Abstract
Insect wings are great resources for studying morphological diversities in nature as well as in fossil records. Among them, variation in wing venation is one of the most characteristic features of insect species. Venation is therefore, undeniably a key factor of species-specific functional traits of the wings; however, the mechanism underlying wing vein formation among insects largely remains unexplored. Our knowledge of the genetic basis of wing development is solely restricted to Drosophila melanogaster. A critical step in wing vein development in Drosophila is the activation of the decapentaplegic (Dpp)/bone morphogenetic protein (BMP) signalling pathway during pupal stages. A key mechanism is the directional transport of Dpp from the longitudinal veins into the posterior crossvein by BMP-binding proteins, resulting in redistribution of Dpp that reflects wing vein patterns. Recent works on the sawfly Athalia rosae, of the order Hymenoptera, also suggested that the Dpp transport system is required to specify fore- and hindwing vein patterns. Given that Dpp redistribution via transport is likely to be a key mechanism for establishing wing vein patterns, this raises the interesting possibility that distinct wing vein patterns are generated, based on where Dpp is transported. Experimental evidence in Drosophila suggests that the direction of Dpp transport is regulated by prepatterned positional information. These observations lead to the postulation that Dpp generates diversified insect wing vein patterns through species-specific positional information of its directional transport. Extension of these observations in some winged insects will provide further insights into the mechanisms underlying diversified wing venation among insects.
Keywords: Drosophila, sawfly, Athalia rosae, bone morphogenetic protein, facilitated transport
1. Introduction: morphological diversities of insects' wing venation
Insects are the most successful and diversified animal group, counting more than two million species. They invade every conceivable ecological niche and dominate on land. Acquisition of flight enabled insects to exploit new habitats and escape from unfavourable environments. It is accepted that wings arose once in the insect lineage, as evidenced by the winged group (Pterygota) being monophyletic [1–5]. The winged insects consist of Paleoptera (the basal winged groups, Ephemeroptera and Odonata) and Neoptera. The latter is divided into Polyneoptera (e.g. Orthoptera, Blattodea), Paraneoptera (e.g. Hemiptera, Thysanoptera) and Oligoneoptera (=Holometabola). Wings are repeatedly lost and gained during insect evolution, while their fundamental characteristics, formed of two epidermal layers, sclerotized, supported by veins and articulated at meso- and metathorax, are conserved on their reappearance. This leads to the concept that the key set of genes governing wing development (the wing gene network) are conserved, and turning on and off these genes by alteration of the regulatory pathway resulted in the presence and absence of wings [6,7]. Many molecular investigations of wing development therefore aim to elucidate the origin of wings: what structure is the serial homologue of the wing? How are wings formed? What are the underlying mechanisms regulating the wing gene network? These concepts have been substantiated in a dipteran Drosophila melanogaster and a lepidopteran butterfly, Precis coenia [8,9]. Moreover, recent gene functional analyses of beetles (Coleoptera) and the milkweed bug (Hemiptera) demonstrated that serially homologous structures of wings in thoracic and abdominal segments are promoted or inhibited to differentiate into wings depending on the regulation of Hox genes and the wing gene network [10–14]. This scenario might be applied to basal winged species, the mayfly Ephoron eophilum (Ephemeroptera) and even to non-winged species, the bristletails Pedetontus unimaculatus (Apterygota, Archaeognatha) [15].
On the other hand, insect wings are subjected to considerable variations in shape, size, marking and vein patterns reflecting their specific functional differences. Venation patterns are the most characteristic structures of wings because of aerodynamic importance associated with specific flight system and of specialized functional purposes, and there are marked differences between forewings and hindwings in a species [4,16,17] (figure 1). Venation patterns are used as an index for the identification of species and for understanding the evolutionary relationships of groups [19,20]. Venation is therefore one of the key traits when considering how diversified morphology is acquired in the insect lineage.
Figure 1.
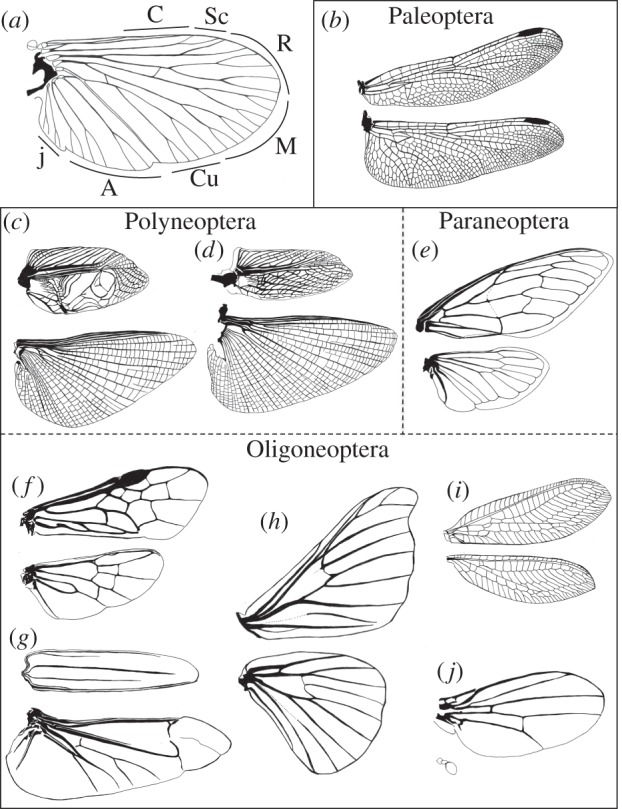
Venation patterns of winged insects (Pterygote). (a) Schematic of the basic vein system shared by winged insects. The nomenclature is based on the Comstock and Needham system with modifications (modified from [18] with permission by John Wiley & Sons Ltd). C, costa; Sc, subcosta; R, radius; M, media; Cu, cubitus; A, annal; j, jugal veins. (b–j) Forewings (top) and hindwings (bottom) with venation patterns of the species representing each group. (b) Sympetrum infuscatum (Odonata), (c) Gryllus bimaculatus (Orthoptera) male and (d) female, (e) Cryptotympana facialis (Hemiptera), (f) Athalia rosae (Hymenoptera), (g) Massicus raddei (Coleoptera), (h) Bombyx mori (Lepidoptera), (i) Chrysoperla carnea (Neuroptera), (j) D. melanogaster (Diptera).
Insect wing veins are composed of longitudinal veins and crossveins. The longitudinal veins are heavily sclerotized, providing conduits for nerves, the tracheae and circulating haemolymph, running alternately on the crests (convexes) or in the troughs (concaves) of wings. Their patterns are basically shared among extant winged insects and named after the Comstock and Needham system with some modifications based on comparative studies of extinct and extant insects [1,16,21–23]. The principal six longitudinal veins are costa, subcosta, radius, media, cubitus and annal from anterior to posterior of the wing (figure 1a). The numbers of these longitudinal veins often increase by branching or decrease by fusion and loss, although the basic pattern of longitudinal veins is consistent within groups such as orders and families. By contrast, the patterns of crossveins are more variable than longitudinal veins, with species characteristics, and are used as landmarks in morphometric analysis [24,25]. The crossveins are generally devoid of a trachea and connect the longitudinal veins to act together as rigid structural supports for the two-layered wing membranes. The primitive winged groups generally have a large number of crossveins, whereas the numbers of crossveins tend to be reduced in more derived groups. An example of rich crossveins is the wings of dragonflies (Odonata) (figure 1b). Irregular networks of crossveins, termed archedictyon, provide strong support for their power flight. One of the most derived groups, the cyclorrhaphan Diptera represented by D. melanogaster, generally has two crossveins (figure 1j): the anterior (radial-medial) crossvein (ACV) and the posterior (medial-cubital) crossvein (PCV), and the PCV is lost in some species group [26,27].
Wings with specialized function recruit unique venation systems. Some orthopteran species have sound-producing forewings, and the arrangement of veins bordering the specialized areas to amplify sounds, termed mirror and harp, are specifically modified (figure 1c,d) [28]. The forewings of Coleoptera transform to elytra for protection of the hindwings and abdomen, and crossveins are generally unrecognized (figure 1g); however, the net-winged beetles retain elytra veins composed of straight longitudinal veins and connecting crossveins to form a characteristic lattice-work appearance [29,30].
The molecular mechanisms of wing venation have been investigated intensively and solely in D. melanogaster [27,31–34]. Formation of the vein patterns is unlikely to occur merely by turning on or off the gene regulatory networks. The long-range signalling of secreted growth factors is critical for wing vein patterning [32]. Recent findings have advanced the understanding of how the directional transport of signalling molecules contributes to wing vein patterns in Drosophila [35,36] and imply that the mechanism is used for establishing complicated wing venation in other insects [37].
2. Lessons from wing vein development in Drosophila
(a). Wing vein development in larval wing imaginal disc
The development of the Drosophila wing is a classic model for understanding the genetic control of tissue size, shape and patterning. The pattern of Drosophila wing veins is relatively simple when compared with other insects, consisting of four main longitudinal veins (L2–L5) and two crossveins (figure 1j) [26,32]. In Drosophila, the development of the wing during larval stages occurs inside the body in an epithelial monolayer called the wing imaginal disc. Systematic studies of wing phenotypes led to a model of vein patterning involving the sequential action of the transcriptional readouts of hedgehog (Hh), decapentaplegic (Dpp)/bone morphogenetic protein (BMP), epidermal growth factor (EGF) and Notch. The first subdivisions are made with reference to compartment boundaries of the signalling molecules Hh and Dpp/BMP in the wing imaginal disc. During the third-instar larval stages, Hh signal is detected at the anterior part of the anterior/posterior boundary in a short-range manner. Positional information about L3 and L4 is provided by Hh signalling [38]. Dpp is expressed between L3 and L4 in response to Hh signalling in the wing imaginal disc and Dpp/BMP forms a long-range morphogen gradient that organizes both patterning and growth of the wing imaginal discs [27,39]. Approaches of functional green fluorescent protein (GFP)-tagged Dpp visualized in the wing imaginal discs provide direct evidence that Dpp/BMP ligands move in the extracellular space to form a gradient of ligand distribution [40,41]. Long-range Dpp/BMP signalling provides positional information about L2 and L5 (figure 2a) [38,42]. Following the subdivision of the wing blade of the imaginal disc into pro-veins and interveins, wing vein development is characterized by the deployment of Notch and EGF signalling pathways at the borders and centre, respectively, of each pro-vein. The activities of these pathways are closely related, and they contribute to restrict (Notch) and maintain (EGF) the vein fate.
Figure 2.
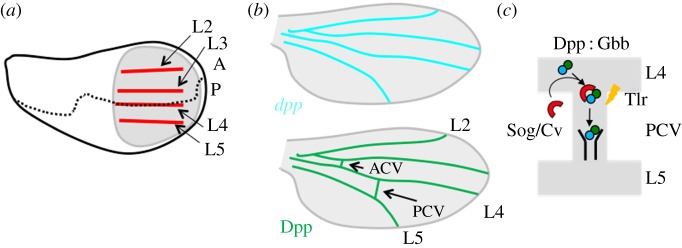
Wing vein development in Drosophila. (a) Schematic diagram of wing vein development of the third-instar wing imaginal disc. Positions of longitudinal veins (L2–L5, red) are identified as pro-veins. Wing pouch is circled (grey). Anterior (A)–posterior (P) boundary (dashed line). (b) Schematic diagram of wing vein development in the pupal wing. Top: dpp mRNA (light blue) is expressed in longitudinal veins but not in crossveins during early pupal stages. Bottom: Dpp/BMP distribution (green) is detected at all wing vein primorida including longitudinal veins and crossveins. L2–L5, ACV and PCV denote longitudinal veins 2–5, anterior and posterior crossveins, respectively. (c) Schematic model of facilitated transport of Dpp/BMP ligands from the longitudinal veins into PCV. A Dpp : Gbb heterodimer (green, blue) forms a complex with transporter BMP-binding proteins (red) such as Sog and Cv and moves by facilitated transport mechanism. A Dpp : Gbb heterodimer is released from the ligand–transporter complex by Tlr (yellow) and binds to the receptor for signalling.
(b). Wing vein development in the pupal wing
The next stage in vein formation occurs in the pupal stage. During the prepupal period, the folded monolayered epithelium of the wing disc is converted into a bilayered wing. Around 18 h after pupariation (AP), dorsal and ventral epithelial cells become closely appose by extending from the basal surfaces of intervein cells. The wing veins emerge progressively as regions that do not extend basal processes and remain as open spaces [43,44]. The critical aspect of this step is the activation of dpp expression in each vein primordia, because signalling mediated by Dpp/BMP is sufficient and necessary for vein differentiation [45]. Unlike larval wing disc development, Dpp/BMP functions as a wing vein determinant. During 18–26 h AP, dpp is only expressed in the longitudinal veins; however, Dpp/BMP signalling is detected in all wing vein cells (figure 2b). These findings suggest that long-range Dpp/BMP signalling is required for crossvein development [46]. Visualizing functional GFP-Dpp in the pupal wing has demonstrated that GFP-Dpp is moved from longitudinal veins into PCVs. In contrast to signalling towards PCVs, the majority of ligands appear to be immobilized in the longitudinal veins of the pupal wing to maintain short-range signalling [36]. Thus, both short-range signalling in longitudinal veins and long-range signalling towards crossveins are required for wing vein formation. How are short-range signalling in longitudinal veins and long-range signalling towards PCVs regulated in the pupal wing? The mobility of BMP ligands is tightly restricted in longitudinal veins by BMP type I receptor Thickveins (Tkv) and a positive feedback mechanism to maintain short-range signalling. This mechanism is designated as ‘active retention of ligands’ and is critical for maintaining vein width [36].
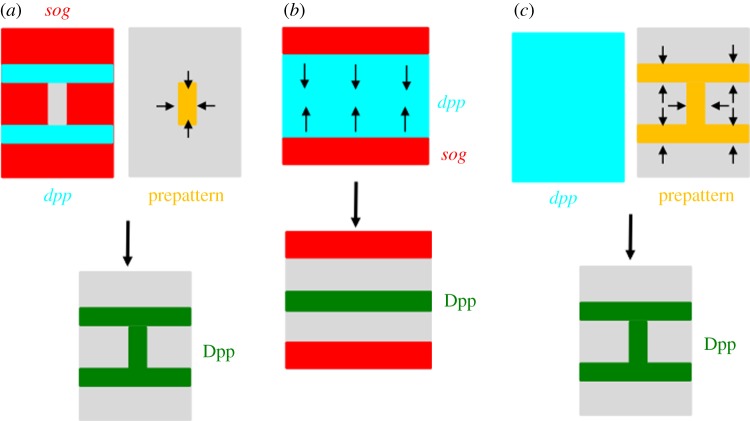
The existence of a class of genes dedicated to the formation of crossveins in Drosophila implies a clear developmental separation between crossveins and longitudinal veins and allows for independent variations in these structures during evolution. For example, mutations found at the crossveinless (cv) locus lead to a complete loss of both ACV and PCV while longitudinal veins remain unaffected [47,48]. The cv gene has been shown to encode a secreted BMP-binding protein [49,50]. Furthermore, several mutants encoding secreted proteins have been identified as components that are required for crossvein development. These include two BMP-type ligands, Dpp and Glass bottom boat (Gbb); a protease, Tolloid-related (Tlr) and two BMP-binding proteins, Short gastrulation (Sog) and Cv [49–52]. Further studies showed that PCV formation needs a facilitated transport mechanism of Dpp/BMP ligands, in which Dpp : Gbb heterodimer produced in the longitudinal veins is moved into the PCV region by a Sog/Cv complex, and Dpp and Gbb are probably freed from the Sog/Cv complex via the activity of Tlr to bind to the receptors for signalling (figure 2c).
Furthermore, another extracellular protein, Crossveinless-2 (Cv-2), is required for PCV development to sustain short-range Dpp/BMP signalling [53,54]. The cv-2 expression in the PCV region is regulated by Dpp/BMP signalling and Cv-2 is a part of the positive feedback mechanism that plays a critical role in PCV formation. Likewise, several mutant alleles have been shown to be required for PCV formation. The detached (det) encoding Drosophila Dystrophin is required for maintaining Dpp/BMP signalling in the PCV region [55]. crossveinless-d (cv-d) encodes a vitellogenin-like lipoprotein. Cv-D is secreted from haemocytes and regulates Dpp/BMP signalling in the PCV region by modulating Dpp/BMP movement as part of a lipid–BMP–lipoprotein complex [56].
(c). Positional information of crossvein development
Assuming that Dpp/BMP ligand transport is primarily responsible for developing crossveins, the direction of ligand transport appears to be critical to where crossveins are being formed. Thus, understanding how positional information about directional transport is established appears to be important for understanding the diversity of crossveins. Although our knowledge about the positional information of directional transport is far from complete, recent studies demonstrated two experimental evidences. First, studies of the sog expression provide evidence that the direction of ligand transport is at least partially manifested by sog transcription [36,46] (figure 3a). During 18–20 h AP, sog expression is repressed in the future PCV region in a Dpp/BMP signalling-independent manner and, thereafter, sog expression in the PCV region is downregulated in a Dpp/BMP signalling-dependent manner. Thus, initial sog transcription reflects prepatterned positional information about Dpp/BMP transport. Second, it has been shown that PCV morphogenesis highlighted by lumen formation and loss of β-integrin accumulation on the basal side occurs prior to the induction of Dpp/BMP signalling in the PCV region during 18–20 h AP. Thereafter, Dpp/BMP signalling is required for maintaining wing vein morphogenesis [35]. PCV morphogenesis and Dpp/BMP signalling towards the PCV region are coordinated through the signalling pathway of the Rho-type GTPases. One of the crossveinless mutant alleles, crossveinless-c (cv-c), encodes Rho GTPase-activating protein (RhoGAP) [57] and Cv-C appears to be the key molecule coupling Dpp/BMP transport and wing vein morphogenesis. Cv-C is induced at PCV primordial cells by Dpp/BMP signalling and mediates PCV morphogenesis cell-autonomously by inactivating Rho-type small GTPases, including Cdc42, Rac1, Rac2 and Rho1. Cv-C is also required non-cell-autonomously for Dpp/BMP transport into the PCV region. The cellular distribution of integrins appears to be the key for regulation of extracellular Dpp/BMP movement, because β-integrin distribution is downregulated on the basal side of PCV epithelial cells by Cv-C and provides an optimal extracellular environment for guiding Dpp/BMP transport. Dpp/BMP ligands then preferentially accumulate on the basal side of the PCV region. These findings indicate the presence of a feed-forward loop that coordinates Dpp transport and PCV morphogenesis. These mechanisms not only play a significant role in achieving precise tissue morphogenesis and tissue homoeostasis, but also may be used for producing variations of crossveins. In fact, ectopic crossvein formations are often observed in weak mutant alleles of Cdc42 [35,58]. In this case, loss of Cdc42 causes ectopic Dpp/BMP transport in the intervein regions during early pupal stages, resulting in ectopic wing vein formation [35]. Thus, modulation of Rho-type GTPases has an instructive role in producing variations of crossveins.
Figure 3.
Schematic diagram of directional transport of Dpp/BMP ligands. (a) Top left: complementary expressions of dpp (light blue) and sog (red). Top right: prepatterned information (orange) that instructs the direction of Dpp transport. Bottom: Dpp proteins move to the prepatterned position (green), e.g. PCV in Drosophila. (b) Top: complementary expression of dpp (light blue) and sog (red). Sog diffusion instructs the direction of Dpp movement. Bottom: Dpp is redistributed to the midline (green), e.g. early embryo in Drosophila. (c) Top left: ubiquitous expression of dpp mRNA (light blue). Top right: prepatterned information (orange) that instructs the direction of Dpp transport. Bottom: Dpp is redistributed to the prepatterned position (green), e.g. wing vein formation in the sawfly.
3. Transport mechanism of decapentaplegic/bone morphogenetic protein ligand in a developing field
Directional transport of Dpp/BMP ligands by BMP-binding proteins widely operates during early embryogenesis in various species. In Drosophila, Dpp/BMP is known to function as a morphogen for dorsal–ventral patterning of the early embryo. Previous genetic analysis has revealed that seven zygotic genes are required for proper dorsal cell fate determination in Drosophila embryos [59]. Among them, five genes encode secreted proteins including two BMP-type ligands, Dpp and Screw (Scw); one protease, Tolloid (Tld) and two BMP-binding proteins, Sog and Twisted gastrulation (Tsg). Intriguingly, a gradient of Dpp/BMP signalling is achieved by a similar mechanism to directional transport of Dpp into the PCV region of the pupal wing. Dpp : Scw heterodimer, which is a primary ligand of morphogen, is moved by a Sog/Tsg complex from lateral to the dorsal midline. Tld cleaves and inactivates Sog in a Dpp : Scw-dependent manner to allow ligands to bind to the receptors for signalling [60]. The reverse gradient formed by Sog appears to provide directionality of Dpp/BMP transport (figure 3).
The regulation of Dpp/BMP signalling by BMP-binding proteins is widely observed in the animal kingdom. Animals ranging from Cnidaria to all phyla of Bilateria have highly conserved signalling pathways connecting Dpp/BMP and Sog (or Chordin) for dorsoventral axis formation in early embryos [61–67]. In fact, similar transport mechanisms of Dpp/BMP ligands have been reported in Xenopus and the beetle Tribolium castaneum (Coleoptera) embryos [64,65]. These findings suggest that the transport mechanism of Dpp/BMP ligands by transporter (BMP-binding proteins) is evolutionarily conserved. Assuming similarities of transport mechanism between early embryogenesis and PCV formation, we hypothesize that gene sets involved in the transport mechanism have been co-opted and contribute to crossvein formation in Drosophila. It remains interesting to address how the Dpp/BMP transport mechanism is used for wing venation among insects.
4. Wing vein development in the hymenopteran sawfly
It has been unexplored to what extent the Dpp/BMP transport mechanism is conserved among insects and whether the mechanisms described in Drosophila could be applied to other insects. Functional analysis of factors involved in the Dpp/BMP transport mechanism revealed that the mechanism is used and required to establish wing venation in the sawfly, Athalia rosae (Hymenoptera) [37]. Sawflies are primitive members of Hymenoptera that is placed in the most basal group of Holometabola [5,68]. The sawfly has complicated vein patterns (figure 1f), as do other species in the order [69]. The wings develop from wing primordia, as in many holometabolous species that evaginate in a very early prepupal stage. The longitudinal veins are first formed similarly in the forewing and hindwing, and then the distinctive patterns of crossveins in the forewing and hindwing are established [37].
Dpp/BMP signalling plays a critical role in sawfly venation, as demonstrated that the accumulation of phosphorylated Mother against dpp (pMad), a transcription factor that mediates Dpp/BMP signalling, is detected in future vein regions, and knockdown of the sawfly dpp gene transcript results in inhibition of localized accumulation of pMad, leading to disruption of vein formation. Intriguingly, the sawfly dpp gene is expressed ubiquitously in the prepupal wings, unlike the expression pattern of Drosophila dpp, which is restricted to longitudinal veins. The sawfly Tsg/Cv, a homologue of BMP-binding protein, is capable of binding Dpp in vivo and in vitro and functions as the transporter of Dpp. The sawfly Tsg/Cv is required for formation of both longitudinal veins and crossveins as loss of the tsg/cv transcript affects all wing veins. In the case of the sawfly, ubiquitous Dpp is probably redistributed by the Tsg/Cv transporter to localize Dpp/BMP ligand precisely in future vein regions (both longitudinal veins and crossveins) to establish complicated venation patterns (figures 3c and 4). These data challenge the view that the patterns of longitudinal veins are defined primarily by genes that are targets of morphogen gradients. Rather, the transport mechanism of BMP ligands may play a primary role in wing vein patterning in general in insects. Other cofactors involved in Dpp transport; e.g. Sog, Tld, remain to be identified in sawfly. Functional analysis and expression of these components in wing development would promote our knowledge for the mechanism involved. Involvement of the transport mechanism in specifying wing venation in basal (Hymenoptera) and derived (Diptera) species also suggests that the mechanism is used among Holometabola at least.
Figure 4.
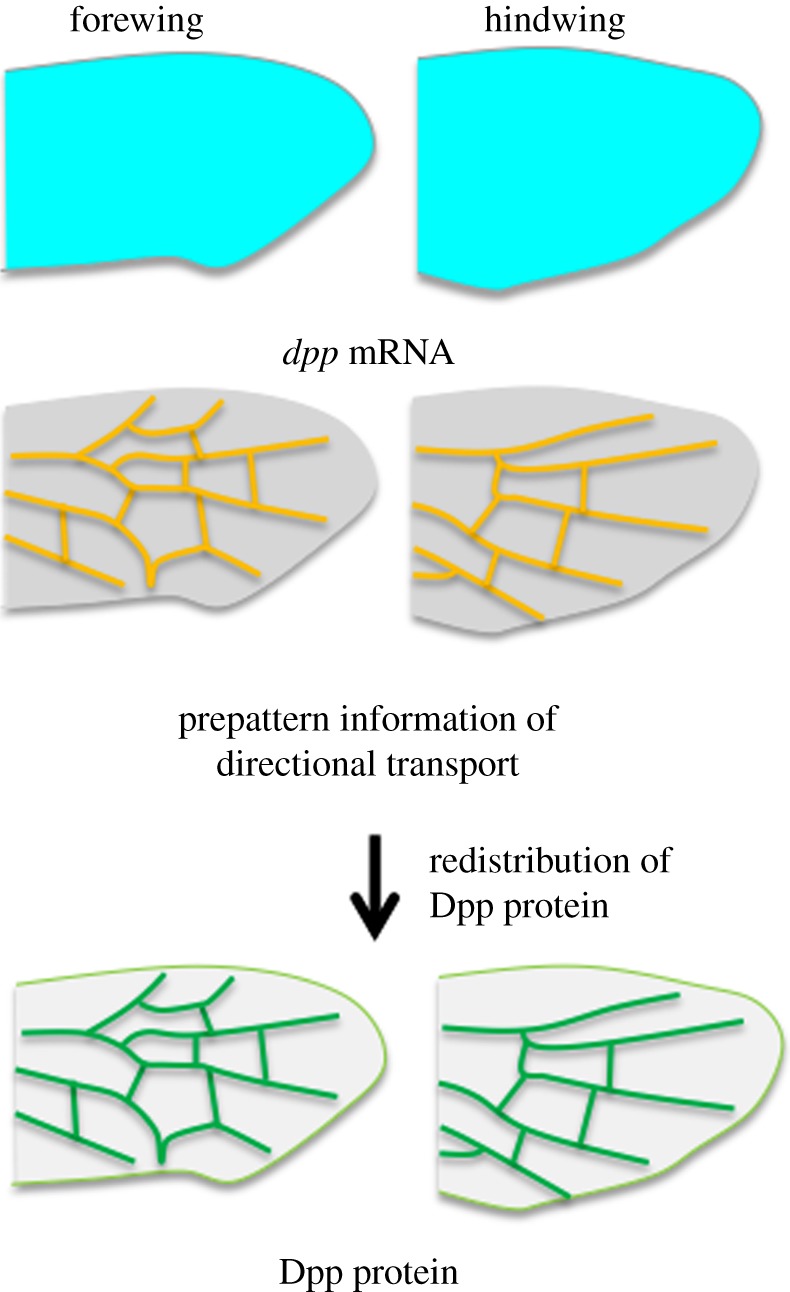
The Dpp transport mechanism is key for sawfly vein development. Dpp produced by ubiquitously expressed transcripts in both fore- and hindwing (top) is moved by the facilitated transport directed by the prepattern information (middle) and redistributed to the positions that reflect distinctive adult fore- and hindwing vein patterns (bottom).
5. Insights into diversified wing venation among insects
Evolutionarily conserved signalling pathways are repeatedly used in a variety of developmental contexts as genetic toolkits. Nevertheless, the shape, size and pattern of homologous organs vary even among closely related species. How conserved signalling systems give rise to diversified morphologies is a fundamental question in biology. Insect wings that arose once in the insect lineage and diversified markedly to acquire specific functions, are excellent resources for studying diversified morphogenesis. Although the molecular mechanisms of wing vein development have been relatively well studied in Drosophila, it has been argued that development of Drosophila is highly derived and different from that of other insects in many cases. In many holometabolans, wing development is initiated by evagination of the primordia in the last larval stage or later [70]. Therefore, conditions in Drosophila development do not always represent characteristics of insects and how the mechanisms found in Drosophila are widely used for wing vein patterning among insects remains to be addressed. Recent observations in Drosophila and the sawfly Athalia provide novel insights into evolutionary conserved mechanisms underlying diversified wing venation among insects. First, Dpp/BMP signalling appears to have a conserved role in wing vein development among insects. Dpp/BMP signal reflects wing vein patterns in both Drosophila and the sawfly during pupal stages. Functional analysis of dpp suggests that Dpp/BMP signalling is required for wing venation not only in Drosophila but also in the sawfly [37,45]. These experimental data suggest that Dpp/BMP signalling may have a conserved instructive role in wing vein development among insects. Second, transcriptional regulation of dpp expression during wing vein development may not provide spatial information about wing vein patterns. In Drosophila, dpp expression is tightly restricted to the primordia of the longitudinal veins during early pupal stages. A cis-regulatory domain called the shortvein (shv) region is responsible for dpp expression in the pupal wing [71,72]. The dpp expression provides spatial information about longitudinal veins in Drosophila. By contrast, dpp is ubiquitously expressed in the sawfly wing; thus, transcription of dpp for wing venation is regulated temporally but not spatially [37]. In coleopteran T. castaneum, dpp is expressed only at the distal tips of the hindwing, which shows relatively simple vein patterns [10]. These findings suggest that transcription of dpp does not always provide spatial information about wing venation even though the Dpp/BMP signal may play a conserved role in wing vein development among insects. How the cis-regulatory domain in the dpp has been changed and its spatial information has been acquired in wing vein development during evolution remain to be addressed. As Dpp/BMP is a key player in many developing tissues, repeated changes in dpp expression seem more likely to interfere with existing Dpp/BMP functions and lead to severe developmental defects.
Therefore, the option of altering Dpp/BMP distribution appears to be less intrusive to adapt to changes in the environment. Although we have to wait for functional analysis in some winged insects, directional transport of Dpp/BMP by BMP-binding proteins may be primarily responsible for redistributing ligands. What is the key mechanism for producing diverse wing vein patterns? Experimental evidence in Drosophila suggests that prepatterned information instructing the direction of ligand transport is crucial for the position of wing veins through altering Dpp/BMP distribution. Although our understanding of prepattern factors is far from complete, spatial control of prepattern factors might be a tool to modify the distribution of signalling molecules. As the deep homology of the Dpp/BMP transport mechanism has been proposed during embryogenesis, the gene sets of the transport mechanism might have been co-opted to wing vein development in primary insects.
To explain how diversity among species is produced by conserved systems, it has been proposed that changes in the gene regulatory network are a major source of novelty [73]. By contrast, less is known about how post-transcriptional mechanisms bring about morphological variations. Further studies of winged insects will provide further insights into how post-transcriptional regulation of secreted growth factors provides the mechanism by which a conserved signalling pathway can generate morphological diversity.
Acknowledgements
We thank T. Yokoyama for illustrations.
Funding statement
S.M. was supported by the Viikki Graduate School in Biosciences. O.S. was supported by grants from the Academy of Finland, the University of Helsinki and the Sigrid Juselius Foundation.
References
- 1.Kukalova-Peck J. 1978. Origin and evolution of insect wings and their relation to metamorphosis, as documented by fossil record. J. Morphol. 156, 53–125. ( 10.1002/jmor.1051560104) [DOI] [PubMed] [Google Scholar]
- 2.Hennig W. 1981. Insect phylogeny. New York, NY: John Wiley and Sons. [Google Scholar]
- 3.Hovmöller R, Pape T, Källersjö M. 2002. The palaeoptera problem: basal pterygote phylogeny inferred from 18S and 28S rRNA sequences. Cladistics 18, 313–323. ( 10.1111/j.1096-0031.2002.tb00153.x) [DOI] [PubMed] [Google Scholar]
- 4.Grimaldi D, Engel MS. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Trautwein MD, Wiegmann BM, Beutel R, Kjer KM, Yeates DK. 2012. Advances in insect phylogeny at the dawn of the postgenomic era. Annu. Rev. Entomol. 57, 449–468. ( 10.1146/annurev-ento-120710-100538) [DOI] [PubMed] [Google Scholar]
- 6.Carroll SB. 2008. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36. ( 10.1016/j.cell.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 7.Carroll SB, Weatherbee SD, Langeland JA. 1995. Homeotic genes and the regulation and evolution of insect wing number. Nature 375, 58–61. ( 10.1038/375058a0) [DOI] [PubMed] [Google Scholar]
- 8.Weatherbee SD, Halder G, Kim J, Hudson A, Carroll S. 1998. Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere. Genes Dev. 12, 1474–1482. ( 10.1101/gad.12.10.1474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weatherbee SD, Nijhout HF, Grunert LW, Halder G, Galant R, Selegue J, Carroll S. 1999. Ultrabithorax function in butterfly wings and the evolution of insect wing patterns. Curr. Biol. 9, 109–115. ( 10.1016/S0960-9822(99)80064-5) [DOI] [PubMed] [Google Scholar]
- 10.Tomoyasu Y, Arakane Y, Kramer KJ, Denell RE. 2009. Repeated co-options of exoskeleton formation during wing-to-elytron evolution in beetles. Curr. Biol. 19, 2057–2065. ( 10.1016/j.cub.2009.11.014) [DOI] [PubMed] [Google Scholar]
- 11.Tomoyasu Y, Wheeler SR, Denell RE. 2005. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433, 643–647. ( 10.1038/nature03272) [DOI] [PubMed] [Google Scholar]
- 12.Chesebro J, Hrycaj S, Mahfooz N, Popadic A. 2009. Diverging functions of Scr between embryonic and post-embryonic development in a hemimetabolous insect, Oncopeltus fasciatus. Dev. Biol. 329, 142–151. ( 10.1016/j.ydbio.2009.01.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohde T, Yaginuma T, Niimi T. 2013. Insect morphological diversification through the modification of wing serial homologs. Science 340, 495–498. ( 10.1126/science.1234219) [DOI] [PubMed] [Google Scholar]
- 14.Clark-Hachtel CM, Linz DM, Tomoyasu Y. 2013. Insights into insect wing origin provided by functional analysis of vestigial in the red flour beetle, Tribolium castaneum. Proc. Natl Acad. Sci. USA 110, 16 951–16 956. ( 10.1073/pnas.1304332110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa N, Akimoto-Kato A, Niimi T, Tojo K, Machida R, Hayashi S. 2010. Evolutionary origin of the insect wing via integration of two developmental modules. Evol. Dev. 12, 168–176. ( 10.1111/j.1525-142X.2010.00402.x) [DOI] [PubMed] [Google Scholar]
- 16.Wootton RJ. 1992. Functional morphology of insect wings. Annu. Rev. Entomol. 37, 113–140. ( 10.1146/annurev.en.37.010192.000553) [DOI] [Google Scholar]
- 17.Wootton RJ, Herbert RC, Young PG, Evans KE. 2003. Approaches to the structural modelling of insect wings. Phil. Trans. R. Soc. Lond. B 358, 1577–1587. ( 10.1098/rstb.2003.1351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gullan PJ, Cranston PS. 2010. The insects: an outline of entomology, 4th edn Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 19.Nel A, Prokop J, Nel P, Grandcolas P, Huang DY, Roques P, Guilbert E, Dostal O, Szwedo J. 2012. Traits and evolution of wing venation pattern in paraneopteran insects. J. Morphol. 273, 480–506. ( 10.1002/jmor.11036) [DOI] [PubMed] [Google Scholar]
- 20.Engel MS, Davis SR, Prokop J. 2013. Insect wings: the evolutionary development of nature's first flyers. In Arthropod biology and evolution (eds Minelli A, Boxshall G, Fusco G.), pp. 269–298. Heidelberg, Germany: Springer. [Google Scholar]
- 21.Comstock JH, Needham JG. 1898. The wings of insects. Am. Nat. 32, 43–48, 81–89, 231–257, 335–340, 413–424, 769–777, 903–911. [Google Scholar]
- 22.Wootton RJ. 1979. Function, homology, and terminology in insect wings. Syst. Entomol. 4, 81–83. ( 10.1111/j.1365-3113.1979.tb00614.x) [DOI] [Google Scholar]
- 23.Comstock JH, Needham JG. 1899. The wings of insects. Am. Nat. 33, 117–126, 573–582, 845–860. [Google Scholar]
- 24.Daly HV. 1985. Insect morphometrics. Anuu. Rev. Entomol. 30, 415–438. ( 10.1146/annurev.en.30.010185.002215) [DOI] [Google Scholar]
- 25.Klingenberg CP. 2002. Morphometrics and the role of the phenotype in studies of the evolution of developmental mechanisms. Gene 287, 3–10. ( 10.1016/S0378-1119(01)00867-8) [DOI] [PubMed] [Google Scholar]
- 26.Stark J, Bonacum J, Remsen J, DeSalle R. 1999. The evolution and development of dipteran wing veins: a systematic approach. Annu. Rev. Entomol. 44, 97–129. ( 10.1146/annurev.ento.44.1.97) [DOI] [PubMed] [Google Scholar]
- 27.De Celis JF. 2003. Pattern formation in the Drosophila wing: the development of the veins. BioEssays 25, 443–451. ( 10.1002/bies.10258) [DOI] [PubMed] [Google Scholar]
- 28.Desutter-Grandcolas L. 1995. Functional forewing morphology and stridulation in crickets (Orthoptera, Grylloidae). J. Zool. 236, 243–252. ( 10.1111/j.1469-7998.1995.tb04491.x) [DOI] [Google Scholar]
- 29.Crowson RA. 1981. The biology of the Coleoptera. London, UK: Academic Press. [Google Scholar]
- 30.Lawrence JF. 1982. Evolution and classification of beetles. Annu. Rev. Ecol. Syst. 13, 261–290. ( 10.1146/annurev.es.13.110182.001401) [DOI] [Google Scholar]
- 31.De Celis JF, Diaz-Benjumea FJ. 2003. Developmental basis for vein pattern variations in insect wings. Int. J. Dev. Biol. 47, 653–663. [PubMed] [Google Scholar]
- 32.Blair SS. 2007. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 23, 293–319. ( 10.1146/annurev.cellbio.23.090506.123606) [DOI] [PubMed] [Google Scholar]
- 33.O'Connor MB, Umulis D, Othmer HG, Blair SS. 2006. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133, 183–193. ( 10.1242/dev.02214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crozatier M, Glise B, Vincent A. 2004. Patterns in evolution: veins of the Drosophila wing. Trends Genet. 20, 498–505. ( 10.1016/j.tig.2004.07.013) [DOI] [PubMed] [Google Scholar]
- 35.Matsuda S, Blanco J, Shimmi O. 2013. A feed-forward loop coupling extracellular BMP transport and morphogenesis in Drosophila wing. PLoS Genet. 9, e1003403 ( 10.1371/journal.pgen.1003403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuda S, Shimmi O. 2012. Directional transport and active retention of Dpp/BMP create wing vein patterns in Drosophila. Dev. Biol. 366, 153–162. ( 10.1016/j.ydbio.2012.04.009) [DOI] [PubMed] [Google Scholar]
- 37.Matsuda S, Yoshiyama N, Kunnapuu-Vulli J, Hatakeyama M, Shimmi O. 2013. Dpp/BMP transport mechanism is required for wing venation in the sawfly Athalia rosae. Insect Biochem. Mol. Biol. 43, 466–473. ( 10.1016/j.ibmb.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 38.Biehs B, Sturtevant MA, Bier E. 1998. Boundaries in the Drosophila wing imaginal disc organize vein-specific genetic programs. Development 125, 4245–4257. [DOI] [PubMed] [Google Scholar]
- 39.Irvine KD, Rauskolb C. 2001. Boundaries in development: formation and function. Annu. Rev. Cell Dev. Biol. 17, 189–214. ( 10.1146/annurev.cellbio.17.1.189) [DOI] [PubMed] [Google Scholar]
- 40.Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. 2000. Gradient formation of the TGF-beta homolog Dpp. Cell 103, 981–991. ( 10.1016/S0092-8674(00)00200-2) [DOI] [PubMed] [Google Scholar]
- 41.Teleman AA, Cohen SM. 2000. Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103, 971–980. ( 10.1016/S0092-8674(00)00199-9) [DOI] [PubMed] [Google Scholar]
- 42.Lunde K, Biehs B, Nauber U, Bier E. 1998. The knirps and knirps-related genes organize development of the second wing vein in Drosophila. Development 125, 4145–4154. [DOI] [PubMed] [Google Scholar]
- 43.Waddington CH. 1940. The genetic control of wing development in Drosophila. J. Genet. 41, 75–139. ( 10.1007/BF02982977) [DOI] [Google Scholar]
- 44.Fristrom D, Wilcox M, Fristrom J. 1993. The distribution of PS integrins, laminin A and F-actin during key stages in Drosophila wing development. Development 117, 509–523. [DOI] [PubMed] [Google Scholar]
- 45.de Celis JF. 1997. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development 124, 1007–1018. [DOI] [PubMed] [Google Scholar]
- 46.Ralston A, Blair SS. 2005. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev. Biol. 280, 187–200. ( 10.1016/j.ydbio.2005.01.018) [DOI] [PubMed] [Google Scholar]
- 47.Diaz-Benjumea FJ, García-Bellido A. 1990. Genetic analysis of the wing vein pattern of Drosophila. Wilhelm Roux's Arch. Dev. Biol. 198, 336–354. ( 10.1007/BF00383772) [DOI] [PubMed] [Google Scholar]
- 48.Bridges CB. 1920. The mutant crossveinless in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 6, 660–663. ( 10.1073/pnas.6.11.660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimmi O, Ralston A, Blair SS, O'Connor MB. 2005. The crossveinless gene encodes a new member of the twisted gastrulation family of BMP-binding proteins which, with short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev. Biol. 282, 70–83. ( 10.1016/j.ydbio.2005.02.029) [DOI] [PubMed] [Google Scholar]
- 50.Vilmos P, Sousa-Neves R, Lukacsovich T, Marsh JL. 2005. crossveinless defines a new family of twisted-gastrulation-like modulators of bone morphogenetic protein signalling. EMBO Rep. 6, 262–267. ( 10.1038/sj.embor.7400347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray RP, Wharton KA. 2001. Context-dependent relationships between the BMPs gbb and dpp during development of the Drosophila wing imaginal disk. Development 128, 3913–3925. [DOI] [PubMed] [Google Scholar]
- 52.Serpe M, Ralston A, Blair SS, O'Connor MB. 2005. Matching catalytic activity to developmental function: tolloid-related processes Sog in order to help specify the posterior crossvein in the Drosophila wing. Development 132, 2645–2656. ( 10.1242/dev.01838) [DOI] [PubMed] [Google Scholar]
- 53.Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O'Connor MB, Blair SS. 2008. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell 14, 940–953. ( 10.1016/j.devcel.2008.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. 2000. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947–3959. [DOI] [PubMed] [Google Scholar]
- 55.Christoforou CP, Greer CE, Challoner BR, Charizanos D, Ray RP. 2008. The detached locus encodes Drosophila Dystrophin, which acts with other components of the Dystrophin associated protein complex to influence intercellular signalling in developing wing veins. Dev. Biol. 313, 519–532. ( 10.1016/j.ydbio.2007.09.044) [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Honeyager SM, Schleede J, Avanesov A, Laughon A, Blair SS. 2012. Crossveinless d is a vitellogenin-like lipoprotein that binds BMPs and HSPGs, and is required for normal BMP signaling in the Drosophila wing. Development 139, 2170–2176. ( 10.1242/dev.073817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denholm B, Brown S, Ray RP, Ruiz-Gomez M, Skaer H, Hombria JC. 2005. crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development 132, 2389–2400. ( 10.1242/dev.01829) [DOI] [PubMed] [Google Scholar]
- 58.Genova JL, Jong S, Camp JT, Fehon RG. 2000. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev. Biol. 221, 181–194. ( 10.1006/dbio.2000.9671) [DOI] [PubMed] [Google Scholar]
- 59.Ray RP, Arora K, Nusslein-Volhard C, Gelbart WM. 1991. The control of cell fate along the dorsal–ventral axis of the Drosophila embryo. Development 113, 35–54. [DOI] [PubMed] [Google Scholar]
- 60.Shimmi O, Umulis D, Othmer H, O'Connor MB. 2005. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120, 873–886. ( 10.1016/j.cell.2005.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Robertis EM. 2008. Evo-devo: variations on ancestral themes. Cell 132, 185–195. ( 10.1016/j.cell.2008.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ. 2004. Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science 304, 1335–1337. ( 10.1126/science.1091946) [DOI] [PubMed] [Google Scholar]
- 63.Saina M, Genikhovich G, Renfer E, Technau U. 2009. BMPs and chordin regulate patterning of the directive axis in a sea anemone. Proc. Natl Acad. Sci. USA 106, 18 592–18 597. ( 10.1073/pnas.0900151106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. 2008. Scaling of the BMP activation gradient in Xenopus embryos. Nature 453, 1205–1211. ( 10.1038/nature07059) [DOI] [PubMed] [Google Scholar]
- 65.van der Zee M, Stockhammer O, von Levetzow C, Nunes da Fonseca R, Roth S. 2006. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc. Natl Acad. Sci. USA 103, 16 307–16 312. ( 10.1073/pnas.0605154103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akiyama-Oda Y, Oda H. 2006. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development 133, 2347–2357. ( 10.1242/dev.02400) [DOI] [PubMed] [Google Scholar]
- 67.Goltsev Y, Fuse N, Frasch M, Zinzen RP, Lanzaro G, Levine M. 2007. Evolution of the dorsal–ventral patterning network in the mosquito, Anopheles gambiae. Development 134, 2415–2424. ( 10.1242/dev.02863) [DOI] [PubMed] [Google Scholar]
- 68.Savard J, Tautz D, Richards S, Weinstock GM, Gibbs RA, Werren JH, Tettelin H, Lercher MJ. 2006. Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the base of the radiation of Holometabolous insects. Genome Res. 16, 1334–1338. ( 10.1101/gr.5204306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goulet H, Huber JT. 1993. Hymenoptera of the world: an identification guide to families. Ottawa, Canada: Canada Communication Group Publishing. [Google Scholar]
- 70.Jockusch EL, Ober KA. 2004. Hypothesis testing in evolutionary developmental biology: a case study from insect wings. J. Hered. 95, 382–396. ( 10.1093/jhered/esh064) [DOI] [PubMed] [Google Scholar]
- 71.Sotillos S, de Celis JF. 2006. Regulation of decapentaplegic expression during Drosophila wing veins pupal development. Mech. Dev. 123, 241–251. ( 10.1016/j.mod.2005.12.002) [DOI] [PubMed] [Google Scholar]
- 72.Stultz BG, Ray RP, Hursh DA. 2005. Analysis of the shortvein cis-regulatory region of the decapentaplegic gene of Drosophila melanogaster. Genesis 42, 181–192. ( 10.1002/gene.20134) [DOI] [PubMed] [Google Scholar]
- 73.Peter IS, Davidson EH. 2011. Evolution of gene regulatory networks controlling body plan development. Cell 144, 970–985. ( 10.1016/j.cell.2011.02.017) [DOI] [PMC free article] [PubMed] [Google Scholar]