 5, 89–104. [Google Scholar]
- 17.Balshine-Earn S, Neat FC, Reid H, Taborsky M. 1998. Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438. ( 10.1093/beheco/9.5.432) [DOI] [Google Scholar]
- 18.Taborsky M. 1985. Breeder–helper conflict in a cichlid fish with broodcare helpers: an experimental analysis. Behaviour 95, 45–75. ( 10.1163/156853985x00046) [DOI] [Google Scholar]
- 19.Bergmüller R, Heg D, Taborsky M. 2005. Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc. R. Soc. B 272, 325–331. ( 10.1098/rspb.2004.2960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruintjes R, Taborsky M. 2008. Helpers in a cooperative breeder pay a high price to stay: effects of demand, helper size and sex. Anim. Behav. 75, 1843–1850. ( 10.1016/j.anbehav.2007.12.004) [DOI] [Google Scholar]
- 21.Heg D, Taborsky M. 2010. Helper response to experimentally manipulated predation risk in the cooperatively breeding cichlid Neolamprologus pulcher. PLoS ONE 5, e10784 ( 10.1371/journal.pone.0010784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLeod KJ, Nielsen JF, Clutton-Brock TH. 2013. Factors predicting the frequency, likelihood and duration of allonursing in the cooperatively breeding meerkat. Anim. Behav. 86, 1059–1067. ( 10.1016/j.anbehav.2013.09.012) [DOI] [Google Scholar]
- 23.Taborsky M. 1984. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 32, 1236–1252. ( 10.1016/s0003-3472(84)80241-9) [DOI] [Google Scholar]
- 24.Zöttl M, Heg D, Chervet N, Taborsky M. 2013. Kinship reduces alloparental care in cooperative cichlids where helpers pay-to-stay. Nat. Commun. 4, 1341 ( 10.1038/ncomms2344) [DOI] [PubMed] [Google Scholar]
- 25.Stiver KA, Dierkes P, Taborsky M, Gibbs HL, Balshine S. 2005. Relatedness and helping in fish: examining the theoretical predictions. Proc. R. Soc. B 272, 1593–1599. ( 10.1098/rspb.2005.3123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zöttl M, Frommen JG, Taborsky M. 2013. Group size adjustment to ecological demand in a cooperative breeder. Proc. R. Soc. B 280, 20122772 ( 10.1098/rspb.2012.2772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zöttl M, Chapuis L, Freiburghaus M, Taborsky M. 2013. Strategic reduction of help before dispersal in a cooperative breeder. Biol. Lett. 9, 20120878 ( 10.1098/rsbl.2012.0878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenig WD, Walters EL. 2011. Age-related provisioning behaviour in the cooperatively breeding acorn woodpecker: testing the skills and the pay-to-stay hypotheses. Anim. Behav. 82, 437–444. ( 10.1016/j.anbehav.2011.05.028) [DOI] [Google Scholar]
- 29.Reeve HK. 1992. Queen activation of lazy workers in colonies of the eusocial naked mole-rat. Nature 358, 147–149. ( 10.1038/358147a0) [DOI] [PubMed] [Google Scholar]
- 30.Cant MA. 2011. The role of threats in animal cooperation. Proc. R. Soc. B 278, 170–178. ( 10.1098/rspb.2010.1241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raihani NJ, Thornton A, Bshary R. 2012. Punishment and cooperation in nature. Trends Ecol. Evol. 27, 288–295. ( 10.1016/j.tree.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 32.Wong M, Balshine S. 2011. The evolution of cooperative breeding in the African cichlid fish, Neolamprologus pulcher. Biol. Rev. 86, 511–530. ( 10.1111/j.1469-185X.2010.00158.x) [DOI] [PubMed] [Google Scholar]
- 33.Taborsky M, Limberger D. 1981. Helpers in fish. Behav. Ecol. Sociobiol. 8, 143–145. ( 10.1007/bf00300826) [DOI] [Google Scholar]
- 34.Dierkes P, Heg D, Taborsky M, Skubic E, Achmann R. 2005. Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 8, 968–975. ( 10.1111/j.1461-0248.2005.00801.x) [DOI] [PubMed] [Google Scholar]
- 35.Heg D, Brouwer L, Bachar Z, Taborsky M. 2005. Large group size yields group stability in the cooperatively breeding cichlid Neolamprologus pulcher. Behaviour 142, 1615–1641. ( 10.1163/156853905774831891) [DOI] [Google Scholar]
- 36.Balshine S, Leach B, Neat F, Reid H, Taborsky M, Werner N. 2001. Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140. ( 10.1007/s002650100343) [DOI] [Google Scholar]
- 37.Cant MA, Hodge SJ, Bell MBV, Gilchrist JS, Nichols HJ. 2010. Reproductive control via eviction (but not the threat of eviction) in banded mongooses. Proc. R. Soc. B 277, 2219–2226. ( 10.1098/rspb.2009.2097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clutton-Brock TH, Brotherton PN, Smith R, McIlrath GM, Kansky R, Gaynor D, O'Riain MJ, Skinner JD. 1998. Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295. ( 10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulder RA. 1995. Natal and breeding dispersal in a cooperative, extra-group-mating bird. J. Avian Biol. 26, 234–240. ( 10.2307/3677324) [DOI] [Google Scholar]
- 40.Johnstone RA, Cant MA. 1999. Reproductive skew and the threat of eviction: a new perspective. Proc. R. Soc. Lond. B 266, 275–279. ( 10.1098/rspb.1999.0633) [DOI] [Google Scholar]
- 41.Bruintjes R, Bonfils D, Heg D, Taborsky M. 2011. Paternity of subordinates raises cooperative effort in cichlids. PLoS ONE 6, e25673 ( 10.1371/journal.pone.0025673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heg D, Hamilton IM. 2008. Tug-of-war over reproduction in a cooperatively breeding cichlid. Behav. Ecol. Sociobiol. 62, 1249–1257. ( 10.1007/s00265-008-0553-0) [DOI] [Google Scholar]
- 43.Heg D, Jutzeler E, Bonfils D, Mitchell JS. 2008. Group composition affects male reproductive partitioning in a cooperatively breeding cichlid. Mol. Ecol. 17, 4359–4370. ( 10.1111/j.1365-294X.2008.03920.x) [DOI] [PubMed] [Google Scholar]
- 44.Heg D, Bergmüller R, Bonfils D, Otti O, Bachar Z, Burri R, Heckel G, Taborsky M. 2006. Cichlids do not adjust reproductive skew to the availability of independent breeding options. Behav. Ecol. 17, 419–429. ( 10.1093/beheco/arj056) [DOI] [Google Scholar]
- 45.Dierkes P, Taborsky M, Kohler U. 1999. Reproductive parasitism of broodcare helpers in a cooperatively breeding fish. Behav. Ecol. 10, 510–515. ( 10.1093/beheco/10.5.510) [DOI] [Google Scholar]
- 46.Doerr ED, Doerr VAJ. 2007. Positive effects of helpers on reproductive success in the brown treecreeper and the general importance of future benefits. J. Anim. Ecol. 76, 966–976. ( 10.1111/j.1365-2656.2007.01280.x) [DOI] [PubMed] [Google Scholar]
- 47.Clutton-Brock TH, Hodge SJ, Flower TP. 2008. Group size and the suppression of subordinate reproduction in Kalahari meerkats. Anim. Behav. 76, 689–700. ( 10.1016/j.anbehav.2008.03.015) [DOI] [Google Scholar]
- 48.Field J, Cant MA. 2009. Social stability and helping in small animal societies. Phil. Trans. R. Soc. B 364, 3181–3189. ( 10.1098/rstb.2009.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duftner N, Sefc KM, Koblmüller S, Salzburger W, Taborsky M, Sturmbauer C. 2007. Parallel evolution of facial stripe patterns in the Neolamprologus brichardi/pulcher species complex endemic to Lake Tanganyika. Mol. Phylogenet. Evol. 45, 706–715. ( 10.1016/j.ympev.2007.08.001) [DOI] [PubMed] [Google Scholar]
- 50.Limberger D. 1983. Pairs and harems in a cichlid fish, Lamprologus brichardi. J. Comp. Ethol. 62, 115–144. [Google Scholar]
- 51.Heg D, Bachar Z, Brouwer L, Taborsky M. 2004. Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. Lond. B 271, 2367–2374. ( 10.1098/rspb.2004.2855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruintjes R, Taborsky M. 2011. Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387–394. ( 10.1016/j.anbehav.2010.10.004) [DOI] [Google Scholar]
- 53.Bruintjes R, Hekman R, Taborsky M. 2010. Experimental global food reduction raises resource acquisition costs of brood care helpers and reduces their helping effort. Funct. Ecol. 24, 1054–1063. ( 10.1111/j.1365-2435.2010.01715.x) [DOI] [Google Scholar]
- 54.Taborsky M. 1982. Brutpflegehelfer beim Cichliden Lamprologus brichardi, Poll (1974): Eine Kosten/Nutzen Analyse. Dissertation, University of Vienna, Austria. [Google Scholar]
- 55.Hamilton IM, Heg D, Bender N. 2005. Size differences within a dominance hierarchy influence conflict and help in a cooperatively breeding cichlid. Behaviour 142, 1591–1613. ( 10.1163/156853905774831846) [DOI] [Google Scholar]
- 56.Reddon AR, O'Connor CM, Marsh-Rollo SE, Balshine S. 2012. Effects of isotocin on social responses in a cooperatively breeding fish. Anim. Behav. 84, 753–760. ( 10.1016/j.anbehav.2012.07.021) [DOI] [Google Scholar]
- 57.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, White JS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 58.Engqvist L. 2005. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971. ( 10.1016/j.anbehav.2005.01.016) [DOI] [Google Scholar]
- 59.R Core Development Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 60.Bates D, Maechler M, Bolker B. 2013. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-2. See http://cran.r-project.org/web/packages/Ime4/index.html. [Google Scholar]
- 61.McDonald PG, Kazem AJN, Wright J. 2009. Cooperative provisioning dynamics: fathers and unrelated helpers show similar responses to manipulations of begging. Anim. Behav. 77, 369–376. ( 10.1016/j.anbehav.2008.10.009) [DOI] [Google Scholar]
- 62.Bell MBV. 2007. Cooperative begging in banded mongoose pups. Curr. Biol. 17, 717–721. ( 10.1016/j.cub.2007.03.015) [DOI] [PubMed] [Google Scholar]
- 63.Wright J, Dingemanse NJ. 1999. Parents and helpers compensate for experimental changes in the provisioning effort of others in the Arabian babbler. Anim. Behav. 58, 345–350. ( 10.1006/anbe.1999.1152) [DOI] [PubMed] [Google Scholar]
- 64.Cant MA, Field J. 2005. Helping effort in a dominance hierarchy. Behav. Ecol. 16, 708–715. ( 10.1093/beheco/ari051) [DOI] [Google Scholar]
- 65.Heinsohn R, Legge S. 1999. The cost of helping. Trends Ecol. Evol. 14, 53–57. ( 10.1016/s0169-5347(98)01545-6) [DOI] [PubMed] [Google Scholar]
- 66.Hert E. 1985. Individual recognition of helpers by the breeders in the cichlid fish Lamprologus brichardi (Poll, 1974). J. Comp. Ethol. 68, 313–325. [Google Scholar]
- 67.Balshine-Earn S, Lotem A. 1998. Individual recognition in a cooperatively breeding cichlid: evidence from video playback experiments. Behaviour 135, 369–386. ( 10.1163/156853998793066221) [DOI] [Google Scholar]
- 68.Carpenter JP. 2007. Punishing free-riders: how group size affects mutual monitoring and the provision of public goods. Game. Econ. Behav. 60, 31–51. ( 10.1016/j.geb.2006.08.011) [DOI] [Google Scholar]
- 69.Boyd R, Richerson PJ. 1988. The evolution of reciprocity in sizable groups. J. Theor. Biol. 132, 337–356. ( 10.1016/S0022-5193(88)80219-4) [DOI] [PubMed] [Google Scholar]
- 70.Santema P, Clutton-Brock TH. 2012. Dominant female meerkats do not use aggression to elevate work rates of helpers in response to increased brood demand. Anim. Behav. 83, 827–832. ( 10.1016/j.anbehav.2011.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baglione V, Canestrari D, Chiarati E, Vera R, Marcos JM. 2010. Lazy group members are substitute helpers in carrion crows. Proc. R. Soc. B 277, 3275–3282. ( 10.1098/rspb.2010.0745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clutton-Brock TH, Brotherton PN, O'Riain MJ, Griffin AS, Gaynor D, Sharpe L, Kansky R, Manser MB, McIlrath GM. 2000. Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc. R. Soc. Lond. B 267, 301–305. ( 10.1098/rspb.2000.1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright J, McDonald PG, te Marvelde L, Kazem AJ, Bishop CM. 2010. Helping effort increases with relatedness in bell miners, but ‘unrelated’ helpers of both sexes still provide substantial care. Proc. R. Soc. B 277, 437–445. ( 10.1098/rspb.2009.1360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clutton-Brock TH. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57. ( 10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 76.Fischer S, Zöttl M, Groenewoud F, Taborsky B. 2014. Group-size dependent punishment of idle subordinates in a cooperative breeder where helpers pay to stay. Dryad Digital Repository ( 10.5061/dryad.s3720) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited in the Dryad digital depository [76].