Abstract
Health economics is a relatively new discipline, though its antecedents can be traced back to William Petty FRS (1623–1687). In high-income countries, the academic discipline and scientific literature have grown rapidly since the 1960s. In low- and middle-income countries, the growth of health economics has been strongly influenced by trends in health policy, especially among the international and bilateral agencies involved in supporting health sector development. Valuable and influential research has been done in areas such as cost–benefit and cost-effectiveness analysis, financing of healthcare, healthcare provision, and health systems analysis, but there has been insufficient questioning of the relevance of theories and policy recommendations in the rich world literature to the circumstances of poorer countries. Characteristics such as a country's economic structure, strength of political and social institutions, management capacity, and dependence on external agencies, mean that theories and models cannot necessarily be transferred between settings. Recent innovations in the health economics literature on low- and middle-income countries indicate how health economics can be shaped to provide more relevant advice for policy. For this to be taken further, it is critical that such countries develop stronger capacity for health economics within their universities and research institutes, with greater local commitment of funding.
Keywords: health economics, health policy, health systems, low- and middle-income countries
1. Introduction
Health economics has risen to public prominence in recent years. It is now a well-established sub-speciality of economics, with its own academics, journals, national and international professional associations, conferences and educational programmes. Health economists also work as practitioners, embedded within ministries of health, health authorities, global agencies such as the World Health Organization (WHO), and industry.
However, the application of economics to guide public policy making in the field of health has not been without controversy. Recently, the editor of the medical journal The Lancet tweeted that economics ‘may just be the biggest fraud ever perpetrated on the world’ [1] arguing that the emphasis placed on markets and competition damages the values of universal health systems. Another controversial area has been the influence of health economics on the introduction of new technologies into publicly funded health services. For example, in England and Wales, the National Health Service is legally obliged to provide funding for medicines and treatments recommended by the National Institute for Health and Care Excellence (NICE; http://www.nice.org.uk/aboutnice (accessed 14 February 2014)). The judgement of NICE is based on the criterion of cost-effectiveness: the health gain of a new treatment relative to its cost. NICE's decisions not to recommend certain high-cost cancer drugs have led to public and professional outcry [2]. In low- and middle-income countries, given extreme resource constraints and health needs, these challenges of prioritization are far greater, and cost-effectiveness has also been the recommended yardstick for determining intervention priorities though has not attracted the same degree of explicit opposition.
Some of this criticism rests on a misunderstanding or partial view of economics, and lack of appreciation of the diversity of views among economists themselves; other critics are reluctant to accept the pervasive problem of shortage of resources and the need to prioritize. But there is also a further criticism relevant to the developing world, that of the appropriateness of the literature, most originating in the rich world, to the circumstances of poorer countries.
This review draws on the author's more than 40 years of experience in research and policy advice to examine the development of health economics over time in low- and middle-income countries, its contributions to health policy and its limitations. The review ends by suggesting how health economics might be strengthened further in such countries.
2. The origins of modern health economics
Appropriately enough given this journal, health economists like to trace the origin of their discipline to William Petty FRS (1623–1687). Petty was an English economist, scientist and philosopher, and a founder member of the Royal Society. His work is noted by modern health economists for his approach to valuing human life based on a person's contribution to national production [3]. In ‘Lessening ye Plagues of London’ [4], he showed that removing people from London during the plague was an excellent financial investment, returning £84 for every pound spent. Echoing much later analyses of the medical care market and the role of government, he also argued that all should have equal opportunity for medical care, and that there should be a state medical service of salaried doctors: ‘it is not in the interests of the state to leave Phisitians and Patients (as now) to their own shifts’ (p. 195).
The focus on people as producers—now termed the human capital approach to valuing human lives—was also advanced by Edwin Chadwick, a Benthamite or Utilitarian who influenced public health legislation in the first half of the nineteenth century. He wrote that:
As the artist for his purpose views the human being as a subject for the cultivation of the beautiful—as the physiologist for the cultivation of his art views him solely as a material organism, so the economist for the advancement of his science may well treat the human being as simply an investment of capital, in productive force. [5, p. 504]
He deployed this thinking to argue that prevention of disease could offer greater benefit than investing in hospitals to treat disease.
The early literature on the economics of health in low- and middle-income countries reflects this emphasis on the economic impact of disease, especially the literature on malaria. From the eighteenth century onwards, malaria was recognized in South and southeast Asia as a major hazard for travellers and expatriates. Landon [6], an English writer and journalist, visited Nepal in 1928:
We are now in the twelve-mile-wide strip of raw forest, which has not unjustly earned for the Terai its famous reputation of being the unhealthiest region in all of Asia. But there is nothing to betray its evil nature unless, perhaps … the extra luxuriance of its vegetation suggests a warm marshy soil and therewith, to a modern mind, mosquitoes … . Throughout the hours of daylight the Terai is safe enough. It is the evening that man may not spend in this beautiful park. Sundown in the Terai has brought to an end more attempted raids into Nepal and buried more political hopes than will ever be known. The English learnt their lesson early … . The English had been told of its dangers but they had to learn from experience what all India had known and feared for centuries … . The tribe of the Tharus alone are immune … . Perhaps after all this zone is only affected by an unusually virulent form of the fever, but of its mortal effects there is no question. The records of Nepal and of the Indian army are crowded with the names of its victims. (pp. 197–173)
Landon goes on to comment on its consequences for economic activity:
From Lady Day (March 25th) to Michaelmas (September 29th) there is nothing in this fever-haunted district of Hetaura but a few houses, deserted by all except a handful of carters and a native traveller or two … . But during the winter it is a well–populated centre from which diverge four or five of the main routes of South Nepal. (pp. 176–177)
Similarly, Rickard Christophers FRS [7], an officer in the Indian Medical Service, wrote that:
The autumn of 1908 in the Punjab was characterised by an epidemic of extraordinary severity. The effects of this epidemic were first prominently brought before the public by a sudden disorganisation of the train services due to ‘fever’ among the employees at the large railway station, Lahore … . At Amritsar … almost the entire population was prostrated and the ordinary business of the city disrupted. For many weeks labour … was unprocurable and even food vendors ceased to carry on their trade. (p. 9)
Later writers sought to quantify the economic cost, for example calculating the cost of treatment and days of work lost due to malaria [8], and noting that the risk of malaria could inhibit the exploitation of fertile land [9]. Such evidence was deployed to help justify the malaria eradication efforts of the 1950s and 1960 s.
3. The modern era of health economics
The development of health economics as a discipline is usually credited to Nobel Laureate Ken Arrow [10], who wrote a seminal paper distinguishing the medical care market from markets for other goods and services, identifying the reasons why private markets might not work well for either healthcare or the provision of health insurance. This seminal paper ushered in a more systematic approach to applying economics to the health sector as a whole—how it is financed, how services are and should be provided and by whom, and the role of government.
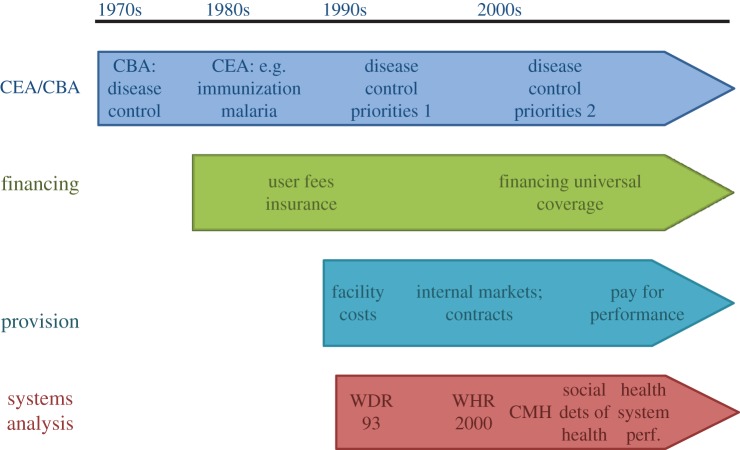
The health economics literature on low- and middle-income countries began to grow in the 1970s, and has expanded since then in both depth and breadth (figure 1). Figure 1 categorizes the field into four broad topic areas: cost–benefit and cost-effectiveness analysis (extending the earlier work on the economic impact of disease to evaluating control options), financing healthcare, provision of healthcare and systems analysis. A characteristic of the literature is that unlike in high-income countries where the discipline of health economics within universities was stimulated by growing academic interest in the economics of public policy, increased national demand for health economists which stimulated new educational programmes, and increased research funding, in low- and middle-income countries the prime drivers for the expansion of health economics were users of health economic analysis at the global level, notably agencies such as the WHO and the multilateral and bilateral aid agencies. This has lent to the development of health economics an especially close link to policy trends in these agencies. A very selective overview of key developments in the literature is given below.
Figure 1.
The development of health economics in low- and middle-income countries: overview. CEA, cost-effectiveness analysis; CBA, cost-benefit analysis; WDR, World Development Report; WHR, World Health Report; CMH, Commission on Macroeconomics and Health.
(a). The development of cost–benefit and cost-effectiveness analysis
Cost–benefit analysis developed originally as a systematic way of assessing the costs and benefits of investments in the public sector where a market test (profitability) could not be applied. In the 1970s, it was a favoured tool for making an economic case for investing in disease control. However, the traditional approach to valuing benefits, the human capital approach referred to earlier, was increasingly recognized as a very partial measure of the value of life. Health is valued not just because it helps people be productive, an instrumental value, but also because it has intrinsic value, as part of general wellbeing. Cost-effectiveness analysis, where benefits are assessed in health terms only rather than in money terms as in cost–benefit analysis, became the preferred technique.
The 1970s and 1980s onwards saw a rapid expansion of the literature on the cost-effectiveness of a variety of interventions, primarily those relating to infectious diseases, childhood illnesses and immunization [11]. For example, in order to help mobilize resources to support the revival of efforts in the late 1990s to control malaria, the WHO commissioned cost-effectiveness studies of the main malaria control tools. These showed that both prevention (e.g. insecticide-treated mosquito nets) and treatment were similarly cost-effective to many of the other low-cost interventions to reduce child mortality [12].
Because of the different measurement units for costs and effects, cost-effectiveness analysis can address only the relative cost-effectiveness of an intervention. It was therefore important to build up a database of studies conducted according to standard methods, so the cost-effectiveness of any new intervention could be compared against the cost-effectiveness of existing interventions. An important initiative was the Disease Control Priorities Project, which in 1993 published a book (DCP1) with chapters covering the major causes of disease in low- and middle-income countries, and providing information on disease burden and the cost-effectiveness of interventions [13]. This was followed in 2006 by DCP2 with an even more extensive set of analyses (summarized in [14]), which is now being updated and extended for the third time (http://www.dcp-3.org (accessed 14 February 2014)).
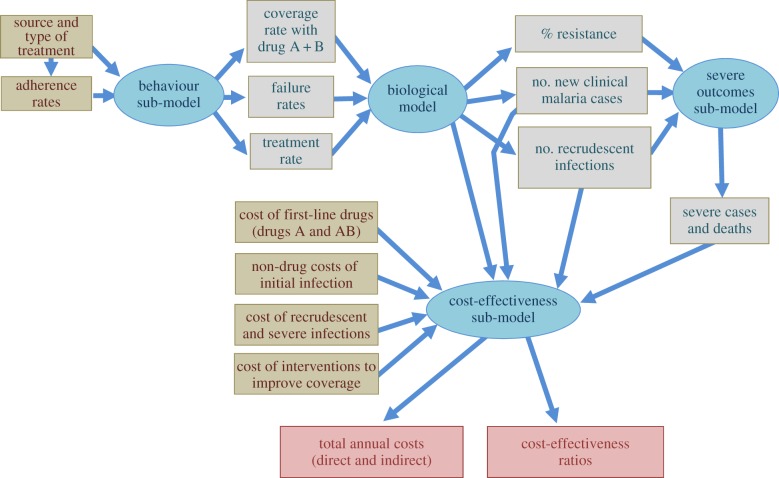
Over the past 30 years, methods of cost-effectiveness analysis have gained in sophistication. Modelling methods are now extensively used both to allow for uncertainty in model parameters and to reflect variations in costs and effects in differing contexts. There have been developments in linking biological models of infectious diseases with models of treatment seeking behaviour and models which generate cost-effectiveness ratios. For example, figure 2 shows a set of models developed to address the question of when to change first-line drugs for malaria treatment, given the development of resistance, and especially to assess the value of introducing combination therapy for malaria [15,16]. At the time, governments were reluctant to switch to combination therapy because of the added cost. The models made explicit the cost of failing first line drugs—increased cases and hence increased treatment costs, as well as the need to retreat treatment failures—and hence helped demonstrate the value of switching first line drugs sooner rather than later.
Figure 2.
A bio-economic model of the spread of antimalarial resistance. Adapted from Yeung [15] with permission.
The main audience for much of this analysis has been multilateral and bilateral agencies, who draw on cost-effectiveness evidence to plan and justify their investments in low- and middle-income countries (in the Department for International Development, for example, economic evaluation principles are embedded in the business case required for any investment decision [17]). A recent study of decision-making processes in relation to vaccines suggests that governments in low- and middle-income countries so far pay much less attention to cost-effectiveness evidence, with funding availability and political factors dominating decision-making [18].
(b). Financing healthcare
During the late 1970s, an increasing number of studies sought to quantify sources of revenue for healthcare and patterns of expenditure [19]. It became evident that direct payments by households made a major contribution to health expenditure even in low-income countries, and that government spending for health was extremely limited.
This led to increased attention being paid to the appropriate role of government in financing healthcare, and the merits of alternative ways of raising revenue. By the early 1980s, the oil price crisis had severely affected government budgets in low- and middle-income countries. Moreover, prevailing economic orthodoxy—a shift away from a state-centric view of development, influenced attitudes towards public spending in general, and on health in particular. Following Arrow's seminal paper, a literature had developed in the USA and UK on the justification for government intervention in the health sector [20,21]. On the one hand were those who followed the traditional economic argument that government intervention is justified only by the failure of private markets to operate efficiently. These failures imply that certain preventive and promotive services should be publicly financed, but that the bulk of curative services can be privately financed, given government regulation. On the other hand were those who argued that equity should receive equal weight to efficiency in social policy, and that social goals would best be achieved by substantial government intervention in the financing of healthcare.
During the 1980s and 1990s, influential publications by the World Bank reflected the view that government intervention in the health sector should be quite limited [22]. Increased government funding for the health sector was not considered either desirable or feasible, and attention focused on the other two main traditional sources of revenue for the health sector: payments by users of government services, and health insurance. Both were considered to have the advantage that they encouraged the exercise of individual responsibility, making users think carefully about the value of using costly services and demand that providers be responsive to their needs.
Given that the policy question was the willingness of individuals and households either to pay for healthcare, or to pay insurance premia, studies were undertaken on demand for healthcare, applying standard methods from economics to identify price elasticities. Initial studies suggested that demand was relatively insensitive to price [23,24], justifying a policy of user fees. However a later study, which allowed price elasticity to differ by income group, found that poorer groups were, not surprisingly, more sensitive to price than richer groups [25].
This finding, plus accumulating evidence that the fee exemptions recommended for the poor were ineffective in practice [26], shifted policy attention to insurance. While there was some exploration of scope for the sort of social insurance arrangements found in the developed world, their application would be limited to the formal sector of employment, very much a minority of the labour force. Hence attention focused rather on community-based health insurance, where communities come together to manage collectively a pre-payment system [27]. As with user fees, studies of willingness-to-pay suggested that households would indeed be willing to pay health insurance premia [28]. However, with very few exceptions schemes have remained small and poor design and management were perennial problems [29].
Most recently, the debate on financing the health sector has moved from issues of specific sources of finance, like user fees or insurance, to drawing on multiple sources of finance to ensure universal coverage of healthcare for an entire population [30]. Since the 1980s, there has been a growing volume of studies on the consequences for households of paying out-of-pocket for healthcare. These have shown, for example, that households who need to pay for hospitalization or for continuing chronic care may go into debt, sell land or other assets, or cut down on food [31]. This evidence has helped put universal coverage on the global policy agenda.
(c). Healthcare provision
Systematic study of the production of health services in low- and middle-income countries first began in the 1980s, in part initially to supply the necessary cost information for cost-effectiveness studies. In the 1990s in the rich world, the approach known as new public management began to influence the provision of public services [32]. Summarized by Kaul as ‘government moves from a concern to do towards a concern to ensure that things are done’ [33, p. 14], proponents argued that the state should be involved in policy, purchasing, and regulation, but not necessarily provision of services. And where the state was involved in provision, there should be a more market-oriented approach to provision of public services, for example giving patients choice of service provider, charging fees, and awarding explicit contracts for service provision through competitive tendering among both public and private providers.
Again, these shifts in the prevailing orthodoxy in high-income countries had their influence on low- and middle-income countries via the advice given by the aid machinery. Governments were recommended to distinguish ‘purchasers’ and ‘providers’ within their health sector, and to introduce formal agreements with funding provided in return for explicit standards of performance [34]. At their most elaborate, such reforms created an ‘internal market’ within the public sector, as was implemented in the UK. Although the introduction of a formal separation between purchaser and providers was described in many countries, very few good quality evaluations were done, so it is impossible to say whether such reforms have benefited the quality or efficiency of service provision [35].
New public management ideas stimulated interest in private providers. The assumption tended to be that public was likely to be inefficient, and private more efficient, because of the effect of market discipline on cost control and responsiveness to users. A number of studies explored the relative efficiency of publicly and privately run facilities, finding that it is not possible to state definitively which type of provider is more efficient [36]. Both government and private sectors can supply services of poor technical quality, though private services tend to be more responsive to patient preferences. Great variation in quality and cost is found within both sectors, but especially within the private sector which can range from small informal and untrained providers through to sophisticated hospitals in the capital city [37].
Further study has focused on whether there are ways in which governments can use the quite substantial resources in many private health sectors. Arrangements studied include using public funds to purchase services from private providers; bringing in private sector firms to manage public hospitals; using franchising to increase demand for private sector services such as contraception; and training drug sellers to provide more appropriate treatment. Evidence indicates that some of these arrangements can work quite well: for example training drug sellers to provide antimalarials, and franchising clearly defined services [38]. Contracts with non-governmental health providers have also been shown to work quite effectively [39]. Contracting with for-profit providers has been less studied, though experience in South Africa suggests that such contracts demand effective public sector management capacity, which may not be available [40].
A recent innovation has been to transfer performance incentives down to the level of individual health facilities or health workers, through the so-called pay-for-performance arrangements [41]. Economists have been active in encouraging and evaluating such reforms through experimental and quasi-experimental methods [42]. These arrangements have indeed been shown to encourage provision of incentivized services [43], but the same review also found they risk diverting attention away from services left out of the prioritized package.
There remains concern about the use of such high-powered incentives in public services where the quality of the interaction between patient and provider is key, and where it can be argued that money should not be an influencing factor at the level of the individual patient–provider interaction, whether in terms of influencing whether the patient seeks care, or the health worker or health facility provides it.
(d). Systems analysis
Analysing individual elements of a health system provides important information for policy, but given the complexity of health systems, it is also important to study the system as a whole and identify what features might be associated with better or worse functioning. Methods to do this are still in their infancy. They range from cross-country analysis of expenditure and health outcomes data, to try and identify whether some countries are better than others at covering spending into health gains [44], to in-depth historical and qualitative studies of how country health systems have evolved over time and what policies seem to be associated with health gains [45].
In the developing world, some landmark studies mark the evolution of analysis at the systems level. In 1993, the World Bank published a World Development Report (WDR 93) which focused on health [46]. This was the first to use analysis of the burden of disease (expressed in Disability Adjusted Life Years or DALYs) and cost-effectiveness of interventions, to propose packages of public health and essential clinical services which country health systems should prioritize. In 2000, the controversial World Health Report (WHR) sought to rank country's health systems in terms of their performance [47]. It struggled with distinguishing the contribution of the health system to improved health, as opposed to the influence of other health determinants such education and diet. In 2001, the WHO Commission on Macroeconomics and Health (CMH) sought to make the case that the line of causality from improved population health to improved economic growth was more important than the line from economic growth to improved population health. It analysed the case for spending far more on health in the developing world, quantified the financial needs and argued that greatly increased funding could be used effectively [48].
Work has continued on studying health systems as a whole, but with greatest focus on the financing issues raised by the goal of universal coverage and less attention paid to other aspects of health systems and especially the influence on systems performance of how services are provided and managed.
4. The corpus of knowledge
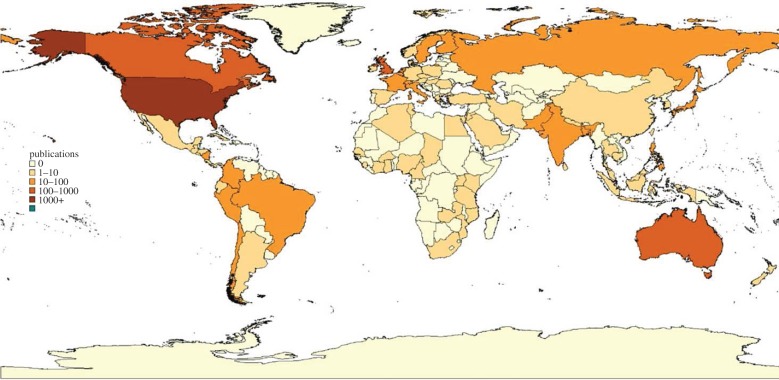
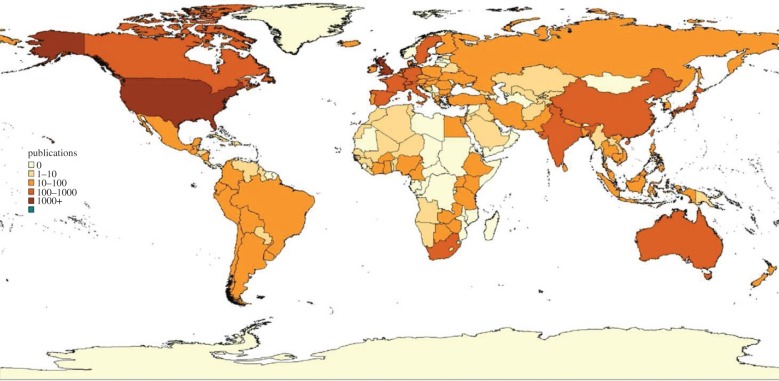
The past 30 years have certainly seen a considerable growth in the health economics literature. Figures 3 and 4 show that coverage of the countries of the world has improved markedly. But there are still many countries in the developing world where health economic analysis is absent. Moreover, research capacity is still quite weak. Capacity for health economics research has not been well documented, but evidence for the related field of health policy and systems research (within which most health economics research fits) shows that only 24% of researchers in health systems research institutions in low-income countries have PhDs (36% in middle-income countries); only 63% of low-income country researchers have access to peer-reviewed journals (89% in middle-income countries); and that international funding dominates national funding (88% of research funding is external in low-income countries; 66% in middle-income countries) [50].
Figure 3.
Country focus of health economics articles 1969–1989. Reprinted from [49], with permission from Elsevier.
Figure 4.
Country focus of health economics articles 1990–2009. Reprinted from [49], with permission from Elsevier.
Does this lack of capacity in low- and middle-income countries matter? Newhouse [51] argued that: ‘…theories and models are typically sufficiently general that they will apply to many institutional contexts’ and ‘the same issues are found to a greater or lesser extent in every medical care financing and delivery system’ (p. 6). On this basis, it could be argued that the developing world can draw its evidence from the richer countries of the world where the body of knowledge in health economics has been rapidly increasing.
This view underestimates the extent to which health economics reflects the context in which it is being developed and applied. There are at least four characteristics of the developing world that mean that health economics theories and policy recommendations cannot necessarily be easily transferred across settings [52].
The first is their economic structure. Severe poverty affects not just household ability to pay for care but also government ability to collectively finance access to healthcare. It was recently estimated that even a basic package of preventive and curative care, plus the necessary systems support, would cost around $50 per capita (as compared to the $31 currently spent, of which only $12 is by government) [53]. All countries face difficulties in affording healthcare, but those faced by the developing world are of a different order of magnitude. The large informal element of the labour force makes it difficult to levy both payroll and income taxes, as well as easily target public subsidies to poorer households. Low country income means that wages are low, and hence retaining health professionals is difficult in the face of high demand from richer countries. The often extreme income inequality within countries means that the wealthy can purchase their own healthcare in the private sector, which attracts health professionals away from the public sector; and the greater the development of the private sector, the more difficult (both politically and financially) it becomes to put in place universal arrangements that pool resources for the benefit of the whole population.
The second is the strength of political and social institutions such as the institutions of democracy and representation, of civil society, and of professional groupings. In the rich world, these go largely unnoticed, but they underpin the performance of health systems. For example, the professional ethos of the medical profession affects how doctors respond to different methods of payment. Capitation (a fixed payment per period of time) carries the risk that volume of care may be minimized, but this has not been a major concern in the UK where traditionally this has been the main way of paying general practitioners. In Thailand, by contrast, capitation payment has aroused considerable concerns about under-provision [54]. A second example is that the governance structure of hospitals affects how they respond to being given greater management autonomy: in parts of the developing world increased autonomy given to encourage efficiency has in fact led to hospitals maximizing income by levying fees on the most profitable services [55]. Where representation of the public interest is missing on hospital governance structures or in the wider community, hospitals may face few constraints on income-maximizing behaviour. Schick [56] summed up the problem of applying developed country public management reforms to countries with much weaker institutions: ‘it would be foolhardy to entrust public managers with complete freedom over resources when they have not yet internalised the habit of spending money according to prescribed rules’ (p. 127).
The third characteristic is weak management capacity in the public sector. In part this reflects limited numbers and training, but more broadly this concerns the management systems that are used to raise revenue, make and implement policy, and deliver services. For example, social health insurance commonly fails to enrol all employers who qualify: in Colombia, for example, revenue lost through evasion was equivalent to 2.75% of gross domestic product [57]. There are numerous examples of policies that fail to be effectively implemented because the ability is lacking to translate policy change at national level into change on the ground [58].
A final characteristic, in low-income countries, is the influence of agencies external to countries. In 2011, 37% of health expenditure in such countries came from external assistance [59]. While providing an important supplement to domestic resources, such external flows bring with them some major complications. For example they are often not predictable, but vary greatly from year to year. External assistance is also fragmented: Vietnam in 2002 had 25 bilateral donors, 19 multilateral donors and around 350 non-governmental organizations providing support through around 8000 projects, one per 9000 people [60], bringing high costs of coordination and reporting. Despite lip service to the importance of ‘country ownership’ of decision-making, in practice donor preferences strongly dictate funding flows. For example, there has been far greater funding in recent years to specific disease programmes than to the broader health system which is needed to underpin healthcare delivery [61], in large part because of donor desires to identify direct health benefits from their funding.
Finally, another strong reason why the development of health economics within the developing world is important is to ensure a close connection with local decision makers. Evidence on the influence of research on policy suggests that close connections between researchers and research users are necessary [62]. Such connections are most likely to develop when research is done within the country setting, rather than in some distant country.
But there are grounds to be optimistic. Those health economists working in or on low- and middle-income countries have responded to the specific country circumstances by rethinking and innovating on methods, prioritizing questions important in the local context, and developing multidisciplinary approaches to studying complex questions. Table 1 lists some of these innovations in relation to the characteristics identified above. For example, a substantial body of literature has developed on measuring payments for healthcare that may have a catastrophic impact on household welfare [63], and studying how households cope with such payments and with what consequences [64]. Studies on health worker incentives to work in rural areas have shown, for example, that those nurses in South Africa who are more altruistic [65] are more likely to be located in a rural area [66]. Methods have been developed to study the informal health markets prevalent in much of the developing world, such as sales by unlicensed drug sellers that supply a significant share of the malaria drug sales in Africa [67]. Theories from disciplines such as political science and public administration have been drawn on to explore government capacity and its implications for ability to implement reforms [36].
Table 1.
Innovations in the developing world literature.
| characteristics | examples of innovations in literature |
|---|---|
| 1. economic structure | |
| household payment for healthcare can have catastrophic implications for household welfare | methods for catastrophic spending analysis; mixing quantitative and qualitative methods; studying coping behaviour |
| low wages and enormous human resource challenges given global market | testing of task shifting; study of health worker motivations |
| large informal sector and challenges for health insurance | testing and analysing community health insurance |
| segmented healthcare markets | exploration of willingness of richer groups to cross-subsidize the poor; options for and implications of mixed systems |
| high heterogeneity; large informal component | methods for studying informal providers |
| 2. weak political and social institutions | |
| effect on reform prescriptions, e.g. voluntary insurance enrolment, contracting, hospital autonomy | exploration of institutional contexts and their influence on reform performance |
| 3. limited management capacity | |
| difficulties in implementation | exploration of factors affecting implementation |
| 4. external dependence for health financing | |
| problems of fragmentation and volatility; external influence on domestic priorities | exploration of fiscal space issues implications of lack of commitment to policies pursued—e.g. fungibility and displacement |
(a). Future developments
The past 40 years have seen the discipline of health economics grow in its ability to provide useful information for policy. But it has not been a neutral tool. Policy prescriptions are often underpinned by ideological positions—for example that government intervention in private markets should be minimized (or vice versa), or at the least reflect the values of their proponents. This is inevitable—economics is concerned not just with describing what is, but also with suggesting what should be. Values inevitably enter into policy prescriptions; what matters is that they be made explicit.
Given the influence of values, it is unfortunate that so much of the health economics research in the developing world has been driven by the policy interests of agencies external to those countries, and that those tend to reflect policy trends in the rich world. It is also unfortunate that most research funding has been directed to specific studies, rather than to building up the capacity of universities and research institutes to provide sources of expertise and training over the longer term.
Governments in low- and middle-income countries need to seize greater ownership of the research agenda, but this will demand not just that they increase their ability to act as informed commissioners of research, but also that they begin to finance their own research programmes. There are some promising signs; for example, Thailand and more recently Kenya have created national research funds. As countries grow richer, it will be important to ensure that part of the fruits of growth are invested in domestic capacity to provide informed input into health systems development.
Acknowledgements
I am most grateful to Kara Hanson for comments on an earlier version. The views expressed here have been developed through many years of interaction with my fellow health economists at the London School of Hygiene and Tropical Medicine and with numerous research collaborators overseas, to all of whom I owe a great debt for 35 years of stimulating research and debate.
References
- 1.Horton R. 2012. Economics, second only to ‘management’, may just be the biggest fraud ever perpetrated on the world. Twitter 31 December 2012. See https://twitter.com/richardhorton1/status/285694937792647168 (accessed 14 February 2014)
- 2.Liver drug too expensive. 2009. BBC News 19 November 2009 See http://news.bbc.co.uk/1/hi/health/8367614.stm (accessed 14 February 2014)
- 3.Fein R. 1980. Social and economic studies shaping American health policy. Milbank Mem. Q. Bull. (Health and Society) 58, 349–385 (doi:10.2307/3349730) [PubMed] [Google Scholar]
- 4.Petty W. 1676. Anatomy Lecture. In The Petty papers: some unpublished writings of Sir William Petty, from the Bowood papers, 1676 (ed. Landsdowne M.), pp. 171–179 London, UK: Constable [Google Scholar]
- 5.Chadwick E. 1862. Opening address as president of section F of the British Association for the Advancement of Science. J. Stat. Soc. Lond. 25, 502–524 (doi:10.2307/2338601) [Google Scholar]
- 6.Landon P. 1928. Nepal. 2 vol London, UK: Constable [Google Scholar]
- 7.Christophers SR.1911. Malaria in the Punjab. Scientific memoirs by officers of the medical and sanitary departments of the Government of India. New Series no. 46, Superintendent Government Printing, Calcutta, India.
- 8.Sinton JA. 1935–6. What malaria costs India nationally, socially and economically. Records of the Malaria Survey of India 5, 223–264, 413–148; 6, 96–169 [Google Scholar]
- 9.Ketterer WA. 1953. Economic benefits of malaria control in the Republic of Indonesia. Public Health Rep. (Washington) 68, 1056–1058 (doi:10.2307/4588633) [PMC free article] [PubMed] [Google Scholar]
- 10.Arrow KJ. 1963. Uncertainty and the welfare economics of medical care. Am. Econ. Rev. LIII, 5, 941–973 [Google Scholar]
- 11.Mills A, Thomas M. 1984. Economic evaluation of health programmes in developing countries. Publication no. 3, Evaluation and Planning Centre, London School of Hygiene and Tropical Medicine [Google Scholar]
- 12.Goodman C, Coleman P, Mills A. 1999. The cost-effectiveness of malaria control in Africa . The Lancet 354, 378–385. (doi:10.1016/S0140-6736(99)02141-8) [DOI] [PubMed] [Google Scholar]
- 13.Jamison DT, Mosley WH. 1993. Disease control priorities in developing countries. Washington, DC: Oxford University Press for The World Bank [Google Scholar]
- 14.Laxminarayan R, et al. 2006. Advancement of global health: key messages from the Disease Control Priorities Project. The Lancet 367, 1193–1208 (doi:10.1016/S0140-6736(06)68440-7) [DOI] [PubMed] [Google Scholar]
- 15.Yeung S. 2006. Antimalarial drug resistance and artemisinin combination therapy: a bio-economic model for the elucidation of policy. PhD thesis, University of London, UK [Google Scholar]
- 16.Yeung S, Pongtavornpinyo W, Hastings I, Mills A, White NJ. 2004. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modelling to elucidating policy choices. Am. J. Trop. Med. Hyg. 71, 179–186 [PubMed] [Google Scholar]
- 17.Department for International Development 2011. Writing a business case. How to note London, UK: Department for International Development; See http://www.ids.ac.uk/files/dmfile/DFID_HowtoNote_BusinessCase_Aug2011.pdf (accessed 26 May 2014) [Google Scholar]
- 18.Burchett HED, et al. 2012. New vaccine adoption: qualitative study of national decision-making processes in seven low- and middle-income countries. Health Policy Plan. 27(Suppl. 2), ii5–ii16 (doi:10.1093/heapol/czs035) [DOI] [PubMed] [Google Scholar]
- 19.Griffiths A, Mills M. 1983. Health sector financing and expenditure surveys. In The economics of health in developing countries (eds Lee K, Mills A.), pp. 43–63 Oxford, UK: Oxford University Press [Google Scholar]
- 20.Lees D. 1976. Economics and non-economics of health services. Three Banks Rev. 110, 3–20 [Google Scholar]
- 21.Culyer AJ. The NHS and the market: images and realities. In The public/private mix for health (eds McLachlan G, Maynard A.), p. 1082 London, UK: Nuffield Provincial Hospitals Trust [Google Scholar]
- 22.World Bank. 1987. Financing health services in developing countries: an agenda for reform. Washington, DC: World Bank; [PubMed] [Google Scholar]
- 23.Akin JS, Griffin CC, Guilkey DK, Popkin BM. 1986. The demand for adult outpatient services in the Bicol region of the Philippines. Soc. Sci. Med. 22, 321–328 (doi:10.1016/0277-9536(86)90130-9) [DOI] [PubMed] [Google Scholar]
- 24.Heller PS. 1982. A model of the demand for medical and health services in Peninsular Malaysia. Soc. Sci. Med. 16, 267–284 (doi:10.1016/0277-9536(82)90337-9) [DOI] [PubMed] [Google Scholar]
- 25.Gertler P, Locay L, Sanderson W. 1987. Are user fees regressive? The welfare implications of health care financing proposals in Peru. J. Econ. 36, 67–88 (doi:10.1016/0304-4076(87)90044-3) [Google Scholar]
- 26.Russell S, Gilson L. 1997. User fee policies to promote health service access for the poor: a wolf in sheep's clothing? Int. J. Health Serv. 27, 359–379 (doi:10.2190/YHL2-F0EA-JW1M-DHEJ) [DOI] [PubMed] [Google Scholar]
- 27.Bennett S, Creese A, Monasch R. 1998. Health insurance schemes for people outside formal sector employment. Current concerns ARA Paper no. 16 World Health Organisation, Geneva, Switzerland [Google Scholar]
- 28.Arhin D. Willingness to pay for rural health insurance: evidence from three African countries, PhD thesis, University of London, UK [Google Scholar]
- 29.Carrin G, Waelkens M-P, Criel B. 2005. Community-based health insurance in developing countries: a study of its contribution to the performance of health financing systems. Trop. Med. Int. Health 10, 799–811 (doi:10.1111/j.1365-3156.2005.01455.x) [DOI] [PubMed] [Google Scholar]
- 30.World Health Organisation 2010. Health systems financing: the path to universal coverage. Geneva, Switzerland: World Health Organisation; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell S. 1996. Ability to pay for health care: concepts and evidence. Health Policy Plan. 11, 219–237 (doi:10.1093/heapol/11.3.219) [DOI] [PubMed] [Google Scholar]
- 32.Walsh K. 1995. Public services and market mechanisms. Competition, contracting and the new public management. Basingstoke, UK: Macmillan [Google Scholar]
- 33.Kaul M. 1997. The new public management: management innovations in government. Public Adm. Dev. 17, 13–26 (doi:10.1002/(SICI)1099-162X(199702)17:1<13::AID-PAD909>3.0.CO;2-V) [Google Scholar]
- 34.Mills A, Bennett S, Russell S, Attanayake N, Hongoro C, Muraleedharan VE, Smithson P. 2001. The challenge of health sector reform: what must governments do? Oxford, UK: Macmillan Press [Google Scholar]
- 35.Mills A, Ranson K. 2012. The design of health systems. In Global public health: diseases, programs, systems and policies, 3rd edn (eds Merson MH, Black RE, Mills AJ.), pp. 615–651, ch. 12 Boston, MA: Jones and Bartlett Publishers [Google Scholar]
- 36.Hanson K, Gilson L, Goodman C, Mills A, Smith R, Feachem R, Feachem N, Koehlmoos TP, Kinlaw H. 2008. Is private health care the answer to the health problems of the world's poor? PLoS Med. 5, 1528–1532 (doi:10.1371/journal.pmed.0050233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills A, Brugha RF, Hanson K, McPake BI. 2002. What can be done about the private health sector in low-income countries? Bull. World Health Org. 80, 325–330 [PMC free article] [PubMed] [Google Scholar]
- 38.Patouillard E, Goodman CA, Hanson KG, Mills AJ. 2007. Can working with the private for-profit sector improve utilization of quality health services by the poor? A systematic review of the literature. Int. J. Equity Health 6, 17 (doi:10.1186/1475-9276-6-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagarde M, Palmer N. 2009. The impact of contracting out on health outcomes and use of health services in low and middle-income countries. Cochrane Database Syst. Rev. 4, CD008133 (doi:10.1002/14651858.CD008133) [DOI] [PubMed] [Google Scholar]
- 40.Broomberg J, Masobe P, Mills A. 1987. To purchase or to provide? The relative efficiency of contracting out versus direct public provision of hospital services in South Africa. In Private health providers in developing countries: serving the public interest? (eds Bennett S, McPake B, Mills A.), pp. 214–236 London, UK: Zed Press [Google Scholar]
- 41.Savedoff W. 2011. Incentive proliferation? Making sense of a new wave of development programs. Washington, DC: Center for Global Development [Google Scholar]
- 42.Basinga P, Gertler PJ, Binagwaho A, Soucat ALB, Sturdy J, Vermeersch CMJ. 2011. Effect on maternal and child health services in Rwanda of payment to primary health-care providers for performance: an impact evaluation. Lancet 377, 1421–1428 (doi:10.1016/S0140-6736(11)60177-3) [DOI] [PubMed] [Google Scholar]
- 43.Oxman AD, Fretheim A. 2009. Can paying for results help to achieve the Millennium Development Goals? Overview of the effectiveness of results-based financing. J. Evid. Based Med. 2, 70–83 (doi:1010.1111/j.1756-5391.2009.01020.x) [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, Verhoeven M, Tiongson E. 2001. Public spending on health care and the poor. Washington, DC: International Monetary Fund Working Paper; [DOI] [PubMed] [Google Scholar]
- 45.Balabanova D, et al. 2013. Good health at low cost 25 years on: lessons for the future of health systems strengthening. Lancet 381, 2118–2133 (doi:10.1016/S0140-6736(12)62000-5) [DOI] [PubMed] [Google Scholar]
- 46.World Bank 1993. World development report. investing in health. World Bank, Washington, DC [Google Scholar]
- 47.World Health Organisation 2000. World health report 2000. Health systems: improving performance. WHO, Geneva, Switzerland [Google Scholar]
- 48.World Health Organisation 2001. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. World Health Organisation, Geneva, Switzerland [Google Scholar]
- 49.Wagstaff A, Culyer AJ. 2012. Four decades of health economics through a bibliometric lens. J. Health Econ. 31, 406–439 (doi:10.1016/j.jhealeco.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 50.Adam T, Ahmad S, Bigdeli M, Ghaffar A, Røttingen J-A. 2011. Trends in health policy and systems research over the past decade: still too little capacity in low-income countries. PLoS ONE 6, e27263 (doi:10.1371/journal.pone.0027263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newhouse J. 2002. Pricing the priceless: a health care conundrum. Cambridge, MA: MIT Press [Google Scholar]
- 52.Mills A. 2011. Health Systems in low- and middle-income countries. In Oxford handbook of health economics (eds Glied S, Smith PC.), pp. 30–57 Oxford, UK: Oxford University Press [Google Scholar]
- 53.Taskforce on Innovative International Financing for Health Systems 2009. Constraints to scaling up and costs. Geneva, Switzerland: World Health Organization [Google Scholar]
- 54.Mills A, Bennett S, Siriwanarangsun P, Tangcharoensathien V. 2000. The response of providers to capitation payment: a case-study from Thailand. Health Policy 51, 163–180 (doi:10.1016/S0168-8510(00)00059-2) [DOI] [PubMed] [Google Scholar]
- 55.Hanson K, Archard L, McPake B. 2001. Creating markets in hospital care: the adoption of developed country health sector reforms by developing countries. Is it appropriate? Trop. Med. Int. Health 6, 747–748 (doi:10.1046/j.1365-3156.2001.00808.x) [DOI] [PubMed] [Google Scholar]
- 56.Schick A. 1998. Why most developing countries should not try New Zealand's reforms. World Bank Res. Obs. 13, 123–131 (doi:10.1093/wbro/13.1.123) [Google Scholar]
- 57.Escobar M-L, Panopolou P. 2003. Health. In Columbia: the economic foundation of peace (eds Giugale M, Lafourcade O, Luff O.), pp. 653–708 Washington, DC: The World Bank [Google Scholar]
- 58.Kamuzora P, Gilson L. 2007. Factors influencing implementation of the community health fund in Tanzania. Health Policy Plan. 22, 95–102 (doi:10.1093/heapol/czm001) [DOI] [PubMed] [Google Scholar]
- 59.WHO Global Health Expenditure Atlas. Geneva, Switzerland: WHO; See http://www.who.int/nha/atlas.pdf (accessed 05 January 2012) [Google Scholar]
- 60.Acharya A, de Lima ATF, Moore M. 2006. Proliferation and fragmentation: transactions costs and the value of aid. J. Dev. Stud. 42, 1–21 (doi:10.1080/00220380500356225) [Google Scholar]
- 61.Balabanova D, McKee M, Mills A, Walt G, Haines A. 2010. What can global health institutions do to help strengthen health systems in low income countries? Health Res. Policy Syst. 8, 22 (doi:10.1186/1478-4505-8-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilson L, McIntyre D. 2008. The interface between research and policy: experience from South Africa. Soc. Sci. Med. 67, 748–759 (doi:10.1016/j.socscimed.2008.02.005) [DOI] [PubMed] [Google Scholar]
- 63.Wagstaff A, van Doorslaer E. 2003. Catastrophe and Impoverishment in paying for health care: with applications to Vietnam 1993–1998. Health Econ. 12, 921–934 (doi:10.1002/hec.776) [DOI] [PubMed] [Google Scholar]
- 64.Goudge J, Gilson L, Russell S, Gumede T, Mills A. 2009. Affordability, availability and acceptability barriers to health care for the chronically ill: longitudinal case studies from South Africa. BMC Health Serv. Res. 9, 75 (doi:10.1186/1472-6963-9-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaauw D, Erasmus E, Pagaiya N, Tangcharoensathein V, Mullei K, Mudhune S, Goodman C, English M, Lagarde M. 2010. Policy interventions that attract nurses to rural areas: a multicountry discrete choice experiment. Bull. World Health Org. 88, 350–356 (doi:10.2471/BLT.09.072918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lagarde M, Blaauw D. 2013. Pro-social preferences and self-selection into rural jobs: evidence from South African nurses RESYST Working Paper no. 3 London School of Hygiene and Tropical Medicine, London, UK: See http://resyst.lshtm.ac.uk/resources/WP3 (accessed 22 February 2014). [Google Scholar]
- 67.Patouillard E, Kleinschmidt I, Hanson K, Pok S, Palafox B, Tougher S, O Connell K, Goodman C. 2013. Comparative analysis of two methods for measuring sales volumes during malaria medicine outlet surveys. Malar. J. 12, 311 (doi:10.1186/1475-2875-12-311) [DOI] [PMC free article] [PubMed] [Google Scholar]