Abstract
Evolutionary theory of plant defences against herbivores predicts a trade-off between direct (anti-herbivore traits) and indirect defences (attraction of carnivores) when carnivore fitness is reduced. Such a trade-off is expected in plant species that kill herbivore eggs by exhibiting a hypersensitive response (HR)-like necrosis, which should then negatively affect carnivores. We used the black mustard (Brassica nigra) to investigate how this potentially lethal direct trait affects preferences and/or performances of specialist cabbage white butterflies (Pieris spp.), and their natural enemies, tiny egg parasitoid wasps (Trichogramma spp.). Both within and between black mustard populations, we observed variation in the expression of Pieris egg-induced HR. Butterfly eggs on plants with HR-like necrosis suffered lower hatching rates and higher parasitism than eggs that did not induce the trait. In addition, Trichogramma wasps were attracted to volatiles of egg-induced plants that also expressed HR, and this attraction depended on the Trichogramma strain used. Consequently, HR did not have a negative effect on egg parasitoid survival. We conclude that even within a system where plants deploy lethal direct defences, such defences may still act with indirect defences in a synergistic manner to reduce herbivore pressure.
Keywords: oviposition-induced plant volatiles, hypersensitive response, defence trade-offs, PR1, Trichogramma, Pieris rapae
1. Introduction
Plants deploy various direct and indirect defences against herbivore attackers. Direct defence traits induced by herbivory such as the production of secondary metabolites have an often systemic and broad effect on herbivores, whereas traits such as the abortion of plant organs and tissue necrosis are more locally targeted against specific attackers [1]. In addition, plant infochemicals may function as an indirect defence trait responsible for attracting natural enemies of herbivores [2,3]. However, a direct defence may not only have a major effect on the herbivore's survival, but also on the survival of the natural enemies of the herbivore, leading to a possible conflict between the plant and the natural enemy that is attracted to the herbivore on that plant [4–12]. Evolutionary theory thus suggests that defence strategies, such as direct and indirect defences, may negatively correlate within a multitrophic community resulting in possible trade-offs [13–15]. Among carnivores, such negative effects are especially relevant for parasitoids that require a single host to complete development. Also, many larval parasitoids have evolved an intimate physiological relationship with their host and, throughout their development, may be exposed to toxic plant defence chemicals ingested by their host [16–21].
Sub-lethal resistance traits, such as toxins or antinutritive agents, seem to be the most common form of plant resistance [22]. The claimed trade-offs between sub-lethal direct and indirect defences that result from deleterious effects on higher trophic levels are expected to be ubiquitous in plant–insect interactions and have been hypothesized to even shape consumer diversity of multitrophic systems [23]. However, sub-lethal plant defences that slow down herbivore growth may both compromise the herbivore's immune response against parasitism [17] and expand the temporal window of vulnerability to parasitoid attack, rather resulting in a higher than lower parasitism [22,24]. Also, plant defences may be shaped by a whole community of interacting herbivores than by single pairwise interactions between species [2,23,25,26]. Therefore, an alternative to the view of trade-offs between defences, is that they act additively or synergistically to combat different herbivore species simultaneously, and do not interfere with each other [22,27].
In contrast to sub-lethal plant defences, lethal traits are less common and mainly known to protect plants against sessile herbivore stages, i.e. egg deposition [28,29], or against fruit predators by fruit abortion [30]. A conflict with the attraction of natural enemies to plants expressing lethal defence traits is especially expected. Experimental evidence for this premise is scarce however, and there is a need for integrative approaches, where the effects of plant defences on fitness proxies across trophic levels are evaluated against the relevant genetic variation present in the plant–insect populations. In this study, we tested the prediction that a direct lethal defence trait killing herbivore eggs will conflict with the indirect defence trait of attracting parasitic wasps.
Egg deposition by herbivorous insects has been shown to trigger several plant responses lethal to herbivore eggs, such as (i) hypersensitive response (HR)—like necrosis and neoplasm formation both leading to egg desiccation and egg dropping [31–35], (ii) egg-crushing plant tissue [36] and (iii) ovicidal substances killing eggs [37,38]. At the same time, herbivore eggs may induce indirect defence traits, such as oviposition-induced plant volatiles (OIPVs) or plant cues perceived by contact, that attract or arrest parasitic wasps killing eggs before larvae hatch [29,39]. The black mustard Brassica nigra is commonly attacked by cabbage white butterflies (Pieris spp.) [40,41], the eggs of which sometimes induce HR-like necrosis [32,34] and serve as hosts for generalist parasitic wasps of the genus Trichogramma [42]. In a previous laboratory study with a B. nigra population originating from Greece, we showed that eggs of the gregarious large cabbage white butterfly (P. brassicae) induced HR-like necrosis [32]. Interestingly, this direct defensive trait against eggs has not been described so far in other crucifer species. One exception is Arabidopsis thaliana (Col-0), where Pieris eggs triggered a transcriptional response similar to HR, including an activation of the salicylic acid signalling and HR marker genes, i.e. pathogenesis-related genes (PR); but HR-like necrosis to eggs was absent [43]. In addition to HR, B. nigra emits volatiles in response to P. brassicae eggs attracting T. brassicae wasps [32]. However, how the direct egg-killing trait correlates with the attraction of natural enemies remains to be investigated.
We conducted a field survey and greenhouse and laboratory experiments to test (i) whether eggs of Pieris butterflies induce HR-like necrosis in different populations of B. nigra and if so, whether there is phenotypic variation therein, (ii) how the expression of HR affects the performance of Pieris eggs and parasitism rates by Trichogramma species and to what extent, (iii) how expression of HR affects preference and performance of Trichogramma wasps and (iv) whether HR necrosis is associated with an activation of the marker gene PR1. We hypothesized that HR-like necrosis, and not oviposition itself, is associated with an induction of the PR1 gene in the plant. Moreover, we expected that HR-like necrosis is a common trait for the black mustard against specialist cabbage whites and that herbivore and parasitoid survival under field conditions will be negatively influenced by the expression of HR. Consequently, Trichogramma wasps were expected to prefer volatiles of plants that did not express HR. This study provides evidence at several levels of biological organization of how insect eggs can shape plant–insect interactions.
2. Material and methods
(a). Plants and insects
Black mustard (B. nigra L.) plants were grown in a greenhouse (18 ± 5°C, 50–70% RH, 16 L : 8 D). Seeds of at least 10 individual plants were collected from a B. nigra population in 2009 at the River Rhine in Wageningen (Steenfabriek), The Netherlands (coordinates: 51.96, 5.68) and pooled. To assess whether HR-like necrosis is common across populations, seeds were also collected from two other locations in The Netherlands namely north of Wageningen (Radix) (coordinates: 51.99, 5.68) and Rhenen (Blauwe Kamer) (coordinates: 51.95, 5.60). The distances between the populations were about 1–6 km. Moreover, seeds collected in West Sacramento, Yolo County, CA, USA (coordinates: 38.58, −121.57) and seeds from Peloponnesus, Greece (collected in 1975 and stored by the Centre for Genetic Resources, Wageningen, The Netherlands) were used. Plants of four weeks old were used in the experiments.
Pieris rapae L. and P. brassicae L. (Lepidoptera: Pieridae) were reared on Brussels sprouts plants (B. oleracea var. gemmifera cv. Cyrus) in a climate room (21 ± 1°C, 50–70% RH, 16 L : 8 D). Two native isofemale Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) strains (RU124 and RU263), originating from P. rapae eggs collected on B. nigra at the River Rhine in Wageningen in 2010, and one isofemale T. brassicae Bezdenko strain (Y175), originating from a Mamestra brassicae egg cluster collected in a cabbage field in Lierop, The Netherlands in 1999, were reared since then on Ephestia kuehniella eggs (Koppert, Berkel en Rodenrijs, The Netherlands) under standardized conditions in a climate chamber (25 ± 1°C, 50–70% RH, 16 L : 8 D). Only mated, 2–3 days old, wasps were used in the preference experiments. All wasps used in the preference experiments lacked previous contact with any plant material or host residues and are referred to as inexperienced.
(b). Field survey
A survey was conducted to record survival and parasitism (by Trichogramma spp.) of Pieris eggs on individual B. nigra plants in a natural population. The survey was conducted at a B. nigra patch along the River Rhine in Wageningen (Steenfabriek), The Netherlands (coordinates: 51.96, 5.68) in three seasons/three butterfly generations: August—September 2010, May—July 2011 (1) and August—September 2011 (2). The total area monitored was approximately 100 m2 consisting of about 1000 plants. In spring, plants were only 5–20 cm tall, whereas they could grow up to more than 1.5 m tall in late summer. Plants were monitored for eggs at the edges of a patch or on isolated growing plants. Eggs were collected on leaves and checked for the presence of a necrotic zone on the leaf and/or parasitism by Trichogramma wasps. In both seasons in 2011, each plant was marked and revisited. Sometimes additional eggs were found on the same plant. After collection, eggs were kept in a climate chamber (25 ± 1°C, 50–70% RH, 16 L : 8 D) until caterpillars or wasps emerged. All hatched, dead and parasitized eggs were recorded. Hatched caterpillars were fed with black mustard leaves until fourth instar for species identification. The emerged wasps were preserved in 100% ethanol and kept at −25°C for standard molecular species identification using ITS-2 markers (for more details, see [44] and the electronic supplementary material).
(c). Greenhouse and laboratory experiments
(i). Plant treatments
To obtain egg-infested plants for monitoring HR-like necrosis induced by Pieris eggs in plants of different B. nigra populations, plants were placed into a cage with more than 100 P. rapae or P. brassicae adults to allow deposition of eggs onto the plants. Plants were exposed to the butterflies, to obtain about 20 single P. rapae eggs or one P. brassicae egg clutch per plant. After egg infestation, plants were kept in a greenhouse compartment (21 ± 2°C, 70% RH, 16 L : 8 D) and checked for HR-like necrosis 72–96 h after egg deposition (see below). For P. rapae egg performances and T. evanescens preferences and gene expression analysis, B. nigra plants from the local population (Steenfabriek) were used. Following P. rapae egg deposition, plants were placed in the same greenhouse compartment for either 24 or 72 h. Control plants, which had not been in contact with P. rapae or any other insects, were kept under the same conditions as treated plants.
(ii). Hypersensitive response-like necrosis
All egg-infested plants and leaves collected from plants in the field were checked for HR-like necrosis. The strength of HR was recorded and the plants were categorized into HR− (no necrotic zone observed) or HR+ (necrotic zone, eggs desiccating). Plants were kept under greenhouse conditions (22 ± 2°C, 70% RH, 16 L : 8 D) and the number of eggs was counted immediately and 5 days after oviposition.
(iii). Egg survival
The survival rate of eggs was calculated based on the total number of eggs and the number of caterpillars. The number of eggs was counted before and the number of caterpillars just after hatching by observation. HR intensity was noted just prior to larval hatching. HR necrosis was categorized into three severities: necrosis visible underneath the egg (HR+), necrosis also visible on other side of the leaf (HR++) and necrosis visible around the egg and underside of leaf (HR+++).
(iv). Two-choice bioassays
We tested the attraction of a laboratory strain of T. brassicae and two native strains of T. evanescens to volatiles of P. rapae egg-induced B. nigra plants. Bioassays were conducted by using a dynamic airflow Y-tube olfactometer. This olfactometer was adapted to small wasps like Trichogramma spp. to be released in groups [32]. We have previously established that group-release did not influence the behaviour of these Trichogramma wasps [32]. Ten adult females of one strain were released and their preference for one of the two odour sources was recorded. Thereafter, the position of the odour sources was exchanged and another group of 10 wasps from the other strain was released to test their preference for the same two odour sources. After 45 min, the wasps present in the collection flasks placed at the end of the arms section of the olfactometer were counted. When a wasp did not make a choice within 45 min, it was recorded as a ‘no response’. The odour sources were plants infested with P. rapae eggs versus uninfested plants. In addition, we recorded the presence or absence of a hypersensitive-like response of the infested plants that were used in the bioassay. Per odour source combination, five to seven different plants with one replicate per experimental day were tested with 10–20 wasps of each strain released per replicate (70–100 wasps/strain/treatment). Each wasp was used only once.
(v). Quantitative RT-PCR analysis of hypersensitive response marker gene
For gene expression, two leaf discs (1.7 cm diameter) were taken from the third and fourth leaf from the top directly next to an egg. Discs of three to five individual plants per treatment were pooled. In total, three to five biological replicates were obtained per treatment. Leaf tissues were snap-frozen in liquid nitrogen and kept at −80°C until analysis. Total RNA was extracted from approximately 100 mg liquid nitrogen-ground leaf powder using the RNeasy Plant Mini kit (Qiagen). One microgram of total RNA was treated with DNaseI (Invitrogen) and subsequently transcribed into cDNA using the iScript cDNA synthesis kit (Bio-rad) following the manufacturer's protocol. The used sequence of B. nigra gene-specific PATHOGENESIS-RELATED GENE1 primers were PR1F 5′-CTTGGCCATGGGTAGCGGCG-3′ and PR1R 5′-ACACCTCGCTTTGCCACATCCA-3′. Quantitative RT-PCR was performed in a Rotor-Gene 6000 machine (Corbett Research) with a 72-well rotor. The amplification reactions were performed in 25 μl final volume containing 12.5 μl iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), 300 nM of the gene-specific forward and reverse primer and 5 μl cDNA. All qRT-PCR experiments were performed in duplicate and average values were used in the analyses. The following PCR program was used for all PCR analyses: 95°C for 3 min, followed by 40 cycles of 95°C for 15 s, 30 s at 60°C and 30 s at 72°C. Normalized gene expression was then calculated as 2–ΔΔCt [45], using GAPDH as a reference gene (primer sequences: F 5′-GCTACGCAGAAGACAGTTGATGG-3′ and R 5′-TGGGCACACGGAAGGACATAC-3′).
(d). Statistics
The effect of a plant showing an HR-like necrosis on Pieris eggs and parasitoid survival, as well as on Trichogramma parasitism was analysed using generalized linear models (GLM) with binomial distribution for errors and a logit link function. In these models, the dispersion parameter was estimated to correct for over-dispersion. The analysis of the data from 2010 could not include plant identity and therefore eggs (instead of plants) were considered as units. We tested whether HR affected Trichogramma clutch size by recording the number of wasps emerging from an egg as a categorical (multinomial) response variable using PROC CATMOD (SAS Institute, Cary, NC, USA). To test whether the number of eggs on a plant affects the induction of HR, a linear regression analysis between the number of eggs/plant/day and the number of eggs inducing HR/plant/day was conducted. Data from the olfactometer bioassays were analysed by expressing the number of animals that chose the test odour as the fraction of all responding wasps and 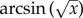 transforming the variable. Subsequently, choices were tested against a 50 : 50 distribution with a one-sample t-test. The distributions in the choices that parasitoids made across the different treatment combinations were compared using a GLM with the
transforming the variable. Subsequently, choices were tested against a 50 : 50 distribution with a one-sample t-test. The distributions in the choices that parasitoids made across the different treatment combinations were compared using a GLM with the 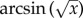 fractions as dependent variables. ‘Time after egg deposition’, ‘HR response’ and ‘parasitoid strain’ were fixed factors. The model used for final inferences contained all main effects and significant interactions terms. The fit of the model was checked by the Kolmogorov–Smirnov test. The gene transcription data were square root-transformed to meet the normality assumption, and analysed with one-way ANOVA for the two time points independently. Then Tukey's test was used for post hoc comparisons.
fractions as dependent variables. ‘Time after egg deposition’, ‘HR response’ and ‘parasitoid strain’ were fixed factors. The model used for final inferences contained all main effects and significant interactions terms. The fit of the model was checked by the Kolmogorov–Smirnov test. The gene transcription data were square root-transformed to meet the normality assumption, and analysed with one-way ANOVA for the two time points independently. Then Tukey's test was used for post hoc comparisons.
3. Results
(a). Field survey
(i). Monitored insects
The majority of the collected eggs were of P. rapae (96%, 191 out of 198 identified caterpillars), and a small fraction of P. napi (4%). Both species deposit single eggs. No egg batches of P. brassicae were found. All wasps that emerged from the collected Pieris eggs belonged to Trichogramma spp. (Hymenoptera: Trichogrammatidae). A group of 25 wasps identified through sequencing of the ITS-2 gene were T. evanescens Westwood.
(ii). Plant response to eggs
In the year 2010, 359 eggs were collected from approximately 50 plants. From all collected eggs in 2010, 211 had induced HR-like necrosis (HR+) (59%). On one exceptional plant, about 80 Pieris eggs were collected, however this plant was excluded from the analysis. In the year 2011 (both generations), 360 eggs were collected from 244 plants out of which 120 plants expressed HR (49%) (figure 1a). A maximum of nine eggs was found on a plant per sampling day (mean eggs/plant/day ± s.d.: 1.4 ± 1.1). There was no linear relationship between egg density and induction of HR-like necrosis (electronic supplementary material, figure S1, R2 = 0.28, F1,48 = 0.06, p = 0.82).
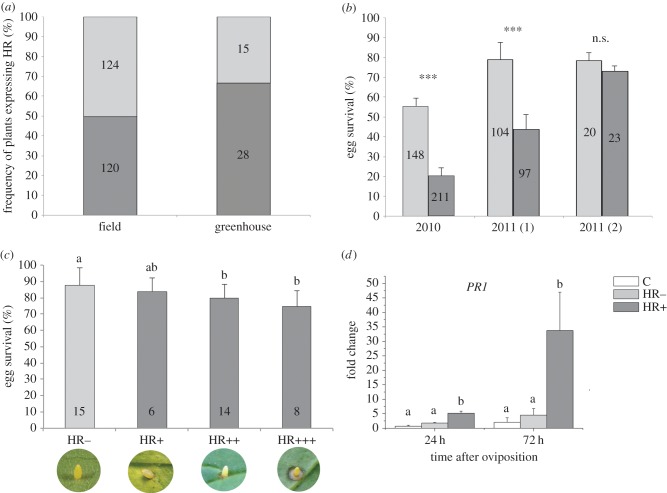
Figure 1.
Frequency of B. nigra plants expressing HR-like necrosis induced by Pieris eggs and effects of HR-like necrosis on Pieris egg survival and PR1 gene expression. (a) Percentage of HR-like necrosis on plants of a Dutch field population (Steenfarbiek) and in the greenhouse. (b) Percentage (estimated mean ± s.e.) of caterpillars emerging from Pieris eggs on plants of a Dutch field population (Steenfarbiek) (2010 and 2011, generation 1 and 2); ***p < 0.001; n.s., not significant (GLM, p > 0.05). (c) Variation in HR severity and effect on P. rapae egg survival (estimated mean ± s.e.) in the greenhouse (different severities were visually characterized as in the pictures); different letters indicate significant differences (GLM and LSD, p < 0.05). (d) Effect of HR-like necrosis induced by P. rapae eggs on expression changes (mean ± s.e.) of HR-marker gene PR1 at 24 and 72 h after oviposition; different letters indicate significant differences (ANOVA, Tukey test p < 0.05, n = 3–5 biological replicates). Numbers inside the bars represent number of plants for data of 2011 and number of eggs for data of 2010. Hypersensitive response (HR), in light grey HR−: no necrotic zone observed, in dark grey HR+: necrotic zone. (Online version in colour.)
(iii). Effect of plant response on egg survival
In two cases out of the three censuses, we found a significant decrease in the egg survival of P. rapae when an HR-like reaction in response to egg deposition was present in the plant (figure 1b). In 2010, significantly more eggs survived on HR− than on HR+ plants (GLM, F1,357 = 37.93, p < 0.001). While a significant effect of HR on egg survival was present in the first generation in 2011 (GLM, F1,199 = 16.84, p < 0.001), there was no effect of HR on egg survival in the second generation (GLM, F1,41 = 0.15, p = 0.70).
(iv). Effect of plant response on egg parasitism
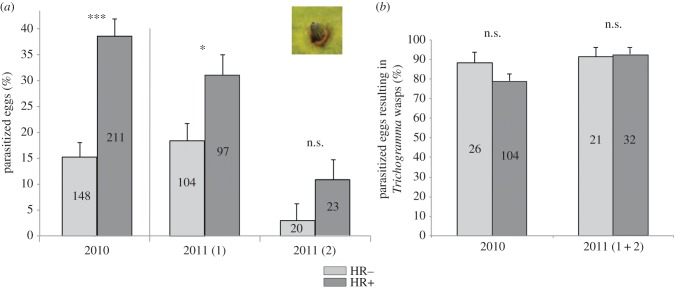
The highest Trichogramma egg parasitism rates were found in 2010: 104 eggs were parasitized (29%), and significantly more parasitized eggs were found with an HR-like necrosis (HR+) than with no necrosis (HR−) (F1,357 = 18.19, p < 0.001, figure 2a). In 2011, significantly more parasitized eggs were found on HR+ than on HR− plants in the first generation (F1,199 = 5.47, p = 0.02), whereas no differences were found in the second generation (F1,41 = 1.57, p = 0.22).
Figure 2.
Effect of Pieris egg-induced HR on Trichogramma parasitism and performance in a natural B. nigra population collected in three different seasons in 2010 and 2011 (generations 1 and 2). (a) Percentage (estimated mean ± s.e.) of eggs that were parasitized by Trichogramma wasps. Picture shows a parasitized egg with HR-like necrosis. (b) Percentage of parasitized eggs (estimated mean ± s.e.) from which Trichogramma wasps emerged. Numbers inside the bars represent number of plants for data of 2011 and number of eggs for data of 2010. Different plant phenotypes: hypersensitive response (HR), HR−: no necrotic zone observed, HR+: with necrotic zone. ***p < 0.001, *p < 0.05; n.s., not significant (GLM, p > 0.05). (Online version in colour.)
(v). Effect of plant response on egg parasitoid survival and clutch size
Trichogramma spp. can successfully parasitize and complete their development inside single Pieris eggs that induced HR in leaves collected from B. nigra plants at the River Rhine. There was no effect of HR-like necrosis on the percentage of wasps emerging from the collected Pieris eggs in 2010 (F1,128 = 1.25, p = 0.27, figure 2b) and in 2011 (F1,51 = 0.06, p = 0.81, figure 2b). Moreover, we showed that up to four wasps emerged per Pieris egg but HR had no effect on clutch size (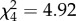 , p = 0.30).
, p = 0.30).
(b). Greenhouse and laboratory experiments
(i). Plant response to eggs
From all six tested B. nigra populations, a fraction of plants responded with HR-like necrosis induced by both singly deposited P. rapae eggs and/or gregariously laid P. brassicae eggs. Within each population expression of HR varied between 27 and 63% of the tested plants (table 1).
Table 1.
Frequency of HR-like necrosis induced by Pieris eggs in different B. nigra populations.
| B. nigra population | Pieris species | N plants | % HR |
|---|---|---|---|
| Peloponnese, Greece | P. brassicae | 74 | 47.3 |
| P. rapae | 38 | 31.6 | |
| South-Wageningen (Steenfabriek), The Netherlands | P. brassicae | 76 | 51.3 |
| P. rapae | 79 | 46.8 | |
| North-Wageningen (Radix), The Netherlands | P. brassicae | 15 | 46.7 |
| Rhenen (Blauwe Kamer), The Netherlands | P. brassicae | 15 | 26.7 |
| West Sacramento, CA, USA | P. brassicae | 11 | 63.6 |
| P. rapae | 15 | 26.7 |
(ii). Effect of plant response on egg survival
Of the 42 B. nigra plants tested for P. rapae egg survival, 28 plants expressed HR (65%) (figure 1a) from which significantly less larvae hatched (22%) than from plants not expressing HR (GLM, F1,41 = 9.51; p = 0.004, figure 1b), probably due to egg desiccation. Only few eggs fell from the plant before hatching (HR−: 0.3%, HR+: 1%). Egg survival depended on the strength of the HR-like necrosis: the larger the area of necrosis, the lesser was the number of eggs that survived. Significantly more eggs survived on non-HR expressing plants (HR−) and on plants with little necrosis (HR+) than on plants expressing medium (HR++) or the strongest necrosis (HR+++) (+++) (GLM, F3,39 = 4.0; p = 0.014, figure 1c).
(iii). Induction of hypersensitive response marker gene PR1
The deposition of single P. rapae eggs strongly induced the expression of the PR1 both at 24 (F2,9 = 18.35, p = 0.002) and 72 h after oviposition (F2,8 = 6.57, p = 0.03) when compared with control plants, and only in HR+ plants, but not on HR− plants (Tukey, p > 0.05, figure 2d). Thus, phenotypic expression of necrosis was clearly correlated with genotypic expression of the HR marker gene PR1.
(iv). Effect of plant response on egg parasitoid-attracting plant volatiles
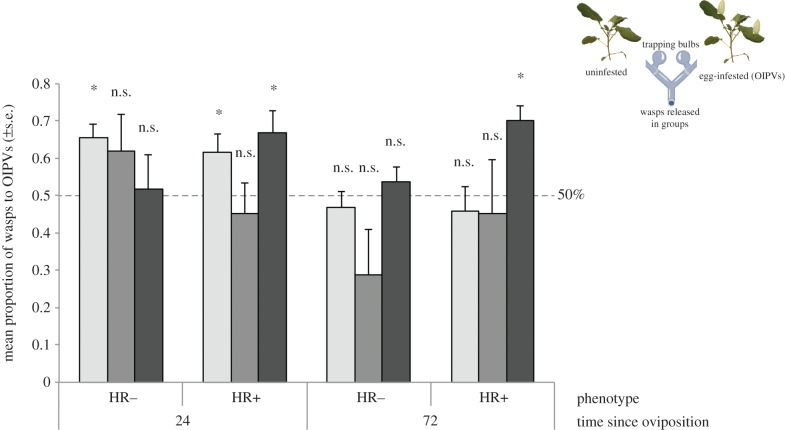
In olfactometer bioassays, two native strains of T. evanescens (RU124 and RU263), both collected from P. rapae eggs in 2010 and a laboratory strain of T. brassicae were tested for attraction to OIPVs (figure 3). Although in the overall model, the presence/absence of HR response did not influence the distribution of the wasps’ choices over the tested plant volatiles (GLM, F1,93 = 0.68, p = 0.41), when exposed to the same odour sources, the Trichogramma strains made different choices (F2,93 = 3.63, p = 0.03). Also, the time after oviposition significantly affected the distribution of the wasps' choices, and the differences between the tested odour blends were more pronounced 24 h after induction than after 72 h (F1,93 = 6.58, p = 0.01).
Figure 3.
Proportion (mean ± s.e.) of female Trichogramma wasps choosing OIPVs of B. nigra plants. Plants were infested with single eggs of P. rapae. Columns represent the mean proportion of choice for OIPVs of isofemale lines from Trichogramma brassicae (lab strain Y175) (light grey) and T. evanescens (native strain RU124, medium grey and native strain RU 263, dark grey) tested in a Y-tube olfactometer. Numbers in the columns represent number of responding wasps. All experiments were conducted in a two-choice situation between plants infested with eggs of different ages (24 and 72 h) and uninfested control plants. *p < 0.05; n.s., not significant (p > 0.05); one-sample t-test. The dashed line indicates 0.5 = no preference. Each treatment combination was replicated with five to seven plant pairs and 10–20 wasps of each strain per plant pair (n = 70–100 wasps per treatment/strain). Different plant phenotypes: hypersensitive response (HR), HR−: no necrotic zone observed, HR+: necrotic zone. (Online version in colour.)
At 24 h after oviposition, only wasps of the T. brassicae laboratory line significantly preferred volatiles of egg-infested plants without a visible necrosis (HR−) (Y175: t4 = 4.55, p = 0.01; RU124: t6 = 1.23, p = 0.27; RU263: t6 = 0.23, p = 0.82; one-sample t-test). However, when OIPVs of plants expressing HR (HR+) were tested 24 h after oviposition, wasps of the laboratory line and of one native line (RU263) were significantly attracted to them (Y175: t5 = 2.92, p = 0.03; RU124: t7 = 1.8, p = 0.11; RU263: t7 = 3.45, p = 0.01). At 72 h after oviposition, none of the wasps were attracted to OIPVs of HR− plants (Y175: t4 = −0.75, p = 0.5; RU124: t6 = −1.67, p = 0.15; RU263: t6 = 1.02, p = 0.35), whereas one native line was significantly attracted to OIPVs of HR+ plants (Y175: t4 = −0.61, p = 0.58; RU124: t6 = −0.29, p = 0.78; RU263: t6 = 4.86, p = 0.003).
4. Discussion
Our study finds a positive correlation between a potentially lethal direct and an information-mediated indirect defence. One tested T. evanescens strain was specifically attracted to volatiles emitted by B. nigra plants expressing HR-like necrosis (HR+). This higher attractiveness to volatiles emitted by plants of the HR+ phenotype could explain the higher egg parasitism rates that were found on the latter compared with plants lacking HR (HR−) in the natural B. nigra population. A synergistic expression of both traits seems to lead to a more effective control of Pieris butterfly eggs leading to up to two times higher egg mortalities on HR+ plants.
We confirmed that HR-like necrosis against P. rapae eggs is a lethal resistance trait that leads to butterfly egg desiccation resulting in fewer caterpillars hatching especially then when necrosis was the strongest. We showed phenotypic variation within the same B. nigra population and between populations in the expression of HR induced by both tested Pieris species ranging from 27 to 64% (table 1). HR-like necrosis is not restricted to B. nigra. Similar necrotic responses have been observed in Solanum hybrids against egg masses of the Colorado potato beetle Leptinotarsa decemlineata or Physalis plants against eggs of the specialist moth Heliothis subflexa [31,33]. In the latter system, both HR-like necrosis sometimes in combination with neoplasm formation leads to 25% lower probability of larvae hatching and 28% lower probability of eggs remaining on the Physalis plants [33]. It is suggested that HR-like necrosis in both Solanum hybrids and Physalis spp. is genotypically determined and this might also be the case in the B. nigra population studied here. Previous studies in Arabidopsis (where several plants were pooled prior to analysis) have shown an upregulation of the classical HR marker gene PR1 in response to eggs [43], but in our study only B. nigra plants that expressed the necrotic response showed an induction of PR1 (figure 2d). This indicates that not all plants can detect the eggs and suggests a genotypic variation in the plants. A possible explanation for the variation in strength of HR could be due to differences in the symbiotic microbial communities associated with the eggs (see also Petzold-Maxwell et al. [33]). The extent to which genotypic variation explains the observed phenotypic variation in HR between populations could be explained, e.g. by differences in herbivore pressure.
Abortion of herbivore-infected fruits is another known lethal defence trait used by plants whose pollinators larvae consume developing seeds and fruits [30]. The white campion (Silene latifolia) can selectively abort flowers that contain larvae of the nursery pollinating moth Hadena bicruris [46]. The plant benefits from this direct defence trait in terms of reduced secondary predation but pays costs in terms of residual fitness of non-aborted infested fruits. The latter is high also due to the presence of larval parasitoids killing larvae before completing fruit consumption showing a possible conflict between the two defence strategies in this nursery pollination system (A. Biere 2014, personal communication). In B. nigra, we expect that plant fitness should covary with presence/absence of the direct defence trait, as a conflict with the indirect defence trait was not shown.
Our study revealed that despite being a lethal trait, HR-like necrosis did neither render the eggs unsuitable for parasitoids nor did it negatively affect parasitoid fitness. Trichogramma wasps could successfully develop in desiccating eggs, without negative effects on their survival and clutch size. In addition, HR-like necrosis does not appear to reduce the window of opportunity during which the eggs are available for parasitism by another Trichogramma species [32]. Egg parasitoids are idiobionts that kill the embryo at the beginning of its development to feed on dead tissue and stored material in the egg. But the egg does not need to be alive for parasitization, as Trichogramma wasps can for example parasitize and develop in irradiated or even artificial eggs [42]. Apparently, Pieris eggs affected by HR-like necrosis provided sufficient conditions for successful development of Trichogramma wasps.
A direct defence trait like the observed necrosis may have evolved as an adaptation to specialist herbivores that are often feeding on reproductive plant tissue, with drastic fitness consequences [47,48]. Additionally, allelochemicals, e.g. glucosinolates, are not the most effective against specialists, because they can sequester or detoxify these compounds [49]. Therefore, HR-like necrosis seems to be targeted against glucosinolate specialists, i.e. Pieris spp., and not generalists such as M. brassicae, the larvae of which are strongly negatively affected by the plant toxins [12,50,51]. Such different lines of defences would support the defensive synergism hypothesis, which states that different defence traits expressed in combination may provide a higher level of resistance against herbivory than when expressed separately [22]. Examples to support this hypothesis have been found in Nicotiana attenuata [52], in rainforest shrubs of Piper cenocladum [9] and in different Umbelliferae species [53]. Multiple defence traits may be adaptive in a complex plant–herbivore–carnivore community, because of the redundancy of defence lines; when one resistance trait fails, another acts as a safety net [54]. This was shown to be the case in a natural belowground tritrophic system involving common milkweed, where no negative correlation between direct (root cardenolides) and indirect defences (nematode attracting-root volatiles) was found [55].
We showed that parasitoid attraction towards volatiles of egg-induced plants was not influenced by HR-like necrosis, and OIPVs of HR+ plants were more attractive for a native strain of T. evanescens. We demonstrate that a synergism between direct and indirect defences may occur even when defence traits are evolved that are lethal for the herbivore and may potentially harm the natural enemies. This suggests that expressing HR is beneficial for B. nigra because it induces higher herbivore egg mortality. However, it remains unclear whether HR-like necrosis incurs fitness costs for the plant. If so, then expression of HR may provide plants with a higher fitness in some individuals over others, based on differences in e.g. herbivore pressure [56], that should result in within- and between population variation in HR responses. It still remains unclear why butterflies are abundant on such plants that kill their eggs by direct or indirect defences. Future studies should investigate how preferences of butterflies correlate with larval performances including the egg phase, to fully understand how directional selection of plant defence traits may shape multitrophic interactions.
Supplementary Material
Acknowledgements
The authors thank Tom van den Beuken, Dani Lucas-Barbosa, Thijs Hoveling, Foteini Pashalidou, Daniela Weber and Jingjue Wang for conducting experiments and helping with fieldwork, Yavanna Aartsma for conducting preliminary experiments, Art Shapiro (UC Davis) and Rieta Gols for providing B. nigra seeds, and Alejandro Lucatti for helping with the RNA isolations and cDNA synthesis. We also thank Rieta Gols and Marcel Dicke for discussion and comments on an earlier version of the manuscript.
Data accessibility
The underlying dataset can be accessed in an Excel file on Dryad doi:10.5061/dryad.q0r11.
Funding statement
The Netherlands Organisation for Scientific Research (NWO/ALW Veni grant no. 863.09.002 to N.E.F and NWO open competition grants) is acknowledged for funding.
References
- 1.Schoonhoven LM, van Loon JJA, Dicke M. 2005. Insect–plant biology, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Dicke M, Baldwin IT. 2010. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15, 167–175. ( 10.1016/j.tplants.2009.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Kessler A, Heil M. 2011. The multiple faces of indirect defences and their agents of natural selection. Funct. Ecol. 25, 348–357. ( 10.1111/j.1365-2435.2010.01818.x) [DOI] [Google Scholar]
- 4.Agrawal AA, Janssen A, Bruin J, Posthumus MA, Sabelis MW. 2002. An ecological cost of plant defence: attractiveness of bitter cucumber plants to natural enemies of herbivores. Ecol. Lett. 5, 377–385. ( 10.1046/j.1461-0248.2002.00325.x) [DOI] [Google Scholar]
- 5.Heil M, Baldwin IT. 2002. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 7, 61–67. ( 10.1016/S1360-1385(01)02186-0) [DOI] [PubMed] [Google Scholar]
- 6.Strauss SY, Agrawal AA. 1999. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 14, 179–185. ( 10.1016/S0169-5347(98)01576-6) [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Duffey SS. 1979. Tomatine and parasitic wasps: potential incompatibility of plant antibiosis with biological control. Science 205, 700–702. ( 10.1126/science.205.4407.700) [DOI] [PubMed] [Google Scholar]
- 8.Ballhorn DJ, Kautz S, Lion U, Heil M. 2008. Trade-offs between direct and indirect defences of lima bean (Phaseolus lunatus). J. Ecol. 96, 971–980. ( 10.1111/j.1365-2745.2008.01404.x) [DOI] [Google Scholar]
- 9.Dyer L, Dodson C, Beihoffer J, Letourneau D. 2001. Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J. Chem. Ecol. 27, 581–592. ( 10.1023/a:1010345123670) [DOI] [PubMed] [Google Scholar]
- 10.Heil M, Fiala B, Linsenmair KE. 1999. Reduced chitinase activities in ant plants of the genus Macaranga. Naturwissenschaften 86, 146–149. ( 10.1007/s001140050589) [DOI] [Google Scholar]
- 11.Gols R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA. 2008. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J. Chem. Ecol. 34, 132–143. ( 10.1007/s10886-008-9429-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gols R, Wagenaar R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA. 2008. Genetic variation in defense chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology 89, 1616–1626. ( 10.1890/07-0873.1) [DOI] [PubMed] [Google Scholar]
- 13.Strauss SY, Rudgers JA, Lau JA, Irwin RE. 2002. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 17, 278–285. ( 10.1016/S0169-5347(02)02483-7) [DOI] [Google Scholar]
- 14.Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83, 176–190. ( 10.1890/0012-9658(2002)083[0176:maosov]2.0.co;2) [DOI] [Google Scholar]
- 15.Koricheva J, Romero GQ. 2012. You get what you pay for: reward-specific trade-offs among direct and ant-mediated defences in plants. Biol. Lett. 8, 628–630. ( 10.1098/rsbl.2012.0271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbosa P, Gross P, Kemper J. 1991. Influence of plant allelochemicals on the tobacco hornworm and its parasitoid, Cotesia congregata. Ecology 72, 1567–1575. ( 10.2307/1940956) [DOI] [Google Scholar]
- 17.Bukovinszky T, Poelman EH, Gols R, Prekatsakis G, Vet LEM, Harvey JA, Dicke M. 2009. Consequences of constitutive and induced variation in plant nutritional quality for immune defence of a herbivore against parasitism. Oecologia 160, 299–308. ( 10.1007/s00442-009-1308-y) [DOI] [PubMed] [Google Scholar]
- 18.Bukovinszky T, Gols R, Smid HM, Bukovinszkine Kiss G, Dicke M, Harvey JA. 2012. Consequences of constitutive and induced variation in the host's food plant quality for parasitoid larval development. J. Insect Physiol. 58, 367–375. ( 10.1016/j.jinsphys.2011.12.017) [DOI] [PubMed] [Google Scholar]
- 19.Harvey JA. 2000. Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: implications for host quality and parasitoid fitness. Ecol. Entomol. 25, 267–278. ( 10.1046/j.1365-2311.2000.00265.x) [DOI] [Google Scholar]
- 20.Ode PJ. 2006. Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. Annu. Rev. Entomol. 51, 163–185. ( 10.1146/annurev.ento.51.110104.151110) [DOI] [PubMed] [Google Scholar]
- 21.Lill JT, Marquis RJ. 2001. The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126, 418–428. ( 10.1007/s004420000557) [DOI] [PubMed] [Google Scholar]
- 22.Agrawal AA. 2011. Current trends in the evolutionary ecology of plant defence. Funct. Ecol. 25, 420–432. ( 10.1111/j.1365-2435.2010.01796.x) [DOI] [Google Scholar]
- 23.Poelman EH, van Loon JJA, Dicke M. 2008. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci. 13, 534–541. ( 10.1016/j.tplants.2008.08.003) [DOI] [PubMed] [Google Scholar]
- 24.Benrey B, Denno RF. 1997. The slow-growth-high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78, 987–999. [Google Scholar]
- 25.Pilson D. 1996. Two herbivores and constraints on selection for resistance in Brassica rapa. Evolution 50, 1492–1500. ( 10.2307/2410886) [DOI] [PubMed] [Google Scholar]
- 26.Pilson D. 2000. The evolution of plant response to herbivory: simultaneously considering resistance and tolerance in Brassica rapa. Evol. Ecol. 14, 457–489. ( 10.1023/A:1010953714344) [DOI] [Google Scholar]
- 27.Agrawal AA. 2007. Macroevolution of plant defense strategies. Trends Ecol. Evol. 22, 103–109. ( 10.1016/j.tree.2006.10.012) [DOI] [PubMed] [Google Scholar]
- 28.Reymond P. 2013. Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta 238, 247–258. ( 10.1007/s00425-013-1908-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilker M, Fatouros NE. Submitted. Plant responses to insect egg deposition. Ann. Rev. Entomol. [DOI] [PubMed] [Google Scholar]
- 30.Holland JN, DeAngelis DL. 2002. Ecological and evolutionary conditions for fruit abortion to regulate pollinating seed-eaters and increase plant reproduction. Theor. Pop. Biol. 61, 251–263. ( 10.1006/tpbi.2001.1571) [DOI] [PubMed] [Google Scholar]
- 31.Balbyshev NF, Lorenzen JH. 1997. Hypersensitivity and egg drop: a novel mechanism of host plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 90, 652–657. [Google Scholar]
- 32.Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME. 2012. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7, e43607 ( 10.1371/journal.pone.0043607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petzold-Maxwell J, Wong S, Arellano C, Gould F. 2011. Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa. Ecol. Entomol. 36, 700–708. ( 10.1111/j.1365-2311.2011.01315.x) [DOI] [Google Scholar]
- 34.Shapiro AM, De Vay JE. 1987. Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera, Pieridae). Oecologia 71, 631–632. ( 10.1007/BF00379310) [DOI] [PubMed] [Google Scholar]
- 35.Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy SR, Clement SL, Williamson RT, Carney JR, DeVilbiss ED. 2000. Bruchins: insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl Acad. Sci. USA 97, 6218–6223. ( 10.1073/pnas.110054697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desurmont GA, Weston PA. 2011. Aggregative oviposition of a phytophagous beetle overcomes egg-crushing plant defences. Ecol. Entomol. 36, 335–343. ( 10.1111/j.1365-2311.2011.01277.x) [DOI] [Google Scholar]
- 37.Seino Y, Suzuki Y, Sogawa K. 1996. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (Horvath) (Homoptera: Delphacidae). Appl. Entomol. Zool. 31, 467–473. [Google Scholar]
- 38.Seino Y, Suzuki Y. 1997. Biotransformation of benzyl benzoate from benzoic acid in rice watery ovipositional lesion tissues induced by Sogatella furcifera (HORVATH) (Homoptera, Delphacidae). Appl. Entomol. Zool. 32, 530–532. [Google Scholar]
- 39.Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M. 2008. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 19, 677–689. ( 10.1093/beheco/arn011) [DOI] [Google Scholar]
- 40.Bos F, Bosveld M, Groenendijk D, van Swaay C, Wynhoff I. 2006. De Dagvlinders van Nederland: Vespreiding en Bescherming, p. 381 Utrecht, The Netherlands: KNNV Uitgeverij. [Google Scholar]
- 41.Feltwell J. 1982. Large white butterfly: the biology, biochemistry and physiology of Pieris brassicae (Linnaeus). The Hague, The Netherlands: Dr. W. Junk Publishers. [Google Scholar]
- 42.Consoli FL, Parra JRP, Zucchi R. 2010. Egg parasitoids in agroecosystems with emphasis on Trichogramma. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 43.Little D, Gouhier-Darimont C, Bruessow F, Reymond P. 2007. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 143, 784–800. ( 10.1104/pp.106.090837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fatouros NE, Huigens ME. 2012. Phoresy in the field: natural occurrence of Trichogramma egg parasitoids on butterflies and moths. BioControl 57, 493–502. ( 10.1007/s10526-011-9427-x) [DOI] [Google Scholar]
- 45.Pineda A, Zheng SJ, van Loon JJA, Dicke M. 2012. Rhizobacteria modify plant–aphid interactions: a case of induced systemic susceptibility. Plant Biol. 14, 83–90. ( 10.1111/j.1438-8677.2011.00549.x) [DOI] [PubMed] [Google Scholar]
- 46.Jolivet C, Bernasconi G. 2006. Experimental analysis of constitutive and induced defence in a plant–seed–predator system. Funct. Ecol. 20, 966–972. ( 10.1111/j.1365-2435.2006.01196.x) [DOI] [Google Scholar]
- 47.Smallegange RC, Loon JJA, Blatt SE, Harvey JA, Agerbirk N, Dicke M. 2007. Flower vs. leaf feeding by Pieris brassicae: glucosinolate-rich flower tissues are preferred and sustain higher growth rate. J. Chem. Ecol. 33, 1831–1844. ( 10.1007/s10886-007-9350-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas-Barbosa D, van Loon JJA, Gols R, van Beek TA, Dicke M. 2013. Reproductive escape: annual plant responds to butterfly eggs by accelerating seed production. Funct. Ecol. 27, 245–254. ( 10.1111/1365-2435.12004) [DOI] [Google Scholar]
- 49.Hopkins RJ, van Dam NM, van Loon JJ. 2009. Role of glucosinolates in insect–plant relationships and multitrophic interactions. Annu. Rev. Entomol. 54, 57–83. ( 10.1146/annurev.ento.54.110807.090623) [DOI] [PubMed] [Google Scholar]
- 50.Harvey JA, Gols R. 2011. Development of Mamestra brassicae and its solitary endoparasitoid Microplitis mediator on two populations of the invasive weed Bunias orientalis. Popul. Ecol. 53, 587–596. ( 10.1007/s10144-011-0267-4) [DOI] [Google Scholar]
- 51.Santolamazza-Carbone S, Velasco P, Soengas P, Cartea M. 2013. Bottom-up and top-down herbivore regulation mediated by glucosinolates in Brassica oleracea var. acephala. Oecologia 174, 893–907. ( 10.1007/s00442-013-2817-2) [DOI] [PubMed] [Google Scholar]
- 52.Steppuhn A, Baldwin IT. 2008. Induced defenses and the cost–benefit paradigm. In Induced plant resistance to herbivory (ed. Schaller A.), pp. 61–83. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 53.Berenbaum M, Neal JJ. 1985. Synergism between myristicin and xanthotoxin, a naturally coocurring plant toxicant. J. Chem. Ecol. 11, 1349–1358. ( 10.1007/BF01012136) [DOI] [PubMed] [Google Scholar]
- 54.Rasmann S, Agrawal AA. 2009. Plant defense against herbivory: progress in identifying synergism, redundancy, and antagonism between resistance traits. Curr. Opin. Plant Biol. 12, 473–478. ( 10.1016/j.pbi.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 55.Rasmann S, Erwin AC, Halitschke R, Agrawal AA. 2011. Direct and indirect root defences of milkweed (Asclepias syriaca): trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J. Ecol. 99, 16–25. ( 10.1111/j.1365-2745.2010.01713.x) [DOI] [Google Scholar]
- 56.Vrieling K, Wijk CM. 1992. Estimating costs and benefits of the pyrrolizidine alkaloids of Senecio jacobaea under natural conditions. In Proc. 8th Int. Symp. on Insect–Plant Relationships (eds Menken SBJ, Visser JH, Harrewijn P.), Wageningen, The Netherlands, August, pp. 77–78. Dordrecht, The Netherlands: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The underlying dataset can be accessed in an Excel file on Dryad doi:10.5061/dryad.q0r11.