Abstract
Trait decoupling, wherein evolutionary release of constraints permits specialization of formerly integrated structures, represents a major conceptual framework for interpreting patterns of organismal diversity. However, few empirical tests of this hypothesis exist. A central prediction, that the tempo of morphological evolution and ecological diversification should increase following decoupling events, remains inadequately tested. In damselfishes (Pomacentridae), a ceratomandibular ligament links the hyoid bar and lower jaws, coupling two main morphofunctional units directly involved in both feeding and sound production. Here, we test the decoupling hypothesis by examining the evolutionary consequences of the loss of the ceratomandibular ligament in multiple damselfish lineages. As predicted, we find that rates of morphological evolution of trophic structures increased following the loss of the ligament. However, this increase in evolutionary rate is not associated with an increase in trophic breadth, but rather with morphofunctional specialization for the capture of zooplanktonic prey. Lineages lacking the ceratomandibular ligament also shows different acoustic signals (i.e. higher variation of pulse periods) from others, resulting in an increase of the acoustic diversity across the family. Our results support the idea that trait decoupling can increase morphological and behavioural diversity through increased specialization rather than the generation of novel ecotypes.
Keywords: coral reef fishes, trait evolution, morphospace, morphological novelty, constraint, sound production
1. Introduction
Trait decoupling, the process by which evolutionary release of constraints via loss of linkage and/or repetition of elements permits formerly integrated structures to become specialized, exists as one of the most important principles of evolutionary morphology for explaining the diversity of organismal form [1–3]. Trait decoupling can be viewed as a mechanism that increases modularity, i.e. an increase in the number of phenotypic subunits on which selection can independently act to diversify the phenotype [4,5]. Although related hypotheses of genetic and developmental modularity have been widely examined [6,7], explicit tests of the evolutionary consequences of decoupling of organismal traits remain scarce. Liem's concept of modular multiplicity is one of the earliest and most influential formulations of this idea [8], and posits that the functional decoupling between the oral jaws (used for prey catching) and the pharyngeal jaws (used to prepare the prey before ingestion) may have been a key to the success of cichlids and other ‘labroid’ fishes [9,10] at multiple stages of their extraordinary radiation. Such a functional decoupling between these jaws allowed them to adapt to multiple prey types [8,11].
Influential, the concept of decoupling has received empirical supports at various organismal levels (see examples in [4,12]). Lauder illustrated decoupling of primitively coupled biomechanical linkage systems of muscles and bones in the head of certain ray-finned fishes is correlated with greater diversity of jaw structure and function than occurs in fishes with coupled system [2]. Muscle duplication has been shown to increase diversity of prey-processing motor patterns within tetraodontiform fishes [13,14], and decoupling is associated with greater morphological variability of associated structures than outgroups having no such decoupled systems [15]. However, key predictions of the hypothesis of decoupled systems regarding shifts in the tempo and the mode of trait evolution remain untested by modern phylogenetic comparative approaches.
The skull of damselfishes (Pomacentridae) provides an opportunity to test hypotheses of morphological trait decoupling. Across most of the 392 species within this family [16], the ceratomandibular (cmd) ligament creates a tight linkage between the hyoid bar and the lower jaw [17,18] (figure 1). This linkage holds biomechanical significance for two different systems. First, the tight connection between the lower jaws and branchial basket couples morphological units that are used during prey capture and prey transport [19,20], and may act as an evolutionary constraint partially explaining the well-documented lack of morphological diversity in oral jaws within damselfishes [21,22]. Indeed, we hypothesize the cmd ligament may act as a physical constraint limiting evolutionary shape variation of oral jaws. Second, the cmd ligament is also involved in sound production during courtship and agonistic interactions [23–26]. During simultaneous neurocranium elevation and hyoid depression, it acts as a cord, forcing rapid mouth closing [18] (figure 1). The forceful contact of the teeth of the upper and lower jaws is responsible for producing pulsed sounds [18,27]. As a result of the cmd ligament, the hyoid and mandible represent a coupled system that is subjected to, at least, two distinct functional demands: feeding and sound production.
Figure 1.
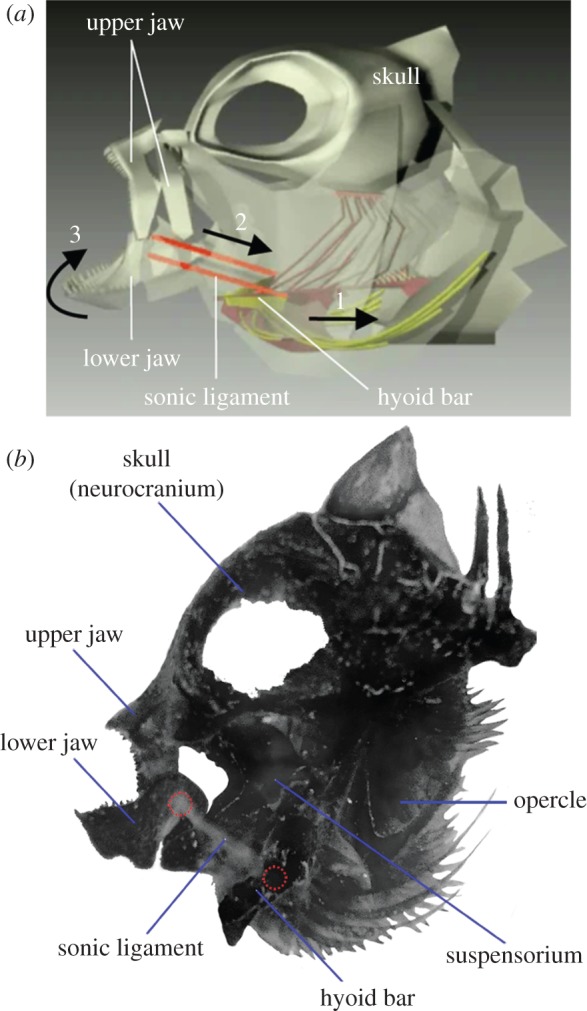
(a) Schematic representation of the sound-production mechanism, illustrating the movement of skeletal components (modified from [18]). Lowering the hyoid bar (1) stretches the sonic ligament (or ceratomandibular ligament) (2), and the jaws close the mouth (3) by rotating around the mandible articulation on the quadrate. Further details and videos illustrating the sound production mechanism are provided in [18]. (b) Lateral view of the skull in Amphiprion clarkii. All the skeletal units from the left side (i.e. jaw, cheek and opercle) and the branchial basket were removed in order to clearly show an internal view of the right side. The insertion points of the sonic ligament on the mandible and the ceratohyal (hyoid bar) are highlighted by dotted red circles.
Although the cmd ligament is considered to be a synapomorphy for the Pomacentridae [17], we recently discovered that it is absent in several species. The loss of this ligament in independent damselfish lineages provides a novel opportunity to test key predictions of the trait decoupling hypothesis. All previous tests of the decoupling hypothesis have been restricted to functionally related trait systems such as the pectoral girdle involved in locomotion of frogs [28] or the musculoskeletal system used for feeding in catfishes [15]. However, if decoupling underlies diversity on the scale envisioned by Liem [8], we should expect to find a strong signal of diversification both within and across disparate functional systems which rely upon the same, previously coupled structures. We tested this prediction in damselfishes by asking if the loss of the cmd ligament relaxes constraint and promotes the diversification of the trophic and acoustic systems of damselfishes. We used comparative phylogenetic analyses combined with new morphological and functional data to test whether the tempo and mode of damselfish evolution changed following the loss of the cmd ligament and to assess levels of acoustic diversity in species with and without the ligament.
2. Material and methods
(a). Morphological and trophic data
We studied a combination of specimens collected in the field (Corsica, French Polynesia, Hawaii, Japan, Madagascar, Papua New Guinea and Taiwan) and accessioned museum specimens in the morphological analyses. The complete list of representatives of species studied and their accession number is available in the electronic supplementary material, table S1. Methods for field collections were described in Frédérich & Vandewalle [29] and followed approved animal care protocols. We dissected and collected information about the presence/absence of the cmd ligament within 124 species (electronic supplementary material, table S1) represented in the Frédérich et al. time-calibrated phylogeny [21], which is used throughout the article. The large size of the cmd ligament (figure 1) makes its presence/absence easy to assess through dissections of fresh, conserved in alcohol or in toto stained specimens [30]. We studied overall body and oral jaws (i.e. mandible and premaxilla) shape from 392 and 519 adult specimens from 107 and 114 damselfish species, respectively. Sample sizes within species ranged between one and 22 individuals (median = three individuals; electronic supplementary material, table S1). We photographed specimens using digital cameras. We first cleared the jaws and then stained them with alizarin red S [30], to study the mandible and the premaxillary bone. We photographed the left unit using a digital camera installed on a binocular microscope. We further conducted geometrical morphometric analyses [31,32] and recorded the x-, y-coordinates of the homologous landmarks (electronic supplementary material, figure S1) using TpsDig [33]. All the details about the generation of shape data are provided in Frédérich et al. [21]. We used species scores along principal component axis for exploring phenotypic diversification during evolution. We assigned the 124 studied species to one of three commonly recognized trophic groups [34] based on literature review: (i) pelagic feeders that suck planktonic copepods, (ii) benthic feeders that graze filamentous algae or bite coral polyps, and (iii) an intermediate group, which feeds on planktonic prey, small benthic invertebrates and algae in variable proportions (electronic supplementary material, table S1).
(b). Phylogeny and stochastic mapping
The time-calibrated phylogeny is described in Frédérich et al. [21], and all details concerning DNA extraction, sequence alignment, fossil calibration, and divergence time estimation can be found therein. From the Bayesian posterior distribution generated by BEAST v. 1.6.2 [35], we randomly sampled 100 trees that we used throughout the article as a way of including uncertainty in tree topology and branch length into our phylogenetic comparative analyses.
We used stochastic character mapping [36] to infer possible histories of the cmd ligament. The stochastic mapping was produced using the function make.simmap in the phytools package (v. 0.2-27) [37] for R [38]. For the parametrization of make.simmap, we used the estimated ancestral state, and a transition matrix with unequal rates estimated from our empirical data. We then sampled 100 character histories for each of the 100 trees generating 10 000 character maps. These character maps allowed us to incorporate the uncertainty associated with the tree topology, branch lengths and timing of the transitions between the morphological states. For an illustrative purpose, we also used the function densityMap in phytools [37] to summarize the generated histories of the cmd ligament on the consensus time-tree.
(c). Lineage diversification
If decoupling permits novel opportunities for ecological diversification through the release of evolutionary constraint, then we predicted that the tempo of lineage diversification should change following the decoupling event. We used the ‘binary state speciation extinction’ (BiSSE) method [39] to test whether the loss of the cmd ligament triggers an elevated rate of speciation during the damselfish evolution. BiSSE provides a likelihood-based test of whether a discrete character (in this case, the presence or absence of the cmd ligament) influences the rate of lineage diversification. We compared the likelihoods of two models (constrained and an unconstrained) based on our empirical data (time-tree and character states at the tips). To test variation in speciation rates, the value of extinction rate was fixed (μlig = μno lig) and we compared the unconstrained model where speciation (λ) and transition (q) rate parameters are free to vary with the constrained model where the speciation rates for both character states are forced to be equal (λlig = λno lig). Then, the same framework was repeated to test variation in extinction rates (unconstrained model: μ and q are free to vary and λlig = λno lig versus constrained model: λlig = λno lig and μlig = μno lig). Difference in log-likelihoods was computed, and a χ2-test was used to test for significance.
(d). Ecological diversification
If decoupling promotes ecological diversification, then we predicted that cmd ligament loss would be associated with an increase in transition rate to new diet types. To test whether the loss of the cmd ligament promotes such a trophic diversification in damselfishes, we compared transition rates with new feeding modes before and after the loss of the cmd ligament using the ‘multiple state speciation extinction’ (MuSSE) method. MuSSE is an extension of the BiSSE likelihood-based test which is described in [39,40]. For this test, we used the Pagel recoding [41] of two state characters where the first trait is related to the ligament (X: 0 = absence, 1 = presence) and the second one to the trophic group (Y: 0 = other diet, 1 = planktivory). Our choice to use two diet codes (instead of the three described above) was justified by the reduction of parameters being estimated. The matrix of transition rates used can be written as
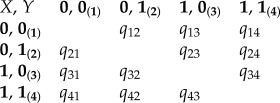 |
2.2 |
The subscripts on the rate parameters refer to the beginning and ending character states for each transition, where 1 = 0, 0; 2 = 0, 1; 3 = 1, 0; 4 = 1, 1. Thus, for example, q12 denotes a transition from state ‘absence of the ligament, other diet’ (0, 0) to ‘absence of the ligament, planktivory’ (0, 1). To test our hypothesis about the impact of the cmd ligament on the transition rates between diet, we followed a similar approach described by FitzJohn [40]. First, we constrained our model assuming that the speciation rates (λ) and the extinction rates (μ) are equal among traits (assumptions based on our BiSSE results). We also assumed that all transition rates including a loss of the cmd ligament are equal (q31 = q41 = q32 = q42) and that all transition rates including a gain of ligament are equal to zero (q13 = q14 = q23 = q24 = 0). Our biological assumptions allow a simplification of the model for which we estimated the probability to regain the ligament during evolution being close to zero. Then, to test whether the loss of the cmd ligament affects the rate of transition among trophic groups, MuSSE was used to compute likelihoods of our empirical data under two models, a constrained and an unconstrained model. The constrained model (null model) forced the evolutionary shifts of diet to be equal with and without the cmd ligament (q12 = q34, q21 = q43) and the unconstrained model allowed these parameters to vary (i.e. the rates of dietary transition are dependent on ligament state). A χ2-distribution with a single degree of freedom was used to test for significance.
We performed BiSSE and MuSSE analyses using the diversitree package [39] for R [36], correcting for incompletely resolved phylogeny without specifying the ligament state of the missing species [40]. Here, we assumed that the missing species are randomly distributed on the phylogenetic tree.
(e). Morphological diversification
To test whether changes in the tempo of morphological evolution through the evolutionary history of damselfishes were explained by the state (present or absent) of the cmd ligament, we used the method recently developed by Adams [42] for comparing evolutionary rates for complex, high-dimensional phenotypic traits such as shape. Under the expectation of a Brownian motion process, this test allows the comparison of shape evolutionary rates by simulations (n = 999 iterations) along the phylogeny and using all the shape data (i.e. all principal components of shape variables).
In addition to the visual exploration of morphospace defined by principal components of shape variables, we used the resampling procedures (n = 2500) implemented in TwoGroup [43] to test whether the shape of damselfishes having no cmd ligament differ significantly from others. By using TwoGroup, we first analysed divergence in shape without taking evolutionary history into account. Then, we compared the shape of damselfishes using phylogenetic MANOVAs in geiger [44].
(f). Sound variation
Functional studies have demonstrated that an intact cmd ligament is necessary for sound production in some damselfishes [18], and thus we predicted that lineages which have lost the cmd ligament may be mute. Alternatively, if the loss of the cmd ligament represents a decoupling for the acoustic system, then we predicted that lineages lacking this structure would show greater diversity in sounds owing to the release of evolutionary constraint on the integrated hyoid and mandible. To test this hypothesis, we compared the sounds of 13 damselfishes: 11 species having the cmd ligament and two phylogenetically closely related species lacking it. The data for Amphiprion and Dascyllus species are from [45] and [46], respectively. Recordings of sound production in the new species followed the protocol of Colleye & Parmentier [23]. Following previous acoustic studies in damselfishes [23,26,45], we carefully selected the sound parameters in our comparative study, because all (i.e. sound duration, number of pulses in a sound, pulse period, pulse length, dominant frequency and relative intensity) may not be relevant for interspecific comparisons. For example, the dominant frequency and the pulse duration are only related to fish size [45,47], and the number of pulses in a sound could be simply owing to its motivational state [24]. Consequently, we focused mainly on the pulse period, i.e. the average peak-to-peak interval between consecutive sounds.
3. Results
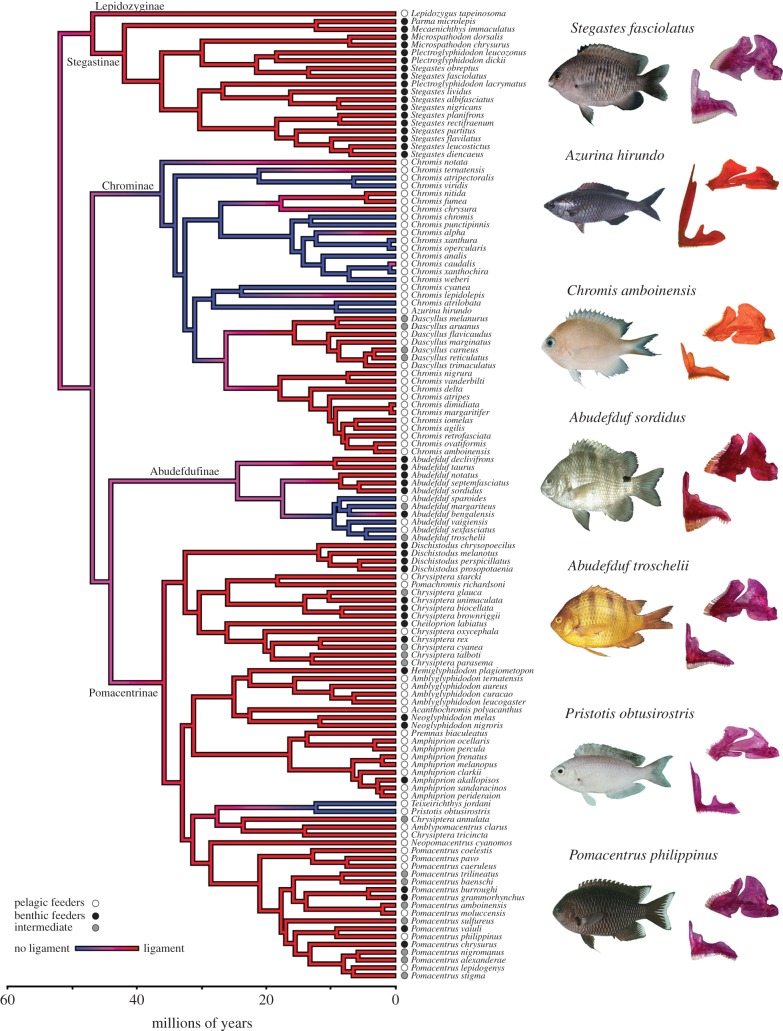
We identified 19 species lacking the cmd ligament (electronic supplementary material, table S1) among the 124 dissected adult specimens. The species lacking the cmd ligament belong to five genera: Abudefduf, Chromis, Azurina and the monotypic Pristotis and Teixeirichthys. Stochastic mapping of this trait on a time-calibrated phylogeny suggested that the cmd ligament was present in the common ancestor of damselfishes (97% posterior probability) and independently lost at least three times (within Chrominae, Abudefdufinae and Pomacentrinae; figure 2). We observed no great anatomical variation among species having the cmd ligament, except in Abudefduf sordidus and Abudefduf septemfasciatus. In both species, the cmd ligament is fused with the geniohyoideus muscle, which is attached to the ventral part of the mandible, and has probably become a tendinous projection of it.
Figure 2.
A time-calibrated phylogeny of damselfishes from [21] with a summary map of morphology (presence of the ceratomandibular (cmd) ligament in red and absence of the cmd ligament in blue) generated through stochastic character mapping using phytools [37]. This trait mapping highly suggests an ancestral damselfish with the cmd ligament as it is the most common root state within the 100 character histories we generated (97% of histories). Circles at the tips of the tree indicate each species diet: mainly pelagic feeders (zooplanktivorous), mainly benthic feeders (including algivorous and coralivorous), and an intermediate group including omnivorous species feeding on planktonic prey, small benthic invertebrates and filamentous algae in variable proportions. Photos illustrating the morphological diversity of body, mandible and premaxilla shapes among damselfishes having or not having the ligament are also shown across the phylogeny. The five subfamilies are highlighted: Lepidozyginae, Stegastinae, Chrominae, Abudefdufinae and Pomacentrinae.
(a). Lineage diversification
We did not record changes in the rate of cladogenesis following the disappearance of the ligament. Indeed, the results of BiSSE analyses provide no support for a two-rate model where the cmd influenced the rate of speciation (lnL unconstrained = −487.51, lnL constrained = −487.75, χ2 = 0.47, p = 0.55; λlig = 0.102 ± 0.006, λno lig = 0.136 ± 0.016) or extinction (lnL unconstrained = −487.75, lnL constrained = −487.76, χ2 = 0.02, p = 0.92; μlig = 0.003 ± 0.004, μno lig = 0.003 ± 0.005) of damselfishes.
(b). Ecological diversification
We found that a model where rates of dietary transitions were dependent on ligament state was significantly better than a model where dietary shifts were independent (lnL-dependent = −569.78, lnL-independent = −563.62, χ2 = 12.32, p = 0.003). However, parameter estimates revealed that only transition rates to zooplanktivory increased by a factor 10 once the ligament was lost (electronic supplementary material, table S2). Visual inspection of diet changes on the pomacentrid phylogeny reinforced the MuSSE results, revealing all but two instances of cmd ligament loss to be associated with a single feeding mode, zooplanktivory (figure 2).
(c). Morphological diversification
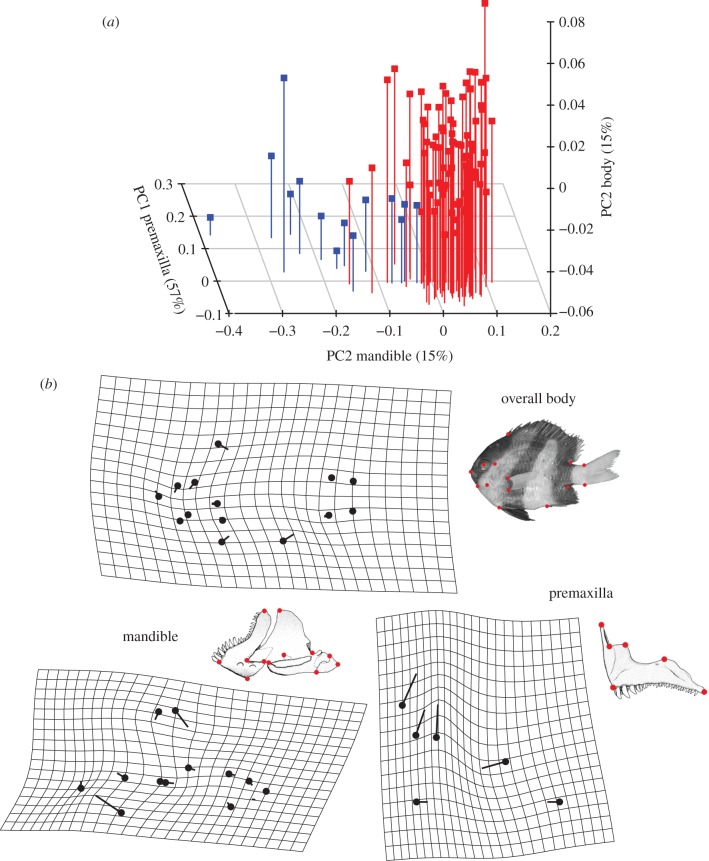
We found that the net morphological evolutionary rates differ significantly between species having and lacking the cmd ligament (table 1). For each morphological entity, the rate of shape evolution (σ2) is higher (more than 1.3x) for the lineages lacking the cmd ligament (table 1). Moreover, visual exploration of morphospace revealed that cmd ligament loss is associated with the evolution of new phenotypes (figure 3). Indeed, species without the cmd ligament occupy a particular subspace of the morphospace (figure 3) and differ significantly in shape from the others (TwoGroup results, body: F-score = 6.6, p < 0.001; mandible: F-score = 21.1, p < 0.001; premaxilla: F-score = 62.4, p < 0.001). Knowing that most of the species lacking the cmd ligament are zooplanktivorous (figure 2 and the electronic supplementary material, table S1), we repeated the tests by only comparing the shape of zooplanktivorous species and once again found significative differences between the species having the ligament and the species without it (TwoGroup results, body: F-score = 6.1, p < 0.001; mandible: F-score = 13.3, p < 0.001; premaxilla: F-score = 50.8, p < 0.001). Phylogenetic MANOVAs strengthen these first results from TwoGroup, showing species lacking the ligament significantly differ in shape from the ones having it (table 2). Within zooplanktivorous species, the ones lacking the cmd ligament observed a strong shape divergence for the premaxilla during evolution (table 2). The species lacking the cmd ligament show slender body with shorter dorsal and anal fins; longer and thinner mandible with an articulo-angular relatively greater; longer ascending process of the premaxilla with a short maxillad spine than other damselfishes and other zooplanktivorous species (figures 2 and 3 and the electronic supplementary material, figure S2).
Table 1.
Results from comparisons of morphological evolutionary rates between the damselfish species lacking the ceratomandibular ligament and the ones having this ligament. The estimated rates of morphological evolution using multivariate data are also provided (mean ± s.d.) and the ratio between them 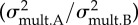 .
.
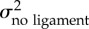 |
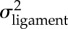 |
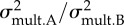 |
p-values | |
|---|---|---|---|---|
| body | 1.51 ± 0.54 × 10−5 | 0.98 ± 0.14 × 10−5 | 1.3920 | 0.001 |
| mandible | 6.96 ± 0.21 × 10−5 | 3.45 ± 0.36 × 10−5 | 1.9139 | 0.001 |
| premaxilla | 11.4 ± 1.94 × 10−5 | 4.94 ± 0.48 × 10−5 | 2.3251 | 0.001 |
Figure 3.
(a) Three-dimensional-morphospace of damselfishes made by principal components of each studied unit (body, mandible and premaxilla). The species lacking the ceratomandibular (cmd) ligament are highlighted in blue and the species having the ceratomandibular ligament are in red. The principal components (PCs) used to produce this morphospace are those revealing the greatest differences between both morphological groups. The percentage of shape variance summarized by each PC are given in brackets. (b) Deformation grids illustrating the shape variation between the zooplanktivorous species having the cmd ligament (black dot) and the ones lacking this ligament (extremity of the vector) for every studied unit. The shape differences have been exaggerated for better visualization (overall body: x2, mandible: x1.7, premaxilla: x1.2).
Table 2.
Results from phylogenetic MANOVA comparing shape divergence between the damselfish species lacking the ceratomandibular ligament and the ones having this ligament. We first compared shape including all the damselfishes and then we tested shape divergence between zooplanktivorous species. Significant p-values, where significance was obtained by multivariate BM simulations (n = 10 000) on the phylogeny, are indicated in italics.
| d.f. | Wilk's lambda | p-values | |
|---|---|---|---|
| all the species | |||
| body | 1;112 | 0.357 | 0.002 |
| mandible | 1;105 | 0.295 | 0.003 |
| premaxilla | 1;105 | 0.292 | 1 × 10−4 |
| zooplanktivorous species | |||
| body | 1;59 | 0.251 | 0.154 |
| mandible | 1;50 | 0.216 | 0.219 |
| premaxilla | 1;50 | 0.265 | 0.001 |
(d). Sound variation
We found that all species produced similar pulsed sounds (figure 4) [24,45], suggesting that species without the ligament still relied upon forceful contact between the teeth of the oral jaws to generate sound. However, consistent with the decoupling hypothesis enabling acoustical diversity, the two species lacking the cmd ligament showed much greater variances in pulse period than other damselfishes (Levene's test, W = 79.18; p < 0.001; figure 4).
Figure 4.
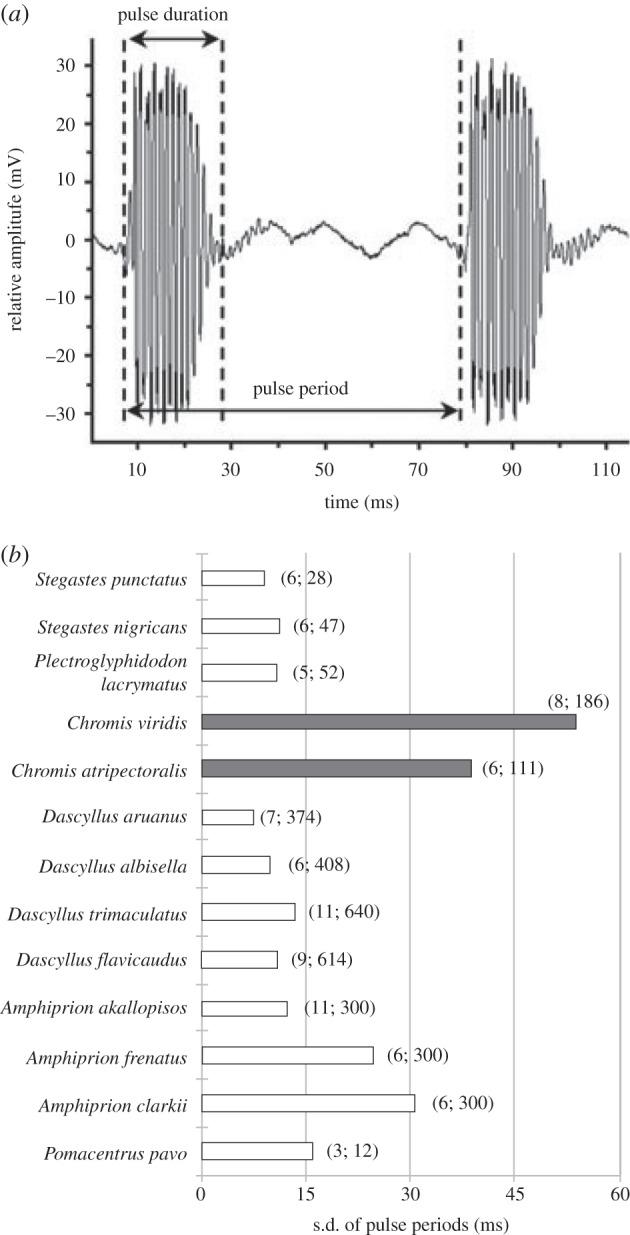
(a) Example of pulsed sounds in Amphiprion akallopisos (modified from [47]). This oscillogram illustrates two main sonic variables: the pulse duration and the pulse period. (b) Standard deviation of the pulse period in sounds produced by 13 species of damselfishes. The two Chromis species, which do not possess the ceratomandibular (cmd) ligament (grey histograms), show highly variable pulse periods during sound emissions in comparison with the species with the cmd ligament (white histograms). The figures between brackets give the number of fish studied and the number of sounds analysed, respectively.
4. Discussion
Our results reveal that the cmd ligament has acted as a major control on ecomorphological and acoustical diversity throughout the evolutionary history of Pomacentridae and shed new insights into the role of decoupling in generating biodiversity.
For the first time, to our knowledge, we have shown that trait decoupling alters the tempo and the mode of phenotypic diversification, leading to greater morphological diversity in trophic structures. However, ecological diversification is not a mandatory consequence of trait decoupling [8]. Although the cmd ligament does not alter the rate of lineage diversification, the loss of this ligament is associated with ecological opportunity for greater specialization. Indeed, MuSSE results (electronic supplementary material, table S2) show that the loss of the cmd ligament has not broadly increased trophic diversity in damselfishes, but instead has led to increased specialization of pelagic feeding. Thus, we suggest that the loss of the ligament obliges greater specialization through loss of feeding versatility that is only possible when the ligament is present.
The study of morphological diversification shows that the ligament loss leads to higher rates of shape evolution (table 1), consistent with the hypothesis that decoupling acts as a release from evolutionary constraints. Moreover, the study of morphospace (figure 3) shows that the ligament loss opens a new axis of morphological variation corresponding to the entrance in a new adaptive zone. Among the various shape differences between the species having and lacking the cmd ligament, the most divergent structure is the premaxilla (table 2). An ecomorphological interpretation of shape variations observed in the damselfishes lacking the cmd ligament suggests an optimization of ram feeding (or ram-jaw feeding sensu [48]) in these zooplanktivorous species. Indeed, as exemplified in many fish groups, a slender body is related to a pelagic lifestyle and probably reduces drag during steady swimming while searching preys [49]. Lengthened mandibles and a long ascending process of the premaxillary bones improve the mouth protrusion which is one way to optimize the catch of zooplanktonic prey [48,50]. Various factors may counter the theoretical expectation that a clade shows an increase in ecological diversification following a decoupling event [8] and differences in evolutionary outcomes probably result from historical or clade-specific factors. Here, we hypothesize that the ecomorphological diversity of damselfish did not increase dramatically after the decoupling event owing to the very high levels of competition present in coral reef environments or related to other internal constraints.
Morphological decoupling of the hyoid and mandible significantly impacts the sound production system in damselfishes. Although experimental transection of the cmd ligament results in the loss of sound production in damselfishes [18], evolutionary loss of this structure does not result in mute lineages. Instead, acoustic diversity within species and across the family is promoted. Our analyses reveal that all the studied species can produce sounds and the species without the cmd ligament show a greater variation of the pulse periods than the species having this ligament (figure 4). This variability in the length of pulse periods can be explained by the mechanism of sound production. As demonstrated by Parmentier et al. [18], damselfishes can produce sounds by rapid mouth closing movements, and this fast jaw slam is allowed by the cmd ligament. We hypothesize that sound producing in species which lack the cmd ligament rely upon actions of the sternohyoidieus, hypaxial, expaxial, levator operculi and jaw adductor muscles to open and close their mouths, and that the variability of the pulse period reflects a smaller degree of coordination among these structures. The studied Chromis species lacking the cmd ligament are sister-species (figure 2) and do not represent evolutionarily independent losses of the ligament. This may limit the power of our statement, but Lobel & Kerr [51] reported similar high variance of pulse periods in Abudefduf sordidus, a species from another subfamily (Abudefdufinae, figure 2) where the cmd linkage became a tendinous projection of the geniohyoideus muscle. The peculiar anatomy of A. sordidus probably does not allow the production of sounds by jaw-slamming involving the cmd ligament [18], inducing higher variations in acoustic signals as in Chromis viridis and Chromis atripectoralis lacking the cmd ligament. Currently, the sounds of species without cmd ligament belonging to the subfamily Pomacentrinae (i.e. Pristotis obtusirostris and Teixeirichthys jordani) are unknown and do not allow further comparisons. However, the same kind of acoustic divergence is observed within two of the three subfamilies, including species without the cmd ligament. Beyond the functional basis of sound production, this study and others [23–26,45,46,51,52] suggest that acoustic signals are a major component of communication in damselfishes and that the ability to produce sounds is an important character underlying their evolution.
Currently, we do not know the forces selecting the damselfishes lacking cmd ligament. Trophic selection could drive the initial divergence by promoting specialization on zooplanktivory, which is then followed by acoustic variation. On the other hand, the theory of social selection, well known to promote rapid trait divergence, could provide another evolutionary scenario [53–55]. Social selection on signals can be very strong in damselfishes, which are territorial and highly aggressive, especially during mating behaviours [56,57]. Thus, it could be possible that social selection on acoustic signals is driving the initial divergence, favouring the ligament loss in some lineages, which is then followed by the observed changes in feeding behaviour and trophic morphology.
Trait decoupling has been thought to influence the pattern of diversification of a clade, but changes in evolutionary rate following decoupling have not been previously documented. Our study illustrates the cmd ligament is a key trait to understanding diversification processes of one of the most ecologically dominant families of reef fishes. We show the cmd ligament clearly impacts the ecomorphological (i.e. trophic adaptation) and the acoustical (i.e. communication) diversity of damselfishes which are two of the main axes of diversification in vertebrates [58]. Although additional examples are needed, damselfishes illustrate that trait decoupling is a source of rapid diversification but does not necessarily allow the production of multiple new ecotypes. Social selection, the availability of untapped niches and the evolvability of the system may be different factors explaining this evolutionary pattern.
Overall, the loss of the cmd ligament can be viewed as having a cascade of diversity promoting effects across discrete trait systems in damselfishes. Although the decoupling hypothesis was originally invoked to explain vast differences in morphology and ecology observed in cichlids [8], tests of this hypothesis have generally focused on diversity patterns within a single trait system [15,28]. Our results help reveal how trait decoupling, through a relatively simple evolutionary release of constraints via the loss of a ligament, can have wide reaching effects on diversity across trait systems, providing a crucial quantitative link between a widely held macroevolutionary idea and empirical patterns of biodiversity.
Supplementary Material
Supplementary Material
Acknowledgements
We thank P. Vandewalle, Y. Nakamura, V. Liu and D. Mann for collecting and providing damselfish species from Papua New Guinea, Japan, Taiwan and Hawaii, respectively. J. E. Randall (Bishop Museum) kindly provided images of damselfishes. We thank A. C. Bentley (KUNHM), M. McGrouther (AM), E. Balart Paez (CIBNOR), M. Sabaj (ANSP), R. Feeney (NHMLA), J. Williams and D. Pitassy (NMNH), D. Catania and M. Hoang (CAS), R. Robins (FMNH) and R. Pyle (Bishop Museum) for kindly providing a part of the specimens examined in this study. We thank Drs J. E. Randall, G. R. Allen and R. Aguilar-Medrano for help with identification of specimens. Helpful suggestions on the manuscript at various stages were provided by David Collar and three anonymous reviewers.
Methods for field collections followed approved animal care protocols.
Funding statement
This research was supported by FRS-FNRS grants (FRFC 2.4.583.05) and by NSF grants (DEB 0842397, 0918748). B.F. is a Postdoctoral Researcher at the F.R.S.-FNRS (Belgium). G.L. is supported by the University of Lausanne research fund and this work received support from the Vital-IT facilities from the Swiss Institute of Bioinformatics.
References
- 1.Vermeij GJ. 1973. Adaptation, versatility, and evolution. Syst. Zool. 22, 466–477. ( 10.2307/2412953) [DOI] [Google Scholar]
- 2.Lauder GV. 1981. Form and function: structural analysis in evolutionary morphology. Paleobiology 7, 430–442. [Google Scholar]
- 3.Wainwright PC. 2007. Functional versus morphological diversity in macroevolution. Annu. Rev. Ecol. Evol. Syst. 38, 381–401. ( 10.1146/annurev.ecolsys.38.091206.095706) [DOI] [Google Scholar]
- 4.West-Eberhard MJ. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 5.Klingenberg CP. 2008. Morphological integration and developmental modularity. Annu. Rev. Ecol. Evol. Syst. 39, 115–132. ( 10.1146/annurev.ecolsys.37.091305.110054) [DOI] [Google Scholar]
- 6.Cheverud JM. 1996. Developmental integration and the evolution of pleiotropy. Am. Zool. 36, 44–50. [Google Scholar]
- 7.Klingenberg CP, Leamy LJ, Cheverud JM. 2004. Integration and modularity of quantitative trait locus effects on geometric shape in the mouse mandible. Genetics 166, 1909–1921. ( 10.1534/genetics.166.4.1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liem KF. 1973. Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst. Zool. 22, 425–441. ( 10.2307/2412950) [DOI] [Google Scholar]
- 9.Liem KF, Sanderson SL. 1986. The pharyngeal jaw apparatus of labrid fishes: a functional morphological perspective. J. Morphol. 187, 143–158. ( 10.1002/jmor.1051870203) [DOI] [PubMed] [Google Scholar]
- 10.Hulsey CD, de Leon FJG, Rodiles-Hernandez R. 2006. Micro- and macroevolutionary decoupling of cichlid jaws: a test of Liem's key innovation hypothesis. Evolution 60, 2096–2109. ( 10.1111/j.0014-3820.2006.tb01847.x) [DOI] [PubMed] [Google Scholar]
- 11.Galis F, Drucker EG. 1996. Pharyngeal biting mechanics in centrarchid and cichlid fishes: insights into a key evolutionary innovation. J. Evol. Biol. 9, 641–670. ( 10.1046/j.1420-9101.1996.9050641.x) [DOI] [Google Scholar]
- 12.Taylor JS, Raes J. 2004. Duplication and divergence: the evolution of new genes and old ideas. Annu. Rev. Genet. 38, 615–643. ( 10.1146/annurev.genet.38.072902.092831) [DOI] [PubMed] [Google Scholar]
- 13.Friel JP, Wainwright PC. 1999. Evolution of complexity in motor patterns and jaw musculature of tetraodontiform fishes. J. Exp. Biol. 202, 867–880. [DOI] [PubMed] [Google Scholar]
- 14.Friel JP, Wainwright PC. 1998. Evolution of motor patterns in tetraodontiform fishes: does muscle duplication lead to functional diversification? Brain Behav. Evol. 52, 159–170. ( 10.1159/000006560) [DOI] [PubMed] [Google Scholar]
- 15.Schaefer SA, Lauder GV. 1996. Testing historical hypotheses of morphological change: biomechanical decoupling in loricarioid catfishes. Evolution 50, 1661–1675. ( 10.2307/2410902) [DOI] [PubMed] [Google Scholar]
- 16.Eschmeyer WN. 2014. Catalog of fishes: genera, species, references (http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp). (accessed 30 April 2014) [DOI] [PubMed]
- 17.Stiassny MLJ. 1981. The phyletic status of the family Cichlidae (Pisces, Perciformes): a comparative anatomical investigation. Neth. J. Zool. 31, 275–314. ( 10.1163/002829681X00013) [DOI] [Google Scholar]
- 18.Parmentier E, Colleye O, Fine ML, Frédérich B, Vandewalle P, Herrel A. 2007. Sound production in the clownfish Amphiprion clarkii. Science 316, 1006 ( 10.1126/science.1139753) [DOI] [PubMed] [Google Scholar]
- 19.Osse JWM. 1969. Functional morphology of the head of the perch (Perca fluvatilis L.): an electromyographic study. Neth. J. Zool. 19, 289–392. ( 10.1163/002829669X00134) [DOI] [Google Scholar]
- 20.Liem KF, Osse JWM. 1975. Biological versatility, evolution, and food resource exploitation in African cichlid fishes. Am. Zool. 15, 427–454. [Google Scholar]
- 21.Frédérich B, Sorenson L, Santini F, Slater GJ, Alfaro ME. 2013. Iterative ecological radiation and convergence during the evolutionary history of damselfishes (Pomacentridae). Am. Nat. 181, 94–113. ( 10.1086/668599) [DOI] [PubMed] [Google Scholar]
- 22.Cooper WJ, Westneat MW. 2009. Form and function of damselfish skulls: rapid and repeated evolution into a limited number of trophic niches. BMC Evol. Biol. 9, 24 ( 10.1186/1471-2148-9-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colleye O, Parmentier E. 2012. Overview on the diversity of sounds produced by clownfishes (Pomacentridae): importance of acoustic signals in their peculiar way of life. PLoS ONE 7, e49179 ( 10.1371/journal.pone.0049179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmentier E, Kever L, Casadevall M, Lecchini D. 2010. Diversity and complexity in the acoustic behaviour of Dacyllus flavicaudus (Pomacentridae). Mar. Biol. 157, 2317–2327. ( 10.1007/s00227-010-1498-1) [DOI] [Google Scholar]
- 25.Parmentier E, Vandewalle P, Frédérich B, Fine ML. 2006. Sound production in two species of damselfishes (Pomacentridae): Plectroglyphidodon lacrymatus and Dascyllus aruanus. J. Fish Biol. 69, 491–503. [Google Scholar]
- 26.Mann DA, Lobel PS. 1998. Acoustic behavior of the damselfish Dascyllus albisella: behavioral and geographic variation. Environ. Biol. Fishes 51, 421–428. ( 10.1023/a:1007410429942) [DOI] [Google Scholar]
- 27.Colleye O, Nakamura M, Frédérich B, Parmentier E. 2012. Further insight into the sound-producing mechanism of clownfishes: what structure is involved in sound radiation? J. Exp. Biol. 215, 2192–2202. ( 10.1242/jeb.067124) [DOI] [PubMed] [Google Scholar]
- 28.Emerson SB. 1988. Testing for historical patterns of change: a case study with frog pectoral girldes. Paleobiology 14, 174–186. [Google Scholar]
- 29.Frédérich B, Vandewalle P. 2011. Bipartite life cycle of coral reef fishes promotes increasing shape disparity of the head skeleton during ontogeny: an example from damselfishes (Pomacentridae). BMC Evol. Biol. 11, 82 ( 10.1186/1471-2148-11-82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor WR, Van Dyke GC. 1985. Revised procedure for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9, 107–121. [Google Scholar]
- 31.Bookstein F. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Adams DC. 2004. Character displacement via aggressive interference in Appalachian salamanders. Ecology 85, 2664–2670. ( 10.1890/04-0648) [DOI] [Google Scholar]
- 33.Rohlf FJ.2004. TpsDig (version 1.40), a software program for landmark data acquisition. (Department of Ecology and Evolution, State University of New York at Stony Brook. See http://life.bio.sunysb.edu/morph/ .
- 34.Frédérich B, Fabri G, Lepoint G, Vandewalle P, Parmentier E. 2009. Trophic niches of thirteen damselfishes (Pomacentridae) at the Grand Récif of Toliara, Madagascar. Ichthyol. Res. 56, 10–17. ( 10.1007/s10228-008-0053-2) [DOI] [Google Scholar]
- 35.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 ( 10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158. ( 10.1080/10635150390192780) [DOI] [PubMed] [Google Scholar]
- 37.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 38.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://R-project.org/. [Google Scholar]
- 39.Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character's effect on speciation and extinction. Syst. Biol. 56, 701–710. ( 10.1080/10635150701607033) [DOI] [PubMed] [Google Scholar]
- 40.FitzJohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 3, 1084–1092. ( 10.1111/j.2041-210X.2012.00234.x) [DOI] [Google Scholar]
- 41.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general-method for the comparative-analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 42.Adams DC. 2014. Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Syst. Biol. 63, 166–177. ( 10.1093/sysbio/syt105) [DOI] [PubMed] [Google Scholar]
- 43.Sheets HD. 2000. TwoGroup: testing the difference in shape between two groups. Buffalo: Department of Physics, Canisius College; See http://www3.canisius.edu/~sheets/morphsoft.html. [Google Scholar]
- 44.Harmon LJ, Weir J, Brock C, Glor R, Challenger W. 2008. Geiger: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 45.Colleye O, Vandewalle P, Lanterbecq D, Lecchini D, Parmentier E. 2011. Interspecific variation of calls in clownfishes: degree of similarity in closely related species. BMC Evol. Biol. 11, 365 ( 10.1186/1471-2148-11-365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmentier E, Lecchini D, Frédérich B, Brie C, Mann D. 2009. Sound production in four damselfish (Dascyllus) species: phyletic relationships? Biol. J. Linn. Soc. 97, 928–940. ( 10.1111/j.1095-8312.2009.01260.x) [DOI] [Google Scholar]
- 47.Colleye O, Frédérich B, Vandewalle P, Casadevall M, Parmentier E. 2009. Agonistic sounds in the skunk clownfish Amphiprion akallopisos: size-related variation in acoustic features. J. Fish Biol. 75, 908–916. ( 10.1111/j.1095-8649.2009.02316.x) [DOI] [PubMed] [Google Scholar]
- 48.Coughlin DJ, Strickler JR. 1990. Zooplankton capture by a coral-reef fish: an adaptive response to evasive prey. Environ. Biol. Fishes 29, 35–42. ( 10.1007/BF00000566) [DOI] [Google Scholar]
- 49.Webb PW. 1984. Body form, locomotion and foraging in aquatic vertebrates. Am. Zool. 24, 107–120. [Google Scholar]
- 50.Liem KF. 1993. Ecomorphology of the teleostean skull. In The skull: functional and evolutionary mechanisms (eds Hanken J, Hall BK.), pp. 422–452. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 51.Lobel PS, Kerr LM. 1999. Courtship sounds of the Pacific damselfish, Abudefduf sordidus (Pomacentridae). Biol. Bull. 197, 242–244. ( 10.2307/1542627) [DOI] [PubMed] [Google Scholar]
- 52.Rice AN, Lobel PS. 2003. The pharyngeal jaw apparatus of the Cichlidae and Pomacentridae: function in feeding and sound production. Rev. Fish Biol. Fish. 13, 433–444. ( 10.1007/s11160-004-8794-0) [DOI] [Google Scholar]
- 53.Lyon BE, Montgomerie R. 2012. Sexual selection is a form of social selection. Phil. Trans. R. Soc. B 367, 2266–2273. ( 10.1098/rstb.2012.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobias JA, Montgomerie R, Lyon BE. 2012. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil. Trans. R. Soc. B 367, 2274–2293. ( 10.1098/rstb.2011.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amundsen T. 2000. Why are female birds ornamented? Trends Ecol. Evol. 15, 149–155. ( 10.1016/S0169-5347(99)01800-5) [DOI] [PubMed] [Google Scholar]
- 56.Myrberg AA, Spanier E, Ha SJ. 1978. Temporal patterning in acoustic communication. In Contrasts in behaviour (eds Reese ER, Lighter FJ.), pp. 137–179. New York, NY: John Wiley and Sons. [Google Scholar]
- 57.Siebeck UE. 2004. Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim. Behav. 68, 273–282. ( 10.1016/j.anbehav.2003.11.010) [DOI] [Google Scholar]
- 58.Streelman JT, Danley PD. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 18, 126–131. ( 10.1016/S0169-5347(02)00036-8) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.