Abstract
The relationships among species' physiological capacities and the geographical variation of ambient climate are of key importance to understanding the distribution of life on the Earth. Furthermore, predictions of how species will respond to climate change will profit from the explicit consideration of their physiological tolerances. The climatic variability hypothesis, which predicts that climatic tolerances are broader in more variable climates, provides an analytical framework for studying these relationships between physiology and biogeography. However, direct empirical support for the hypothesis is mostly lacking for endotherms, and few studies have tried to integrate physiological data into assessments of species' climatic vulnerability at the global scale. Here, we test the climatic variability hypothesis for endotherms, with a comprehensive dataset on thermal tolerances derived from physiological experiments, and use these data to assess the vulnerability of species to projected climate change. We find the expected relationship between thermal tolerance and ambient climatic variability in birds, but not in mammals—a contrast possibly resulting from different adaptation strategies to ambient climate via behaviour, morphology or physiology. We show that currently most of the species are experiencing ambient temperatures well within their tolerance limits and that in the future many species may be able to tolerate projected temperature increases across significant proportions of their distributions. However, our findings also underline the high vulnerability of tropical regions to changes in temperature and other threats of anthropogenic global changes. Our study demonstrates that a better understanding of the interplay among species' physiology and the geography of climate change will advance assessments of species' vulnerability to climate change.
Keywords: biodiversity, macrophysiology, global change, birds, mammals, macroecology
1. Introduction
Species' thermal physiology and the variation of climatic conditions in time and space play central roles in determining species distributions and the spatial variation of diversity on the Earth [1–5]. Understanding the association among these factors is crucial for predicting the potential impacts of climate change [6], one of the major threats to biodiversity [7–9]. To study these associations, a powerful framework is provided by the climatic variability hypothesis [10], which states that species occurring in more variable climates should have broader thermal tolerances. The framework of the climatic variability hypothesis may also help predict which species may become threatened by climate change [11]: the hypotheses predict that species currently experiencing a low variation in ambient temperatures (Ta) should be particularly threatened by rising and more extreme Ta, as their thermal tolerances should be narrower than that of species living under highly variable temperature conditions [3,10,12].
While perhaps generally applicable to ectotherms [1,5], the climatic variability has only received mixed support for endotherms (mammals and birds) [6,10,13]. In contrast to ectotherms, endotherms maintain a high and constant body temperature, which is thus decoupled to a large degree from the direct influence of ambient climatic conditions [14]. The breadth of the thermal tolerance of a given endotherm can be estimated empirically via its energetic requirements [12]. The amount of energy needed to compensate for the difference between body temperature and Ta is minimal when Ta is within the thermoneutral zone (TNZ)—the range of temperatures within which the metabolism of an endotherm is lowest and almost independent of Ta. Species with a broad TNZ are able to maintain their basal metabolism over a wide range of temperature conditions (electronic supplementary material, figure S1). Beyond their TNZ, species need to allocate additional metabolic energy to maintain their body functions [15]. Even though endotherms can cope with short-term thermal stress, prolonged periods of increased energy expenditure should ultimately decrease their fitness [16]. TNZ breadth therefore gives an estimate of the long-term thermal tolerance of a species.
Species' responses to anthropogenic changes of the global climate system are, in simplified terms, usually summarized as evolutionary adaptation, dispersal and extinction [17–19]. However, whether species may be physiologically buffered against projected future increases of Ta, i.e. whether species might be able to tolerate higher temperatures, because these fall within their TNZ, remains a largely unexplored question. Recently, it has been suggested that heat tolerance is more strongly conserved across lineages than cold tolerance [20]. This may imply that heat tolerance imposes a rather hard physiological limit which is not easily changeable by evolutionary adaption, and it has also been proposed that ectotherm species in tropical regions may live closer to their heat tolerance limits [5,20]. In birds, many species appear to lag behind in tracking their climatic envelopes via shifts of their geographical ranges [21,22]. This observation possibly suggests that birds may either be able to adapt rapidly to novel environmental conditions (but see [23]), or that the levels of climate change so far do not exceed their tolerance limits. However, we still lack quantitative estimates of endotherms' physiological potential to tolerate ongoing and future Ta increases. Furthermore, an improved understanding of how the relationship between thermal tolerances and Ta varies geographically will also help quantifying species' vulnerability to climate change.
Here, we compiled, to our knowledge the largest set of endotherm TNZ data to date in order to: (i) investigate the variation in species' thermal tolerances across the globe and thus to provide the most comprehensive test of the climatic variability hypothesis for endotherms, and to (ii) assess the potential vulnerability of endotherms to climate change, based on their thermal tolerances. We compiled data on experimental measurements of TNZ for 255 bird and 297 mammal species from the literature (see the electronic supplementary material, table S1), along with geographical information as well as data on ambient climatic variability [24]. We tested the climatic variability hypothesis by analysing the relationships between TNZ breadth and climatic variability while accounting for species' evolutionary history, i.e. their phylogenetic relationships. To assess species' vulnerability to climate change, we quantified the concordance between the upper TNZ limit and maximum Ta for current as well as for future conditions.
2. Methods
(a). Data
As thermal tolerance data, we compiled information on TNZ for 255 bird and 297 mammal species from the literature (see the electronic supplementary material, table S1). We used studies that measured species' metabolism in relation to ambient temperature under laboratory conditions (electronic supplementary material, figure S1; [25]), and that provided data on both upper and lower TNZ limits as well as body mass. To enhance comparability with mammals, 94 migratory bird species were excluded from the analyses as they experience varying climatic environments throughout the year and may have developed different physiological adaptations than all-year-resident birds. For studies in which upper and lower TNZ limits were not calculated, but a graph of suitable data was given, we extracted the values from plots using DataThief III [26]. We classified species into two categories: wild and acclimatized. Species were classified as acclimatized when kept in captivity for more than one month. While the validity of laboratory measurements of TNZ breadth as estimates of fundamental temperature niches has been debated [27], TNZ breadth is an approximation of thermal tolerance based directly on physiological measurements. Therefore, it is an improvement over inferring species' climatic preferences from their geographical distributions [20]. We acknowledge that TNZ may vary within the same species, either seasonally [28], or among different populations across its geographical distribution [15]. However, interspecific variation in TNZ breadth in our dataset ranges from 1°C to 40°C, and is therefore likely to exceed any intraspecific variation which might potentially confound our analyses.
The final dataset contained 161 resident bird species (from 17 orders and 50 families) and 297 mammal species (from 24 orders and 75 families; see the electronic supplementary material, table S1). Thus, even though some orders or families might be over- or under-represented, we assume that the data cover a representative selection of the physiological diversity within the avian and mammalian trees of life. Furthermore, the data represent the majority of the world's biomes. From each study that provided physiological data, we extracted information on the geographical coordinates of the capture site of the individuals used in the experiments. Latitude and longitude coordinates of the sites were mentioned in most of the studies; if coordinates were not given, we retrieved them using ACME Mapper 2.0 (http://mapper.acme.com). We also compiled distribution data for all species from public databases provided by BirdLife International for birds and by the International Union for Conservation of Nature for mammals [29,30]. Phylogenetic information for all species were compiled from published supertrees for birds [31] and mammals [32,33].
Climatic data were derived from the CliMond dataset for current conditions and future climate change scenarios [24]. Climatic data for current conditions were averaged across 30 years from 1961 to 1990, centred on 1975. For the projections of future climate, we used two different global climate models (or general circulation models; GCMs—CSIRO-MK 3.0 and MIROC-H) for 2080, and two different greenhouse gas emission scenarios—the A1B (balanced) scenario and the A2 (extreme) scenario (see [24] for information on the choice of GCMs; for more details on the scenario outlines, see [34,35]). All climatic data were resampled to a global 50 × 50 km grid using weighted averaging. For transferring species distribution polygons to gridded distribution data, we defined species presence within grid cells if any part of the distribution overlapped with the respective cell.
(b). Climatic variability hypothesis
To test the climatic variability hypothesis, we assessed the relationship between TNZ breadth in birds and mammals and climatic variables representing the variability of climatic conditions (temperature, precipitation and radiation) within a year (variables ‘Bio04’, ‘Bio15’ and ‘Bio23’ in [24]). These variables are calculated as the coefficient of variation of monthly mean temperature, monthly sum of precipitation and monthly solar irradiance across 12 months. As an additional measure for temperature variability, we used the annual temperature range (‘Bio07’), calculated as the difference of the hottest temperature of the warmest month and the coldest temperature of the coldest month [24,36]. TNZ breadth and all climatic variables were log-transformed to linearize the relationships among variables.
To estimate the association between TNZ breadth and climatic variability while accounting for the non-independence of species owing to their joint evolutionary history, we used phylogenetic generalized least-squares (PGLS) regression using the package caper [37] in R [38]. This approach allows flexibility in the underlying evolutionary assumptions via the use of a parameter λ (Pagel's lambda [39]) that reflects the amount of phylogenetic constraint on the phenotype [40,41]. We modelled log-transformed TNZ breadth using PGLS as a function of log-transformed body mass and acclimatization while we estimate λ, and set it to its maximum-likelihood value. After controlling for phylogeny, body mass and acclimatization, we individually added absolute latitude, temperature seasonality, temperature annual range, precipitation seasonality or radiation seasonality (all climatic variables log-transformed) to the model (table 1; also see the electronic supplementary material, table S2). For both birds and mammals, we randomly sampled 100 trees from the pseudo-posterior distribution of the used supertrees [31,32] and ran the PGLS analyses for these 100 trees. For the mammal trees, polytomies were resolved using a birth–death model of diversification [33].
Table 1.
Association of thermoneutral zone (TNZ) breadth of birds and mammals with latitude and climatic variability. (TNZ breadth was modelled using PGLS, as a function of body mass and acclimatization while we estimate Pagel's λ, and set it to its maximum-likelihood value (see Methods). After controlling for phylogeny, body mass and acclimatization, we individually added absolute latitude, temperature seasonality, annual temperature range, precipitation seasonality or radiation seasonality to the model. We show medians as well as 90% confidence intervals (in brackets) of parameters obtained from 100 randomly sampled trees from the pseudo-posterior distribution of the supertrees used (see Methods). Italicized values indicate associations where estimated parameters (B) are significantly different from 0. Climatic variables were averaged for a global 50 × 50 km equal-area grid; for the statistical analyses, we took the climatic values for the grid cell where the capture sites of the species' individuals used in the physiological experiments were located. TNZ breadth, body mass and all climatic variables were log-transformed. B, estimated parameter ± s.e.m. λ, Pagel's Lambda, set to its maximum-likelihood value.)
| birds (n = 161) |
mammals (n = 297) |
|||||||
|---|---|---|---|---|---|---|---|---|
| B | λ | R2 | p | B | λ | R2 | p | |
| latitude | 0.012 (0.0119, 0.0122) | 0.23 (0.189, 0.281) | 0.43 (0.419, 0.433) | <0.001 | 0.004 (0.0033, 0.0037) | 0.22 (0.175, 0.244) | 0.19 (0.185, 0.191) | 0.13 (0.111, 0.156) |
| temperature seasonalitya | 0.086 (0.0829, 0.0881) | 0.25 (0.166, 0.342) | 0.35 (0.337, 0.364) | 0.018 (0.0142, 0.0239) | 0.010 (0.0081, 0.0113) | 0.22 (0.164, 0.248) | 0.18 (0.179, 0.186) | 0.76 (0.73, 0.80) |
| annual temperature range | 0.10 (0.095, 0.109) | 0.31 (0.195, 0.414) | 0.32 (0.312, 0.341) | 0.23 (0.194, 0.270) | −0.08 (−0.079, 0.006) | 0 (0, 0.247) | 0.22 (0.179, 0.217) | 0.28 (0.28, 0.993) |
| precipitation seasonalitya | −0.18 (−0.178, −0.172) | 0.38 (0.304, 0.442) | 0.36 (0.350, 0.367) | <0.001 | −0.048 (−0.058, −0.042) | 0.19 (0.138, 0.23) | 0.186 (0.182, 0.191) | 0.36 (0.27, 0.43) |
| radiation seasonalitya | 0.22 (0.214, 0.220) | 0.24 (0.183, 0.305) | 0.39 (0.379, 0.396) | <0.001 | 0.072 (0.068, 0.077) | 0.22 (0.184, 0.249) | 0.187 (0.184, 0.190) | 0.15 (0.125, 0.177) |
aSeasonality variables are calculated as the coefficient of variation of monthly climate variables (see Methods).
As the spatial structure in geographical data may affect the analyses and their interpretation, we tested for spatial autocorrelation in the residuals of the regression models using Moran's I [42]. With the exception of a single model (mammals, radiation seasonality), we did not find any evidence for spatial autocorrelation. Accounting for spatial autocorrelation using generalized least-squares as implemented in the software spatial analysis in macroecology (SAM, version 4.0 [43]) did not significantly influence the results for any of the regression models (birds, all p-values > 0.11; mammals, all p-values > 0.06); therefore, we present the original models.
(c). Vulnerability to climate change
To assess species' potential vulnerability to climate change, we quantified the concordance between upper TNZ limit (upper critical temperature, UCT) and maximum ambient temperature (Ta) for current as well as for future conditions under different climate change scenarios. As an estimate of maximum Ta, we used the monthly average of daily maximum temperatures of the warmest month. When maximum Ta exceeded UCT, we recorded a ‘thermal mismatch’, whereas ‘thermal safety’ was recorded when maximum Ta was below UCT [44]. Thus, thermal safety represents a buffer zone in degrees Celsius, with higher safety values representing a larger buffer to current and projected future temperatures. Thermal mismatch is an estimate for the potential degree of vulnerability in degrees Celsius that a species experiences under current or projected future temperatures, with higher mismatch values representing higher degrees of vulnerability. While we are aware that the lower TNZ limit (lower critical temperature, LCT) is also an influential metabolic parameter, we do set our focus on UCT, because rising temperatures as projected in climate change scenarios should have a larger impact on the relationship between UCT and Ta. This is underlined by the heat dissipation limit theory [45] which suggests that metabolic heat generated in order to compensate for higher Ta could be particularly detrimental for endotherm species.
We calculated three different measures for thermal safety and mismatch. First, we calculated the difference between Ta and UCT at the sites of animal capture (see above). Second, to account for the geographical variation in climatic conditions across species distributions, we calculated thermal mismatch as the proportion of grid cells across the species' current distribution where it experiences a mismatch between Ta and UCT at any time of the year under current and projected future conditions. Third, to account for both the spatial and temporal variation in climatic conditions that a species experiences across its geographical range, we calculated a ‘temperature mismatch index’. To do so, we first calculated for each grid cell of the species' current distribution the number of months in which the species experiences a mismatch under current or projected future conditions. Then, we summed the mismatching months across all grid cells within the distribution and divided them by the total number of grid cells of the distribution, multiplied by 12; this value was then multiplied by 100:
where MMi is the number of mismatching months in grid cell i, and n represents the total number of grid cells of the distributional range. A value of 100 indicates that a species experiences a mismatch in each month and in all grid cells across its distribution, whereas a value of 0 indicates that the species does not experience a mismatch anytime and anywhere across its distribution. To account for the variability in climate change projections for future time periods [46], we used combinations of two different GCMs (MIROC-H and CSIRO-MK 3.0 [34]) and greenhouse gas emission scenarios (A1B and A2 [35]).
3. Results
We found contrasting patterns in the global variation of thermal tolerances for birds and mammals (figure 1; electronic supplementary material, figure S2). For birds, TNZ breadth increased with increasing latitude of species occurrences, whereas we found no relationship between TNZ breadth and latitude for mammals (table 1). For birds, TNZ breadths increased with temperature and radiation variability and decreased with precipitation variability, whereas mammals did not show any significant relationship between TNZ breadth and climatic variability (table 1). The differences between birds and mammals were largely consistent across regions (electronic supplementary material, figure S3) and also robust to varying the spatial extent of the study (electronic supplementary material, table S2). The results were also robust to including or excluding the largest bird or mammal orders (Passeriformes, Rodentia, Chiroptera; results not shown). All effects were significantly different between birds and mammals (Wald test, all p < 0.05) except for annual temperature range, which did not significantly affect TNZ breadth for either group (table 1). Overall, mammals had smaller TNZ breadths than birds (t = 9.11, p < 0.0001).
Figure 1.

Geographical variation in thermal tolerances of birds and mammals. Breadths of thermoneutral zone (TNZ, see electronic supplementary material, figure S1 for details) of (a) birds and (b) mammals are plotted in relation to latitude and climatic variability (grey lines) at the capture sites of the species' individuals used in the physiological experiments. Major orders in the datasets are highlighted by different colours (shading). Here, climatic variability is the annual range between maximum (monthly average of daily maximum temperatures of the warmest month) and minimum temperatures (monthly average of daily minimum temperatures of the coldest month). (Online version in colour.)
TNZ breadths were strongly influenced by the lower TNZ limits (LCT) in birds (rPearson = −0.83, p < 0.001, n = 161) and mammals (rPearson = −0.83, p < 0.001, n = 297), i.e. species with lower LCT had larger TNZ breadths. LCT was positively related to annual minimum temperature at the site of animal capture for both birds and mammals, but after restricting the datasets to a latitudinal band between 60°S and 60°N (an area where data availability is evenly distributed, see also the electronic supplementary material, table S2), this relationship was positive only for birds (rPearson = 0.17, p = 0.038, n = 157) and not for mammals (rPearson = 0.03, p = 0.592, n = 294).
Our comparison of upper TNZ limits (UCT) and maximum Ta at the sites where the animals were captured for the physiological experiments revealed that 15% of bird and 16% of mammal species are currently experiencing maximum Ta levels above UCT (‘mismatch’; figure 2a,b). Under projected climate change, these mismatches increased to proportions of 36% (birds) and 47% (mammals) for the year 2080 (MIROC-H GCM, A2 emission scenario; figure 2c,d; for other GCM × scenario combinations, see electronic supplementary material, figure S4).
Figure 2.
Latitudinal variation in differences between species' thermal tolerance and ambient temperature (ΔT) for birds and mammals. ΔT is calculated as the difference between upper TNZ limit (UCT) and maximum ambient temperature (Ta). Thermal safety is defined as UCT > Ta, thermal mismatch is defined as UCT < Ta. Histograms indicate the distribution of the numbers of species that experience thermal safety and mismatch. Latitudes and maximum Ta are estimated for the capture sites of the species' individuals used in the physiological experiments. (a) Birds (n = 161) and (b) mammals (n = 297) under current conditions; (c) birds and (d) mammals under projected future conditions (year 2080, MIROC-H global climate model, the A2 emission scenario; for other GCM × scenario combinations, see the electronic supplementary material, figure S4). (Online version in colour.)
When we accounted for the geographical variation in climatic conditions across species distributions, we found that 63% of the bird and 76% of the mammal species currently experience thermal mismatch in at least one grid cell across their distribution (electronic supplementary material, figure S5). Under projected future conditions, these proportions are projected to increase to 83% for birds and 96% for mammals. Thermal mismatches across more than 50% of their current distribution are projected for about 54% of the bird and 62% of the mammal species. These values are largely consistent independent of different measures used for maximum Ta (electronic supplementary material, figure S5).
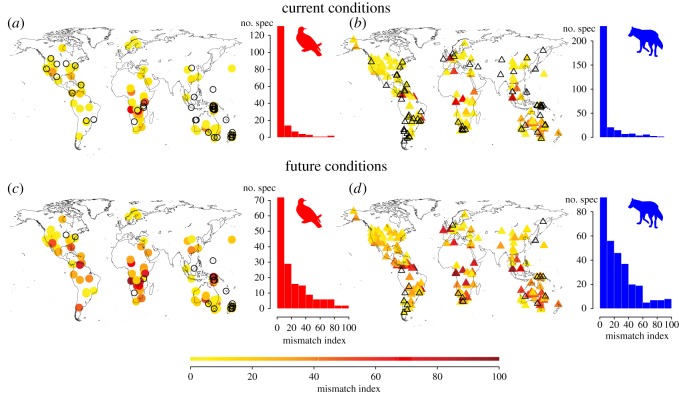
When additionally considering the intra-annual temporal variation of Ta across species distributions, we found that most species in both birds and mammals experience thermal mismatches across relatively small proportions of their current range and also for only relatively short time periods per year (figure 3). The mismatch index, which takes into account both spatial and temporal variation in thermal mismatch, ranged between 0 and 20 for 89% of the bird and 85% of the mammal species under current conditions (figure 3a,b). Under projected future conditions, these values decrease to 55% of the bird and 49% of the mammal species, whereas the mismatch index exceeds a level of 50 for 16% of the bird and 20% of the mammal species (figure 3c,d). These results showed very little variation among the different GCM and scenario combinations (electronic supplementary material, figure S6).
Figure 3.
Global geographical variation in species vulnerability to climate change for birds and mammals, based on their physiological thermal tolerances. We show the global variation in species' vulnerability to climate change for current conditions (a,b) and for projected future conditions (c,d; year 2080, MIROC-H global climate model, the A2 emission scenario), for birds (a,c) and mammals (b,d). Vulnerability is quantified by the temperature mismatch index (see Methods). In the maps, warmer colours (darker shadings) indicate higher potential vulnerability of species to projected temperature changes. Species for which maximum Ta projections are below UCT (i.e. mismatch index = 0) are indicated as empty symbols. Histograms depict frequency distributions of the temperature mismatch index for all species. See the electronic supplementary material, figure S6, for other global climate models and emission scenarios. (Online version in colour.)
Degrees of mismatch increased from polar and temperate towards tropical regions for both birds and mammals (electronic supplementary material, table S3), largely independent of whether considering current Ta at the sites of animal capture (figure 2) or future Ta across species geographical ranges (figure 3 and the electronic supplementary material, figure S6).
4. Discussion
While our results support the climatic variability hypothesis for birds, they contradict studies suggesting that it also applies to mammals [12,13,47]. The relationship between thermal tolerance breadth and climatic variability was stronger for birds than for mammals, independently of the variability measure used. This indicates that, on the one hand, distributions of birds may be more strongly governed by their thermal tolerances (i.e. their TNZ) than mammalian distributions. On the other hand, thermal tolerances of birds may be more closely adapted to the ambient temperatures experienced than those of mammals. Although with our data and analyses we cannot identify the relative importance of these two mechanisms or their potential interactions, we believe that our findings provide support for the assumption that birds' thermal physiology is more directly linked to their ambient climatic conditions than that of mammals (see also [48, p. 134]). This is also underlined by our finding that LCTs are more closely related to annual temperature minima in birds than in mammals, suggesting that the discrepancy between ambient temperature variability and thermal tolerance in mammals is mainly driven by the decoupling of their cold tolerances from low ambient temperatures [12]. These differences may be due to differing behaviours and lifestyles. To avoid extreme climatic conditions, many mammals are able to create their preferred microclimates such as burrows and dens [49], whereas only few birds use such strategies to avoid climatic extremes [48]. In other words, mammals may have developed behavioural strategies to cope with challenging thermal conditions, whereas in birds, physiological adaptations appear to predominate their strategies to cope with extreme temperatures.
We found that most of the endotherm species in our dataset are currently experiencing maximum ambient temperatures within their tolerance limits for most months within a year. Under future climate change projections, our analyses suggest that the majority of endotherm species will probably find suitable temperature conditions within their current geographical ranges. These results may provide one line of explanation for the suggestion that despite their comparatively strong dispersal capacities, many bird species appear to lag behind in tracking their climatic envelopes via shifts of their geographical ranges [21,22].
Overall, we show that the potential vulnerability to higher future ambient temperature increases from polar towards tropical regions, even though increases of temperature projected for temperate and polar regions exceed those in the tropics. Species in tropical regions tend to live closer to their upper temperature limits and even small increases in ambient temperatures may challenge their long-term survival [44,50,51].
It is important to note that our analyses represent a purely physiological perspective on species' potential vulnerability to rising ambient temperatures. Nevertheless, we assume that our results are conservative estimates of species' vulnerability to increasing temperatures for four reasons. First, to assess vulnerability to climate change we used species' UCT, i.e. the upper limit of the TNZ, instead of the lethal heat tolerance limit [52]. As lethal tolerance limits well exceed UCT of most species, using lethal temperatures would have further increased the thermal safety margins of most species. Second, various metabolic, behavioural or even evolutionary pathways enable endotherms to avoid extremely hot temperatures. Third, our coarse temporal and spatial averaging of climatic data largely ignores the microclimatic variation in heterogeneous habitats where small areas of suitable climates may allow species to endure adverse conditions [18,53]. Such opportunities further support our conclusions that temperature increases alone may not impose a severe threat on many endotherms. Finally, while, in our study, we have not explicitly considered additional species' responses to climate change such as dispersal or evolutionary adaptation—processes which may only further increase species' potential to cope with changing climatic conditions [18].
However, while relatively high UCT levels may buffer many species against rising mean temperatures, recent studies on ectotherms [54,55] suggest that the potential increase of temperature variation in the future may still negatively affect species, especially because of the sharp decline in fitness at the warm thermal limit. Additionally, rising temperatures might have indirect effects via biotic interactions [56]: for instance, temperature increase may improve ambient climatic conditions for competing species [57] or pathogens [58], or have negative impacts on the occurrence of food resources or mutualists [59].
In terms of geographical and taxonomic coverage, our study is, to the best of our knowledge, the most comprehensive to date that investigates the global variation in experimentally derived thermal tolerances of endotherms in a spatially explicit context. With regard to endotherms' potential vulnerability to climate change, three conclusions arise, relating to: (i) the differences in thermal tolerance patterns between birds and mammals, (ii) the spatial variation in vulnerability to climate change, and (iii) the consequences for studies aiming to predict climate change impacts on species distributions.
First, owing to their generally narrower thermal tolerances and their seemingly higher levels of projected mismatch with ambient temperatures in comparison with birds, the challenges from climate change may appear more severe for mammals than for birds. However, the high independence of their thermal physiology from their ambient climatic conditions suggests that projections of mammal responses to climate change [60] may contain a substantial component of uncertainty.
Second, the mismatch between physiological thermal limits and ambient climatic conditions is highest in tropical regions, which also harbour the highest amount of biodiversity worldwide. Projections of decreasing precipitation in areas where rising temperature alone may already jeopardize bird and mammal populations [61] worsen the perspective for tropical species, as water availability is crucial for endotherms to compensate thermal stress (electronic supplementary material, figure S1b) [62]. Assuming that our dataset is representative for a wide selection of endotherms in general, this coincidence of high sensitivity to even small levels of temperature increase with potential precipitation decrease, high levels of species richness and endemism, and especially the unhalted loss of natural habitats, underlines the threats from global change for biodiversity in tropical regions [7,51,63].
Third, we show that the majority of bird and mammal species in our dataset are likely to experience increases of temperature that fall within their thermal tolerance limits across more than 50% of their geographical ranges and for the majority of months per year. Thus, from a macrophysiological point of view, many endotherms might not be stressed by rising temperatures [21]. These findings call for a careful reinterpretation of predictions of extinction risk that are based solely on statistical models correlating species occurrences with climatic variables and that do not take into account that physiological tolerances may buffer projected changes in ambient climatic conditions. However, our results suggest considerable variation of potential vulnerability among regions and between major taxonomic groups (birds and mammals). Furthermore, additive or synergistic effects of climate change with other threats, such as land-use change, the spread of invasive species or overexploitation [18,63,64], may counteract the potential opportunities given by species' physiological tolerances.
Our analyses are a step forward towards a better quantification of species' chances and challenges to cope with climate change, by explicitly considering their fundamental physiological capacities. With our study, we demonstrate how a joint analytical framework combining species' thermal physiology, their ecology and the geography of climate change may help to improve understanding and predicting the potential futures of species and biodiversity in an age of global change.
Supplementary Material
Acknowledgements
We are grateful to Diana Bowler, Marcel Cardillo, Robert Colwell, Catherine Graham, Susanne Fritz and Martin Plath, as well as two anonymous reviewers for comments on earlier versions of the paper. We also thank Tanja Caprano, Cornelia Weist, Susanne Fritz and Jan Schnitzler for support with data preparation and analyses.
Funding statement
I.K. is supported by the Higher Education Commission of Pakistan and the German Academic Exchange Service (DAAD). This work was supported by the research funding programme ‘LOEWE—Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz’ of Hesse's Ministry of Higher Education, Research and the Arts.
References
- 1.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaston KJ, et al. 2009. Macrophysiology: a conceptual reunification. Am. Nat. 174, 595–612. ( 10.1086/605982) [DOI] [PubMed] [Google Scholar]
- 3.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 223–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 4.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 6.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaumont LJ, Pitman A, Perkins S, Zimmermann NE, Yoccoz NG, Thuiller W. 2011. Impacts of climate change on the world's most exceptional ecoregions. Proc. Natl Acad. Sci. USA 108, 2306–2311. ( 10.1073/pnas.1007217108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sala OE. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 9.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 10.Stevens GC. 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256. ( 10.1086/284913) [DOI] [Google Scholar]
- 11.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 12.Scholander PF, Hock R, Walters V, Johnson F, Irving L. 1950. Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 99, 237–258. ( 10.2307/1538741) [DOI] [PubMed] [Google Scholar]
- 13.Buckley LB, Hurlbert AH, Jetz W. 2012. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 21, 873–885. ( 10.1111/j.1466-8238.2011.00737.x) [DOI] [Google Scholar]
- 14.McNab BK. 2012. Extreme measures: the ecological energtics of birds and mammals. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 15.McNab BK, Morrison P. 1963. Body temperature and metabolism in subspecies of Peromyscus from arid and mesic environments. Ecol. Monogr. 33, 63–82. ( 10.2307/1948477) [DOI] [Google Scholar]
- 16.Oswald SA, Huntley B, Collingham YC, Russell DJF, Anderson BJ, Arnold JM, Furness RW, Hamer KC. 2011. Physiological effects of climate on distributions of endothermic species. J. Biogeogr. 38, 430–438. ( 10.1111/j.1365-2699.2010.02435.x) [DOI] [Google Scholar]
- 17.Holt RD. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315. ( 10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 18.Hof C, Levinsky I, Araújo MB, Rahbek C. 2011. Rethinking species’ ability to cope with rapid climate change. Glob. Change Biol. 17, 2987–2990. ( 10.1111/j.1365-2486.2011.02418.x) [DOI] [Google Scholar]
- 19.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 20.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 21.La Sorte FA, Jetz W. 2012. Tracking of climatic niche boundaries under recent climate change. J. Anim. Ecol. 81, 914–25. ( 10.1111/j.1365-2656.2012.01958.x) [DOI] [PubMed] [Google Scholar]
- 22.Devictor V, Julliard R, Couvet D, Jiguet F. 2008. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B 275, 2743–2748. ( 10.1098/rspb.2008.0878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintero I, Wiens JJ. 2013. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. ( 10.1111/ele.12144) [DOI] [PubMed] [Google Scholar]
- 24.Kriticos DJ, Webber BL, Leriche A, Ota N, Macadam I, Bathols J, Scott JK. 2012. CliMond: global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 3, 53–64. ( 10.1111/j.2041-210X.2011.00134.x) [DOI] [Google Scholar]
- 25.Prinzinger R, Lubben I, Schuchmann KL. 1989. Energy metabolism and body temperature in 13 sunbird species (Nectariniidae). Comp. Biochem. Physiol. A, Mol. Integr. Physiol. 92, 393–402. ( 10.1016/0300-9629(89)90581-1) [DOI] [Google Scholar]
- 26.Tummers B. 2006. Data thief III See http://www.datathief.org/.
- 27.Porter WP, Kearney M. 2009. Size, shape, and the thermal niche of endotherms. Proc. Natl Acad. Sci. USA 106, 19 666–19 672. ( 10.1073/pnas.0907321106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson A-L, Brown M, Downs CT. 2011. Seasonal variation in metabolic rate of a medium-sized frugivore, the Knysna turaco (Tauraco corythaix). J. Therm. Biol. 36, 167–172. ( 10.1016/j.jtherbio.2011.01.004) [DOI] [Google Scholar]
- 29.BirdLife International and NatureServe 2011. Bird species distribution maps of the world. BirdLife International, Cambridge, UK and Arlington, VA: NatureServe. [Google Scholar]
- 30.IUCN. 2008. IUCN Red list of threatened species. International Union of Conservation of Nature See http://www.iucnredlist.org/mammals
- 31.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 32.Fritz SA, Bininda-Emonds ORP, Purvis A. 2009. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 12, 538–549. ( 10.1111/j.1461-0248.2009.01307.x) [DOI] [PubMed] [Google Scholar]
- 33.Kuhn TS, Mooers AØ, Thomas GH. 2011. A simple polytomy resolver for dated phylogenies. Methods Ecol. Evol. 2, 427–436. ( 10.1111/j.2041-210X.2011.00103.x) [DOI] [Google Scholar]
- 34.Meehl GA, Covey C, Delworth T, Latif M, McAvaney B, Mitchell JFB, Stouffer RJ, Taylor KE. 2007. The WCRP CMIP3 mutimodel dataset: a new era in climate change research. Bull. Am. Meteorol. Soc. 88, 1383–1394. ( 10.1175/BAMS-88-9-1383) [DOI] [Google Scholar]
- 35.IPCC. 2007. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change: summary for policymakers. IPCC. [Google Scholar]
- 36.Hijmans RJ, Cameron SE, Parra JL, Jones G, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 1978, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 37.Orme CDL, Freckleton RP, Thomas GH, Petzoldt T, Frits SA, Issac N, Pearse W. 2012. Caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5.
- 38.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 40.Martins EP, Hansen TF, Url S. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 41.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726. ( 10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 42.Moran PAP. 1950. Notes on continuous stochastic phenomena. Biometrika 37, 17–23. ( 10.1093/biomet/37.1-2.17) [DOI] [PubMed] [Google Scholar]
- 43.Rangel TF, Diniz-Filho JAF, Bini LM. 2010. SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33, 46–50. ( 10.1111/j.1600-0587.2009.06299.x) [DOI] [Google Scholar]
- 44.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speakman JR, Król E. 2010. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746. ( 10.1111/j.1365-2656.2010.01689.x) [DOI] [PubMed] [Google Scholar]
- 46.Diniz-Filho JAF, Mauricio Bini L, Fernando Rangel T, Loyola RD, Hof C, Nogues-Bravo D, Araujo MB. 2009. Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography 32, 897–906. ( 10.1111/j.1600-0587.2009.06196.x) [DOI] [Google Scholar]
- 47.Rodríguez-Serrano E, Bozinovic F. 2009. Interplay between global patterns of environmental temperature and variation in nonshivering thermogenesis of rodent species across large spatial scales. Glob. Change Biol. 15, 2116–2122. ( 10.1111/j.1365-2486.2009.01854.x) [DOI] [Google Scholar]
- 48.Bicudo JEPW, Buttermer WA, Chappell MA, Pearson JT. 2010. Ecological and environmental physiology of birds. New York, NY: Oxford University Press. [Google Scholar]
- 49.Boyles JG, Seebacher F, Smit B, McKechnie AE. 2011. Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 51, 676–690. ( 10.1093/icb/icr053) [DOI] [PubMed] [Google Scholar]
- 50.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706. ( 10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 51.Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261. ( 10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- 52.Monahan WB. 2009. A mechanistic niche model for measuring species’ distributional responses to seasonal temperature gradients. PLoS ONE 4, e7921 ( 10.1371/journal.pone.0007921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scherrer D, Körner C. 2011. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 38, 406–416. ( 10.1111/j.1365-2699.2010.02407.x) [DOI] [Google Scholar]
- 54.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 1–6. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CDG, McCann KS, Savage V, Tunney TD, O'Connor MI. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612 ( 10.1098/rspb.2013.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cahill AE, et al. 2013. How does climate change cause extinction? Proc. R. Soc. B 280, 20121890 ( 10.1098/rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warren RJ, Chick L. 2013. Upward ant distribution shift corresponds with minimum, not maximum, temperature tolerance. Glob. Change Biol. 19, 2082–2088. ( 10.1111/gcb.12169) [DOI] [PubMed] [Google Scholar]
- 58.Benning TL, LaPointe D, Atkinson CT, Vitousek PM. 2002. Interactions of climate change with biological invasions and land use in the Hawaiian Islands: modeling the fate of endemic birds using a geographic information system. Proc. Natl Acad. Sci. USA 99, 14 246–14 249. ( 10.1073/pnas.162372399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweiger O, Heikkinen RK, Harpke A, Hickler T, Klotz S, Kudrna O, Kühn I, Pöyry J, Settele J. 2012. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob. Ecol. Biogeogr. 21, 88–99. ( 10.1111/j.1466-8238.2010.00607.x) [DOI] [Google Scholar]
- 60.Levinsky I, Skov F, Svenning J-C, Rahbek C. 2007. Potential impacts of climate change on the distributions and diversity patterns of European mammals. Biodivers. Conserv. 16, 3803–3816. ( 10.1007/s10531-007-9181-7) [DOI] [Google Scholar]
- 61.McCain CM, Colwell RK. 2011. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecol. Lett. 14, 1236–1245. ( 10.1111/j.1461-0248.2011.01695.x) [DOI] [PubMed] [Google Scholar]
- 62.McKechnie AE, Wolf BO. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256. ( 10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hof C, Araújo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–519. ( 10.1038/nature10650) [DOI] [PubMed] [Google Scholar]
- 64.Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. ( 10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.