Abstract
Modern cities represent one of the fastest growing ecosystems on the planet. Urbanization occurs in stages; each stage characterized by a distinct habitat that may be more or less susceptible to the establishment of disease vector populations and the transmission of vector-borne pathogens. We performed longitudinal entomological and epidemiological surveys in households along a 1900 × 125 m transect of Arequipa, Peru, a major city of nearly one million inhabitants, in which the transmission of Trypanosoma cruzi, the aetiological agent of Chagas disease, by the insect vector Triatoma infestans, is an ongoing problem. The transect spans a cline of urban development from established communities to land invasions. We find that the vector is tracking the development of the city, and the parasite, in turn, is tracking the dispersal of the vector. New urbanizations are free of vector infestation for decades. T. cruzi transmission is very recent and concentrated in more established communities. The increase in land tenure security during the course of urbanization, if not accompanied by reasonable and enforceable zoning codes, initiates an influx of construction materials, people and animals that creates fertile conditions for epidemics of some vector-borne diseases.
Keywords: land tenure security, Chagas disease, Trypanosoma cruzi, Triatoma infestans, urbanization
1. Introduction
Modern cities represent one of the fastest growing ecosystems on the planet. Urbanization is especially pronounced in Latin America, where the proportion of the population living in cities has increased from 41% in 1950 to 80% today [1]. Historically, urbanization has led to sanitation and hygiene revolutions that have decreased the incidence of infectious diseases [2]. However, urbanization also leads to a greater density of people as well as domestic and peridomestic animals, creating conditions that can propagate, rather than reduce, disease transmission [3,4].
Chagas disease is arguably the most important vector-borne disease in the Americas. Millions of individuals are infected with Trypanosoma cruzi, the aetiologic agent. Initial infection with this parasite is typically mild with non-specific symptoms. Some 20–30% of those infected will progress, over a period of decades, to develop more severe clinical manifestations such as cardiomyopathy [5]. The transmission of T. cruzi by the insect vector, Triatoma infestans, is now recognized as a serious urban problem in Peru [6,7] and elsewhere [8,9]. Several reservoir hosts, including guinea pigs and dogs, contribute to T. cruzi transmission in Peru [10], and certain building materials, especially stacked brick, have been associated with large vector populations [6]. While most vector-borne epidemics spread through populations on a timescale of weeks or months, T. cruzi spreads over the course of decades [11], making Chagas disease an ideal system through which to observe the movement of a vector-borne pathogen through a city.
A number of socio-political drivers of urbanization have coalesced in southern Peru. The initiation of urbanization in the region is often attributed to ill-planned agrarian reform laws in the 1960s, which, along with drought and earthquakes, led to a drastic drop in agricultural production, and subsequent food scarcity in rural regions [12]. The rate of migration to large cities such as Arequipa increased drastically during the 1980s and early 1990s; much of the migration during this period was the result of actions from terrorist movements that led to an exodus from the Peruvian highlands [12]. Concurrently, legislation aimed at formalizing property rights was adopted in Peru [13]. A newly created agency, the Committee for the Formalization of Private Property, issued nearly 1.4 million new land titles between 1996 and 2004 [14,15]. Conference of land titles likely accelerated the ongoing urbanization of Arequipa and other large Peruvian cities, as access to property rights can be a factor in determining where and when rural migrants settle [16].
Geographically, Arequipa has expanded outwardly from its traditional centre, principally through the settlement of desert hills on the periphery of the city, and secondarily along major roads connecting the city to Lima and other destinations. New communities often arise when groups of individuals, often landless or displaced families [17], occupy vacant land. The nascent settlement is known as an invasion [18], and may remain in this stage of development for many years. Some authors, most recognizably the Peruvian economist Hernando de Soto [16], have argued that community-wide titling initiatives set in motion a metamorphosis within invasions, as greater land tenure security allows residents to build more permanent structures without fear of eviction. Others, especially Gilbert [19], provide an alternative and perhaps more nuanced view in which security of land tenure, and the accompanying changes to the built environment, may proceed in the absence of formal title.
Over time, and generally following the infilling of construction in residential lots in Arequipa, communities come to be referred to as pueblos jovenes (young communities). Eventually greater road, sewage and water infrastructure connect the pueblos jovenes to established sections of the city. In common usage, the terms ‘invasion’ and pueblo joven mark a perceived difference in land tenure security of communities. When a community is referred to as a young community (pueblo joven), it is implied that it is expected to develop; by contrast, referring to a community as an ‘invasion’ implies that its trajectory is uncertain: it may be successful, with residents establishing themselves on the land, or it may be repelled, and vanish altogether.
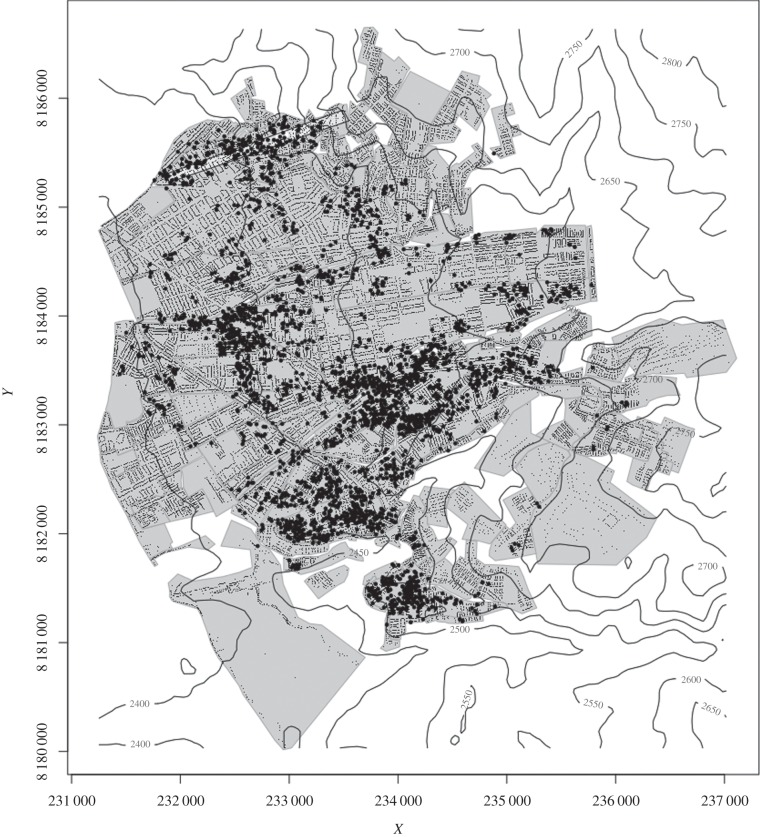
Since 2003, the Arequipa office of the Peruvian Ministry of Health has been conducting a campaign to control T. infestans in the city. The campaign, which comprises education and health communication, followed by insecticide application and continued community-based surveillance for vector recurrence [7,20], is a consolidated attempt to eliminate the insect from the city. The campaign has moved from west to east, from rural to periurban, and finally in 2009, to fully urban neighbourhoods. Over the course of the urban portion of the campaign, a pattern has emerged—the prevalence of household vector infestation is large in communities proximate to, but not a part of, the traditional centre of the city, but very low in more distal communities to the east (figure 1) [21].
Figure 1.
A map of the district of Mariano Melgar and the neighbouring district of Paucarpata, Arequipa, Peru. The study transect is shown as the lightly shaded rectangle towards the top of the map. Contour lines (50 m) represent the elevation. Houses surveyed for the presence of Triatoma infestans are shown as small black dots. Households in which insects were detected are represented in larger black circles; the coordinates of these have been spatially jittered to mask the exact location of infestations.
From 2009 to 2011, insecticide procurement issues at a national level interrupted the control campaign; this delay provided an opportunity to conduct longitudinal studies of the vector populations in the city. We designed an urban transect to test two alternative hypotheses to explain the observed pattern in vector populations. Our first hypothesis was that the vector was still moving through the city, and had yet to reach the outskirts on the eastern extreme. Our second hypothesis was that the vector had reached an environmental limit, and perhaps owing to some combination of temperature, humidity or other factors, was unable to establish itself at the eastern edge of the city. We describe below the results of repeated sampling along this urban transect, and how these have led us to a third hypothesis: the process of urbanization itself is constraining the expansion of the vector population. We discuss how a key step in this process—the transition from invasion to pueblo joven—may inadvertently create conditions that favour the emergence of T. infestans and eventually Chagas disease. A full Spanish translation of the text is provided in the electronic supplemental material.
2. Methods
(a). Study site: a transect of Arequipa, Peru
We conducted a series of household surveys from February 2010 to September 2011 along a 1900 × 125 m transect of Arequipa, Peru, a major city in which T. cruzi transmission by the insect vector T. infestans has emerged. The transect encompasses 445 households that span the gradient of development in Arequipa, spanning nine localities of the district of Mariano Melgar. Twenty-six of these households are in a recent land invasion, 272 are in pueblos jovenes, and 147 are in established communities (figures 1 and 2).
Figure 2.
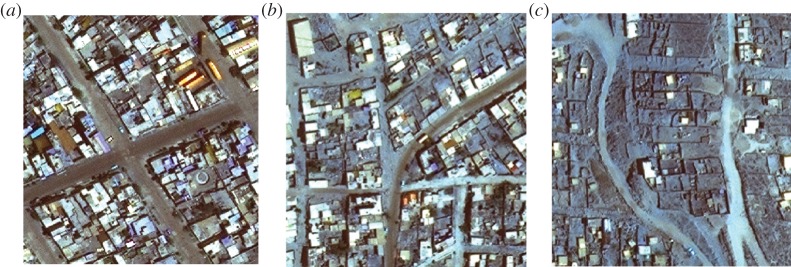
(a) Satellite photographs of households in an established community, (b) a pueblo joven and (c) a recent invasion along a transect of Arequipa, Peru, 2011. (Online version in colour.)
(b). Survey techniques
We first conducted entomological and veterinary surveys along the transect between February and April 2010. We returned to the houses every six months for a total of four visits. At each visit, we conducted a timed search for triatomine insects for one person-hour [6]. We used a tetramethrin spray (Sapolio, Mata Moscas) to flush out the insects from their hiding places [22]. All insects, except first-instar nymphs, were examined for the presence of T. cruzi at the Universidad Peruana Cayetano Heredia/University of Pennsylvania Chagas Disease Field Laboratory in Arequipa following published protocols [6]. We recorded a full description of the domestic animal populations and domiciliary construction materials in each household. We determined the geographical location of all animal enclosures, human dwelling places and vector colonies by referencing a high-resolution satellite image. Weather conditions (temperature and relative humidity) in two locations within the transect, one at the base and one at top, were measured hourly using a Hobo sensor.
The fourth and final sampling was conducted between June and September of 2011 in coordination with the first of two applications of insecticide (deltamethrin) by the Ministry of Health of Arequipa in the district of Mariano Melgar. At this point, we invited all individuals over 2 years of age living along the transect to participate in a serological survey. Three to five millilitres of blood was drawn from all consenting individuals and tested in our laboratory using ELISA (Chagatek, Quimica Suiza). Positive samples were additionally tested by immunofluorescence assay (IFA) and the trypomastigote excreted-secreted antigen assay [23]. Individuals positive to at least one of the confirmatory assays were considered confirmed for T. cruzi infection. We have found previously that the IFA confirmatory test has a very low negative predictive value among ELISA+ individuals in Arequipa [7,24]. For this reason, all ELISA+ individuals, both those confirmed for T. cruzi infection and those with discordant results, were referred to the Ministry of Health for clinical evaluation and treatment when indicated. We gathered detailed information about the migration history of participants in this survey and used event history calendars [25] to elicit recall of the time of the first appearance of vectors in each household. We asked residents directly if they had title to their land, and when the title was conferred. Intuitional review board approval was obtained from the Universidad Peruana Cayetano Heredia and collaborating institutions.
(c). Statistical analysis
We used Fisher's exact test to compare the proportion of houses infested in the invasions, pueblos jovenes and established communities. We then performed a more detailed analysis on the presence or absence of vectors in each habitat (animal enclosure or room of the human dwelling) sampled. We used a multivariate logistic regression to evaluate the effects of the level of development on infestation, controlling for the host populations and construction materials present in the habitat. We reduced the dimensionality of our models by constructing principal components [26] to describe the covariates (figure 3). Models were fit with generalized estimating equations [27] with an exchangeable correlation structure to account for the multiple observations in each habitat over the course of the study. All analyses were performed in R [28].
Figure 3.
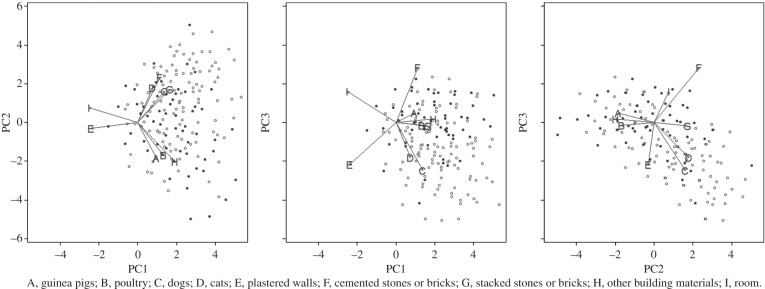
A visual representation of the principal components (PCs) of data on animal populations and construction materials present in animal enclosures and human dwelling areas along the transect study site. The first principal component is plotted against the second (a), the first against the third (b) and the second against the third (c). Coloured dots represent infested sites; open dots represent uninfested sites. These principal components are included in the models presented in table 3.
3. Results
(a). The built environment density, land titles and reservoir species
There are a number of differences in the built environment, possession of land titles and composition of species along the transect. The density of the built environment increases with development (figure 2). The median number of habitats (rooms and animal enclosures) per property increased from five in the invasions to eight in the pueblos jovenes and then to 10 in the established communities. The increase in density within households is evident in the satellite photographs presented in figure 2. The predominant building material in the invasions was the basalt rubble naturally occurring on the volcanic hillsides surrounding the city. Brick construction was more common in pueblos jovenes, and fully mortared walls were the most common in the established communities. However, unmortared brick and loose stones were present in 4.2% of rooms and 26% of animal enclosures surveyed in the established communities (table 1). Access to basic services increased with level of development. Only 7% of households in the invasions had running water, compared with 88% and 100% of households in the pueblos jovenes and established communities, respectively. Half of 20 households interviewed in the invasion reported holding title to their land; these households reported receiving title in 2006 or 2007, shortly before our sampling in 2009. By contrast, 88 of 91 households interviewed in the neighbouring pueblo joven reported holding land title; the median and mode year of receiving title were 1997 and 1998, respectively.
Table 1.
Building materials present in rooms and animal enclosures in communities of different development stages along a transect of Arequipa, Peru, in 2010. p-values are calculated using Fisher's exact test comparing the proportion of each building material in each development type with that in the other two types. Only major building materials were considered, and most habitats were comprised of several materials (columns may sum to >100%).
| rooms |
animal enclosures |
|||||
|---|---|---|---|---|---|---|
| invasion | pueblo joven | established communities | invasion | pueblo joven | established communities | |
| fully plastered walls | 34%*** | 58%*** | 75%*** | 3.4% | 13% | 19%* |
| cemented brick or stone | 32% | 29%*** | 21%*** | 17% | 31% | 35% |
| unmortared brick or stone | 30%*** | 14%*** | 4.2%*** | 69%*** | 44%*** | 26%*** |
| wire mesh | n.a. | n.a. | n.a. | 21% | 31%* | 41%** |
| N | 53 | 1459 | 1206 | 29 | 556 | 312 |
*p < 0.05, **p < 0.01, ***p < 0.001.
Animal husbandry practices also transition along the transect. Guinea pigs and dogs are the major reservoirs of T. cruzi in southern Peru [10]. Dogs were found in fairly consistent densities along the transect. Guinea pigs, while common in the pueblos jovenes and the established communities at lower altitudes, were rarer in the invasions at the top of the transect. Chickens, an important host for the vector, though not a reservoir of T. cruzi [29], were similarly absent from the recent invasions. Animals were typically housed in enclosures of unmortared stone or bricks in the invasions and pueblos jovenes, and wire enclosures supported by wood frames in more developed communities (table 1).
(b). Entomological collections
Over the four sampling periods, we uncovered vectors in 85 of 258 houses (32.9%) in the pueblos jovenes (table 2). Ten of these 85 households (11.8%) harboured vectors carrying T. cruzi. Infested households were slightly less common in the established communities with 36 of 147 (24.5%) sampled households harbouring insects, but among the infested households, a greater proportion harboured T. cruzi-infected insects (17 of 36 infested houses (47.2%); p-value = 0.0017, two-sided Fisher's exact test comparing established communities with pueblos jovenes). Not a single vector was collected from the 19 households sampled in the recent invasion at the top of the transect (p-value less than 0.003; Fisher's test comparing the proportion of infested households in the invasion with other communities). Over subsequent sampling, vector densities nearer the top of the transect, from metre 1500 to 1800, increased (figure 4), whereas those in the lower portions remained constant or decreased. Still, no vectors crossed the invisible line between the topmost pueblo joven and the most recent invasion.
Table 2.
The number of households infested by Triatoma infestans and Triatoma infestans carrying Trypanosoma cruzi over three development stages along a transect of Arequipa Peru, 2010–2011.
| development stage | no. houses surveyed | no. houses w/vectors (%) | no. houses w/vectors carrying parasites (%) |
|---|---|---|---|
| invasion | 19 | 0a,b (0.0) | 0 (0.0) |
| pueblo joven | 258 | 85 (32.9) | 10 (11.8) |
| established communities | 147 | 36 (24.5) | 17c (47.2) |
| total | 424 | 121 | 27 |
aNumbers represent presence of vectors or vectors carrying parasites during any of the four sampling periods.
bThe proportion of houses infested with vectors in invasions is significantly lower than that in other communities (p-value < 0.003; Fisher's exact test).
cThe proportion of houses harbouring vectors carrying parasites in established communities was significantly higher than that in pueblos jovenes (p-value = 0.0017; Fisher's exact test).
Figure 4.
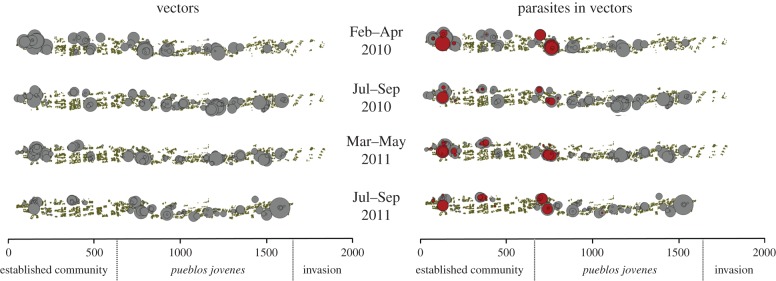
Spatial locations of Triatoma infestans (left) and Triatoma infestans carrying Trypanosoma cruzi along a transect of the city of Arequipa, Peru, over four samplings between 2010 and 2011. Points representing vector colonies (in grey) are scaled by the log of the number of vectors collected at each site; T. cruzi-infected vector colonies are shown in red on the same scale. (Online version in colour.)
The most important covariates positively associated with infestation were those we have described previously in Arequipa [6]: the presence (but not number) of guinea pigs, dogs and stacked or cemented (but not fully plastered) building materials. After controlling for the principal components describing these and other covariates related to animal hosts and construction materials, the odds of infestation in invasions compared with the reference group (established communities) was highly significant (p < 0.001), and incalculably small owing to the complete absence of vectors in the invasions (table 3). The odds of infestation among habitats in the pueblos jovenes were less than the reference (OR = 0.86), though the association was not significant (p = 0.21) after controlling for covariates. The first three principal components describing materials and animals were all significant; inclusion of additional components did not affect the model estimates.
Table 3.
Multivariate logistic regression describing risk factors for Triatoma infestans infestation in habitats along a transect of Arequipa, Peru, 2010–2011. Details of the principal components are shown in figure 3.
| coefficient | s.e. | Pr(>|W|) | |
|---|---|---|---|
| principal component 1 | 0.52089 | 0.03123 | <2 × 10 −16*** |
| principal component 2 | 0.08798 | 0.02938 | 0.002747** |
| principal component 3 | 0.14317 | 0.03957 | 0.000296*** |
| invasion | −39.90747 | 0.12496 | <2 × 10−16*** |
| pueblo joven | −0.15432 | 0.12349 | 0.211428 |
**p < 0.01, ***p < 0.001.
(c). Recall of first incurrence of vectors
Event history calendars provided a range of times during which participants first recalled observing the presence of T. infestans in their households (figure 5). The majority of residents nearer to the bottom of the transect reported the first occurrence of the vector in their households a long time ago, reaching back to the establishment of the communities in the early-twentieth century. The ranges reported were broad, probably owing to greater uncertainty associated with the recall of long-ago events compared with more recent ones [30]. Around metre 1400 of the transect, the pattern of recall in event history calendars transitioned. Residents consistently reported the incurrence of the vector into their homes within the past 20 years, and there is a clear positive and linear correlation between the reported times of incurrence and the geographical position of households. In the invasions, only two residents had ever seen the insects in their households, and both reported that they had first observed the vector very recently.
Figure 5.
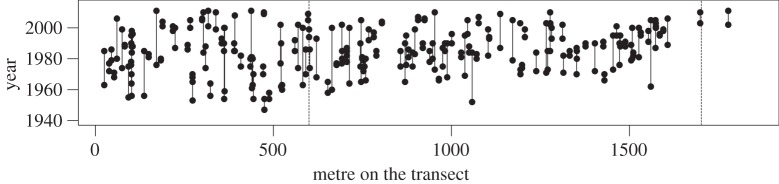
Time of first incurrence of T. infestans into households along a transect of Arequipa, Peru, as delimited through event history calendars. The first dotted vertical line marks the boundaries between established communities and pueblos jovenes; the second marks the boundary between the pueblos jovenes and the new invasion.
(d). Trypanosoma cruzi transmission foci and cases of Chagas disease
We initially observed two large foci of T. cruzi-infected insects (around metres 100 and 750), as well as an isolated occurrence of the parasite in one vector colony at metre 300. All of the foci of transmission were in the lower half of the transect. Over subsequent sampling, the isolated occurrence of the parasite expanded from a single colony to a focus of several colonies. On the fourth and final sampling, we observed an additional isolated instance of the parasite at metre 1100 (figure 4).
Fourteen of the 993 individuals tested for T. cruzi infection (1.41%) were positive by ELISA; nine were confirmed by a second test, and one moved from the study area and was lost to follow-up. Six of the nine confirmed cases lived in the lower sector of the transect and near the observed foci of vector-borne T. cruzi transmission. Three individuals lived in an area in which T. cruzi was absent from vectors, and, based on migration histories, were likely infected prior to resettling in the transect.
4. Discussion
Migrants to developing cities are often marginalized geographically as well as economically, so that gradients in the developing urban environment follow natural landscape clines. Pathogens such as T. cruzi, arriving in urban environments, are thus superimposed upon a very complex substrate. Our results suggest that the process of urbanization is constraining the dispersion of Triatoma infestans, the principal vector of T. cruzi, in southern Peru. Specifically, the insect is unable to establish itself in new land invasions.
We cannot pinpoint any single or set of factors that might protect land invasions from vector infestation. The materials and animals which provide habitat to T. infestans elsewhere are present in invasions, yet the association between invasions and the absence of insects remains strongly significant even after controlling for their presence. Perhaps the protection provided by the invasions is not on the level of the individual habitats, but an emergent property of the environment as a whole. T. infestans must survive as a metapopulation [31] in an unstable and patchy environment, as its food sources are transient throughout the year. The density of suitable habitats in the invasions may simply be too low to support the insect.
The density of habitats within a household is the link we propose between land tenure security and triatomine infestation. During the transition from invasion to pueblo joven, the greater land tenure security afforded by property formalization spurs the growth of the built environment as well as human and animal populations [16]. We hypothesize that this growth provides vectors with a sufficient range of habitats and meal sources to establish colonies and persist in the new environment. The properties in the land invasion we surveyed had on average only five habitats (rooms and animal enclosures), whereas the pueblos jovenes and established communities that were infested by the vector had eight and 10, respectively. We were not able to measure land tenure security among residents of different communities directly, but rather indirectly through the imperfect surrogate [19] of land titles. Half of the households we interviewed in the invasion did not hold title to the land on which they reside, and those that did had obtained it only very recently. By contrast, nearly all residents of pueblos jovenes held title to their land, many for over a decade.
We had initially hypothesized that the vector may have been spreading outwardly through the city, and had simply not yet arrived at the invasions on the outskirts. The data from event history calendars are consistent with an outward spread of the vector. However, the spread is far too slow to be attributed to the dispersion of the insect. According to the trend in the event history calendars, it took two decades for the insects to advance a distance of several hundred metres. By contrast, in earlier field studies, we found insects to infest newly placed sentinel enclosures in about two weeks [32]. We also hypothesized that the vector may have been excluded from the eastern edge of the city owing to local environmental conditions. The difference in altitude between infested houses in the pueblo joven and the uninfested households of the invasion is only 5 m. While we cannot rule out an environmental block to the insects, we can say that, if such a block exists, then it would be rather exceptional. Additionally, at the edge separating the highest pueblo joven from the invasion, insect populations increased at a faster rate than anywhere else along the transect (figure 4). Generally, we would not expect insect populations to increase if they were abutting an environmental limit (although see [33] for a discussion of this point). Altitude itself is not a definitive barrier to the insect, as we have observed populations of T. infestans in more developed communities at higher altitudes than the invasion we studied, as is demonstrated in figure 1.
Our study is limited by a number of factors. Our ability to make inferences on the dynamics of vectors and parasites was limited by our own observations—as we removed thousands of insects at each sampling, we perturbed the vector populations that remained. It is possible that, by disrupting insect habitats, our sampling led to greater scatter of vector populations. Equally possible, by removing insects and decreasing density-dependent competition among them, we created a net decline in the total number of insects migrating from each colony we disturbed. Whatever the net effect of our actions, they were not substantial enough to lead to the presence of insects in the new invasion. We also were not able to obtain detailed information on the date of acquisition of land title from all participants, and therefore could not consider this variable directly.
We have shown previously that urban T. cruzi transmission occurs through a series of spatially focal microepidemics [11]. We witnessed the growth of one such microepidemic over the course of repeated sampling of the transect. The rapid formation of this microepidemic suggests that the foci of transmission we observe in urban Arequipa are very recent, potentially only a few years old. The hypothesis that T. cruzi transmission in the city is recent is consistent with the relatively low prevalence of Chagas disease observed among individuals tested along the transect, and across the city [34] as well as with the lack of late-stage cases of Chagas disease in hospitals in the city [11].
Our results serve as a warning for the unintentional outcomes of formalization of property in Peru and elsewhere. Prior to the 1996 reforms, obtaining a land title in Peru was a prohibitively expensive and lengthy process [15,35]. Following the reforms, title could be obtained as quickly as six weeks [15,36], and the direct costs associated with obtaining title reduced as much as 40-fold (from $2000 to $50) [35]. Some critics of the Peruvian mass titling initiative have noted unsatisfactory effects on housing markets and credit availability for the poor [19]. Nevertheless, the initiative has been generally well received, as formalization of land invasions brings numerous social, economic and health benefits [37] to residents of these communities, as well as increased tax revenue to local and regional governments [38]. However, when such formalization is not accompanied by reasonable and enforceable zoning codes, it leads to an influx of building materials, people, and animals that creates prime habitats for triatomine bugs and other insects of epidemiological importance. The costs associated with control of these insects are high [39,40] and can quickly erase the economic gains engendered by the formalization of property.
The zoning codes currently in effect in Arequipa (provided in Spanish and English in the electronic supplementary material) consist of a list of fines, some of which are unrealistically high relative to the income of homeowners in Mariano Melgar and similar communities. Among the fines are some for the husbandry of large animals (cows and pigs) in urban settings. There are no restrictions or fines regarding the husbandry of guinea pigs, a practice known to be associated with T. infestans infestations [10]. There is a recent revival of architecture as a means to prevent insect infestations [41] and alternative structures and materials have been shown to decrease the susceptibility of households to mosquito vectors of malaria [42]. Still, such protective factors of individual dwellings have not, to any great extent, been incorporated into zoning codes as a means to protect a community from vector-borne Chagas disease. Zoning codes need not be perfect. Just as complete coverage of vaccination is not needed to eliminate most infectious diseases (as a result of herd immunity [43]), partial adherence to a zoning code may still be sufficient to protect a whole community from a disease vector [44].
Chagas disease is often described as a disease of the poorest of the poor [45]. In Arequipa, and perhaps many other cities, this characterization is not entirely accurate. It is not the poorest of the poor—recent migrants and others who reside in land invasions or newer pueblo jovenes—who are at greatest risk of Chagas disease. According to our results, it is the relatively wealthier residents of more established sections of the city who have been living with vectors for much longer, and those who currently live in greater proximity to insects transmitting T. cruzi. That the parasite affects wealthier citizens, who may have more political clout, could facilitate zoning changes if the disease were more visible. But the symptoms of infection are entirely absent in the city owing to the short history of transmission and long asymptomatic period of Chagas disease [11]. New zoning codes will likely be unpopular, but given their potential to maintain the lower densities of human and animal habitats that we show here to protect against triatomine infestations, they are badly needed.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
This work is dedicated to the memory of Dr Eleazar Cordova Benzaquen. We gratefully acknowledge the invaluable contributions of the Ministerio de Salud del Perú (MINSA), the Dirección General de Salud de las Personas (DGSP), the Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxenicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), the Dirección General de Salud Ambiental (DIGESA), the Gobierno Regional de Arequipa, the Gerencia Regional de Salud de Arequipa (GRSA), the Pan American Health Organization (PAHO/OPS) and the Canadian International Development Agency (CIDA). We also thank Robert Gilman, Caryn Bern, Natalie Bowman and Michael Harhay for their input on the study. The Chagas Disease Working Group in Arequipa in 2009–2011 included: Fernando Malaga-Chavez, Andy Catacora, Karina Oppe, Katelyn Levy, Miranda Hillyard, Megan Christenson, Lina Mollesaca-Riveros, Jose Ylla-Velásquez, Javier Quintanilla-Calderón, Jorge Apaza-Perez, Roger Quispe-Apaza, Danitza Pamo-Tito and Oscar Carrion.
Funding statement
Funding for this study came from National Institutes of Health P50 AI074285. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. De-identified data can be made available upon request; please email mzlevy@mail.med.upenn.edu.
References
- 1.Spencer N, Butler D. 2010. Cities: the century of the city . Nature 467, 900–901. ( 10.1038/467900a) [DOI] [PubMed] [Google Scholar]
- 2.Gross R, Schell B, Molina MC, Leao MA, Strack U. 1989. The impact of improvement of water supply and sanitation facilities on diarrhea and intestinal parasites: a Brazilian experience with children in two low-income urban communities . Rev. Saude Publ. 23, 214–220. ( 10.1590/S0034-89101989000300006) [DOI] [PubMed] [Google Scholar]
- 3.Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. 2011. Urbanisation and infectious diseases in a globalised world . Lancet Infect. Dis. 11, 131–141. ( 10.1016/S1473-3099(10)70223-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enserink M. 2008. Entomology. A mosquito goes global. Science 320, 864–866. ( 10.1126/science.320.5878.864) [DOI] [PubMed] [Google Scholar]
- 5.Mady C, Cardoso RH, Barretto AC, da Luz PL, Bellotti G, Pileggi F. 1994. Survival and predictors of survival in patients with congestive heart failure due to Chagas’ cardiomyopathy . Circulation 90, 3098–3102. ( 10.1161/01.CIR.90.6.3098) [DOI] [PubMed] [Google Scholar]
- 6.Levy MZ, Bowman NM, Kawai V, Waller LA, del Carpio JGC, Benzaquen EC, Gilman RH, Bern C. 2006. Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru . Emerg. Infect. Dis. 12, 1345 ( 10.3201/eid1209.051662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman NM, et al. 2008. Chagas disease transmission in periurban communities of Arequipa, Peru . Clin. Infect. Dis. 46, 1822–1828. ( 10.1086/588299) [DOI] [PubMed] [Google Scholar]
- 8.Santana Kde S, Bavia ME, Lima AD, Guimaraes IC, Soares ES, Silva MM, Mendonca J, Martin Mde S. 2011. Spatial distribution of triatomines (Reduviidae: Triatominae) in urban areas of the city of Salvador, Bahia, Brazil . Geospat. Health 5, 199–203. [DOI] [PubMed] [Google Scholar]
- 9.Medrano-Mercado N, Ugarte-Fernandez R, Butrón V, Uber-Busek S, Guerrai HL, de Araújo-Jorge TC, Correa-Oliveira R. 2008. Urban transmission of Chagas disease in Cochabamba, Bolivia . Mem. Inst. Oswaldo Cruz 103, 423–430. ( 10.1590/S0074-02762008000500003) [DOI] [PubMed] [Google Scholar]
- 10.Córdova E, Montesinos J, Náquira F. 1969. Estudio epidemiológico sobre la enfermedad de Chagas en el valle de Vitor . Arch. Per Pat. Clin. 23, 257–270. [Google Scholar]
- 11.Levy MZ, et al. 2011. Retracing micro-epidemics of Chagas disease using epicenter regression . PLoS Comput. Biol. 7, e1002146 ( 10.1371/journal.pcbi.1002146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen D, Tremblay J, Errazuriz C, Gamarra J. 2008. The sequelae of political violence: assessing trauma, suffering and dislocation in the Peruvian highlands . Soc. Sci. Med. 67, 205–217. ( 10.1016/j.socscimed.2008.03.040) [DOI] [PubMed] [Google Scholar]
- 13.De Soto H. The other path: the invisible revolution in the Third World. 1990 New York, NY: Harper & Row. [Google Scholar]
- 14.Martine G. 2008. The new global frontier: urbanization, poverty and environment in the 21st century. London, UK: Earthscan. [Google Scholar]
- 15.Field E. 2007. Entitled to work: urban property rights and labor supply in Peru . Q. J. Econ. 122, 1561–1602. ( 10.1162/qjec.2007.122.4.1561) [DOI] [Google Scholar]
- 16.De Soto H. 2003. Mystery of capital: why capitalism triumphs in the West and fails everywhere else. New York, NY: Basic books. [Google Scholar]
- 17.Bayer AM, Hunter GC, Gilman RH, del Carpio JGC, Naquira C, Bern C, Levy MZ. 2009. Chagas disease, migration and community settlement patterns in Arequipa, Peru . PLoS Negl. Trop. Dis. 3, e567 ( 10.1371/journal.pntd.0000567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torribio M. 1995. Recipe for a house. In The Peru reader: history, culture, politics (eds Starn O, Degregori C, Kirk R.), pp. 278–281. Durham, UK: Duke University Press. [Google Scholar]
- 19.Gilbert A. 2002. On the mystery of capital and the myths of Hernando de Soto: what difference does legal title make? Int. Dev. Plan. Rev. 24, 1–19. ( 10.3828/idpr.24.1.1) [DOI] [Google Scholar]
- 20.Levy MZ, Malaga Chavez FS, del Carpio JGC, Vilhena DA, McKenzie FE, Plotkin JB. 2010. Rational spatio-temporal strategies for controlling a Chagas disease vector in urban environments . J. R. Soc. Interface 7, 1061–1070. ( 10.1098/rsif.2009.0479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado S, Ernst KC, Pumahuanca ML, Yool SR, Comrie AC, Sterling CR, Gilman RH, Náquira C, Levy MZ. 2013. A country bug in the city: urban infestation by the Chagas disease vector Triatoma infestans in Arequipa, Peru . Int. J. Health Geogr. 12, 48 ( 10.1186/1476-072X-12-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood E, De Licastro SA, Casabé N, Sivori J, Zerba E. 1993. Evaluation of the flushing out activity of pyrethroids on Triatoma infestans . Int. J. Trop. Insect Sci. 14, 651 ( 10.1017/S1742758400018075) [DOI] [Google Scholar]
- 23.Umezawa ES, Bastos SF, Coura JR, Levin MJ, Gonzalez A, Rangel-Aldao R, Zingales B, Luquetti AO, da Silveira JF. 2003. An improved serodiagnostic test for Chagas’ disease employing a mixture of Trypanosoma cruzi recombinant antigens . Transfusion 43, 91–97. ( 10.1046/j.1537-2995.2003.00279.x) [DOI] [PubMed] [Google Scholar]
- 24.Tustin AW, et al. 2012. Use of individual-level covariates to improve latent class analysis of Trypanosoma cruzi diagnostic tests . Epidemiol. Methods 1, 33–54. ( 10.1515/2161-962X.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belli RF, Shay WL, Stafford FP. 2001. Event history calendars and question list surveys: a direct comparison of interviewing methods . Publ. Opin. Q. 65, 45–74. ( 10.1086/320037) [DOI] [PubMed] [Google Scholar]
- 26.Pearson K. 1901. LIII. On lines and planes of closest fit to systems of points in space. London, Edinburgh, and Dublin Philos. Mag. J. Sci. 2, 559–572. ( 10.1080/14786440109462720) [DOI] [Google Scholar]
- 27.Diggle P, Heagerty P, Liang K, Zeger S. 2013. Analysis of longitudinal data. New York, NY: Oxford University Press. [Google Scholar]
- 28.Team RC. 2005. R: a language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing. [Google Scholar]
- 29.Cohen JE, Gürtler RE. 2001. Modeling household transmission of American trypanosomiasis . Science 293, 694–698. ( 10.1126/science.1060638) [DOI] [PubMed] [Google Scholar]
- 30.Roy J, Stewart WF. 2010. Estimation of age-specific incidence rates from cross-sectional survey data . Stat. Med. 29, 588–596. [DOI] [PubMed] [Google Scholar]
- 31.Hanski I. 1999. Metapopulation ecology. New York, NY: Oxford University Press. [Google Scholar]
- 32.Levy MZ, et al. 2008. Impregnated netting slows infestation by Triatoma infestans . Am. J. Trop. Med. Hyg. 79, 528. [PMC free article] [PubMed] [Google Scholar]
- 33.Antonovics J, McKane AJ, Newman TJ. 2006. Spatiotemporal dynamics in marginal populations . Am. Nat. 167, 16–27. ( 10.1086/498539) [DOI] [PubMed] [Google Scholar]
- 34.Hunter GC, et al. 2012. A field trial of alternative targeted screening strategies for Chagas disease in Arequipa, Peru. PLoS Negl. Trop. Dis. 6, e1468 ( 10.1371/journal.pntd.0001468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaritis E, Weiss M, Lam AH. 2001. Do property rights matter? An urban case study from Peru . Global Outlook: Int. Urban Res. Monit. 1, 20–22. [Google Scholar]
- 36.Field E. 2003. Property rights, community public goods, and household time allocation in urban squatter communities: evidence from Peru . Wm. & Mary L. Rev. 45, 837. [Google Scholar]
- 37.Galiani S, Schargrodsky E. 2004. Effects of land titling on child health . Econ. Hum. Biol. 2, 353–372. ( 10.1016/j.ehb.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 38.de Soto H. 2000. El misterio del capital: por qué el capitalismo triunfa en occidente y fracasa en el resto del mundo. Lima, Peru: Empresa Editora de Comercio. [Google Scholar]
- 39.Vazquez-Prokopec GM, Spillmann C, Zaidenberg M, Kitron U, Gurtler RE. 2009. Cost-effectiveness of chagas disease vector control strategies in northwestern Argentina . PLoS Negl. Trop. Dis. 3, e363 ( 10.1371/journal.pntd.0000363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurtler RE. 2009. Sustainability of vector control strategies in the Gran Chaco region: current challenges and possible approaches . Mem. Inst. Oswaldo Cruz. 104(Suppl. 1), 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geiger CA, Cox C. 2012. Pest prevention by design: authoritative guidelines for designing pests out of structures. SF Environ. 1, 6–17. [Google Scholar]
- 42.Atieli H, Menya D, Githeko A, Scott T. 2009. House design modifications reduce indoor resting malaria vector densities in rice irrigation scheme area in western Kenya . Malaria J. 8, 108 ( 10.1186/1475-2875-8-108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson RM, May RM. 1990. Immunisation and herd immunity . Lancet 335, 641–645. ( 10.1016/0140-6736(90)90420-A) [DOI] [PubMed] [Google Scholar]
- 44.John TJ, Samuel R. 2000. Herd immunity and herd effect: new insights and definitions . Eur. J. Epidemiol. 16, 601–606. ( 10.1023/A:1007626510002) [DOI] [PubMed] [Google Scholar]
- 45.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. 2008. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination . PLoS Negl. Trop. Dis. 2, e300 ( 10.1371/journal.pntd.0000300) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.