Abstract
This study compared inhibitory functioning among ADHD subtype groups on manual and visual versions of the stop task. Seventy-six children, identified as ADHD/I (n = 17), ADHD/C (n =43), and comparison (n = 20) completed both tasks. Results indicated that both ADHD groups were slower to inhibit responses than the comparison group on both tasks. Comparison children were faster to inhibit than activate responses on both tasks. Children in the ADHD groups also demonstrated this robust pattern on the manual task. However, on the visual task, the ADHD groups evidenced slowed inhibition comparable to the time required to activate responding. This implies that the visual task is more sensitive than the manual task to inhibitory deficits associated with ADHD. The ADHD/I and the ADHD/C groups did not differ on most measures, suggesting that neither stop task is effective in differentiating the subtypes. These findings extend work highlighting the role of disinhibition in ADHD, and contrast recent work suggesting divergence between ADHD subtypes.
Keywords: ADHD, subtypes, inhibition, oculomotor
The impairment of basic inhibitory processes is widely recognized as a central deficit in attention-deficit/hyperactivity disorder (ADHD; American Psychiatric Association, 1994; Barkley, 1997; Nigg, 2001, 2005; Pennington & Ozonoff, 1996; Quay, 1997; Schachar, Tannock, & Logan, 1993; Schachar, Mota, Logan, Tannock, & Klim, 2000). Current ADHD research is largely focused on understanding these inhibitory deficits and how they may influence or interact with other facets of executive functioning (i.e., working memory, self-regulation of affect and motivation, internalization of speech, and reconstitution), which, in turn, influence behavioral symptoms (Barkley, 1997; Berlin, Bohlin, & Rydell, 2003; Castellanos & Tannock, 2002; Nigg, 2005). Despite a growing body of research, several questions remain regarding how these deficits operate in ADHD. The current study aims to address two of these questions, namely: (1) do the observed abnormalities in response inhibition underlying ADHD differ as a function of response requirement (i.e., hand or eye movements), and (2) how do these findings compare across clearly defined ADHD subgroups relative to comparison children?
Measuring Response Inhibition
When defined broadly, inhibition is the process of suppressing an inappropriate response, but the term may refer to a number of related processes, including inhibiting prepotent responses, stopping ongoing responses, and controlling interference (Barkley, 1997). According to Barkley’s (1997) influential model, deficits in response inhibition have downstream effects on other executive functions, resulting in the characteristic behavioral and academic impairments observed in ADHD. Abundant empirical evidence supports a role for inhibitory deficits in ADHD in both children and adults (Carr, Nigg, & Henderson, 2006; Nigg, 2001, 2006).
A number of assessment tools have been developed over the years to measure response inhibition (Nichols & Waschbusch, 2004; also see Nigg, 2001, Table 1). Among the most popular of these measures are variants of Logan and Cowan’s (1984) stop task (Lijffijt, Kenemans, Verbaten, & van Engeland, 2005; Oosterlaan, Logan, & Sergeant, 1998). The basic premise of this task is that two separate but interactive processes—a response execution or “go” process and a response inhibition or “stop” process—are engaged in an interactive “race” every time one wishes to inhibit a response (Boucher, Palmeri, Logan & Schall, 2007; Logan, 1994; Logan & Cowan, 1984; Osman, Kornblum, & Meyer, 1986). If the stop process runs to completion before the go process finishes, then the response is successfully inhibited; otherwise, a response occurs. Inherent in this model is the notion that these processes occur at different rates and thus require different amounts of time to complete. More specifically, the stop or inhibitory process must be faster than the go process, otherwise responses could not be successfully inhibited.
Table 1.
Diagnostic Information by Group
| Comparison | ADHD/I | ADHD/C | |||
|---|---|---|---|---|---|
| Variable | (n = 20) | (n = 17) | (n = 43) | F | t result |
| Age diagnosed with ADHD (years) | 8.2 (0.9) | 7.3 (2.3) | 1.88 | ||
| DSM-IV attention | 0.5 (1.1) | 6.2 (2.6) | 7.3 (1.9) | 89.60*** | a |
| DSM-IV hyperactivity | 0.3 (0.6) | 1.5 (1.5) | 5.5 (2.4) | 61.80*** | b |
| DSM-IV oppositional/defiant | 0.2 (0.4) | 1.1 (1.3) | 3.3 (2.2) | 26.04*** | b |
| Connors oppositionality | 42.8 (4.0) | 50.4 (8.3) | 61.5 (12.0) | 26.77*** | b |
| Connors cognitive problems/inattention | 45.1 (3.9) | 71.5 (9.3) | 72.9 (8.6) | 91.62*** | a |
| Connors hyperactivity | 47.8 (5.3) | 53.1 (11.6) | 76.9 (10.3) | 78.02*** | b |
| Connors ADHD total | 44.7 (3.4) | 68.5 (8.5) | 74.7 (6.6) | 149.62*** | c |
| CBCL aggression | 51.1 (4.1) | 54.4 (5.9) | 62.4 (9.7) | 15.93*** | b |
| CBCL delinquency | 51.5 (2.9) | 53.2 (5.7) | 58.9 (8.3) | 9.65*** | b |
Note. p< .05,
p< .01,
p< .001.
ADHD/I = ADHD inattentive. ADHD/C = ADHD combined. DSM-IV scores based on semistructured interview.
Comparison significantly different from 2 ADHD groups.
ADHD/C significantly different from comparison and ADHD/I groups.
Comparison significantly different from ADHD/I, and ADHD/I significantly different from ADHD/C.
In the typical stop task, participants are involved in a primary task, such as quickly and accurately making differential responses to stimuli as they appear on a screen. Here, reaction times are thought to reflect the speed of the underlying go process, with mean go reaction times (RTGo) typically reported for each individual or group (Logan, 1994). Occasionally, a tone or image is presented after the initial stimulus, signaling that participants should withhold responding on those specific trials. This stop signal presumably initiates the inhibitory process (Logan & Cowan, 1984). Stop signal reaction time (SSRT) is defined as the difference between the time the stop signal is presented and the end of the inhibitory process, and can be estimated mathematically based on observed proportion of inhibition at various stop signal delays (Logan & Cowan, 1984; Logan, 1994).
Two meta-analytic studies incorporating 35 independent samples have reviewed performance on this task specifically for children with ADHD (Lijffijt et al., 2005; Oosterlaan et al., 1998). Across these studies, a consistent pattern is that SSRTs are faster than RTGos for both ADHD and comparison groups (i.e., stopping is faster than going). Beyond this, a relatively stable group effect is observed whereby children with ADHD have slower SSRTs relative to comparison children, consistent with the notion that ADHD is at least partly attributable to deficits in response inhibition.
An additional reliable finding in the ADHD stop task literature is that children with ADHD tend to demonstrate greater variability in reaction time than children without the disorder (Castellanos & Tannock, 2002; Derefinko et al., 2008; Nigg, 2006). This variability has been described as a marker of inattention, reflecting children’s inability to sustain attention from trial to trial. Castellanos and Tannock argue that this variability in responding is one of the core defining features of ADHD. A recent study found increased variability on reaction time trials to be the most influential mediator of story comprehension deficits among children with ADHD/C (Flory et al., 2006), reflecting its importance as a core deficit of the disorder.
Manual vs. Oculomotor Countermanding
Despite widespread use of the stop task, it is important to note that a great deal of variability exists in the procedures used (Lijffijt et al., 2005; Oosteralaan et al., 1998). Though the traditional stop task is a valuable tool and reflects an important model for understanding inhibition, it puts great emphasis on ballistic hand movements as an indicator of inhibition. An alternative version of the stop task, called the oculomotor or visual stop task, requires participants to respond by moving their eyes rather than their hands (Armstrong & Munoz, 2003; Hanisch, Radach, Holtkamp, Herpertz-Dahlmann, & Konrad, 2006; Logan & Irwin, 2000; Schall, Hanes, & Taylor, 2000). Here, quick eye movements called saccades constitute responses. When a stop signal is presented, participants are instructed to withhold eye movement and instead fixate on a designated point or object on the screen. The oculomotor countermanding task allows researchers to explore whether the problems with response inhibition observed in ADHD via the typical stop task apply across response modalities or if these deficits are unique to hand-motor responding. According to Logan and Irwin (2000), investigating response inhibition for eye movements is important, because (a) eye movements are controlled by separate anatomical pathways from hand movements, suggesting separate physiological mechanisms for response inhibition, and (b) visual movements often precede and improve accuracy of hand movements, implying that inhibition of eye movements may mediate inhibition of hand movements. Additionally, by measuring one’s ability to appropriately inhibit one’s eye movements, the visual stop task more directly represents an effort to tap into the intersection between attentional processes and inhibitory processes, which is of particular relevance to the study of ADHD.
The oculomotor countermanding paradigm has received far less scrutiny in the research literature than those tasks that require some form of hand-oriented response, particularly with respect to ADHD. Armstrong and Munoz (2003) found that adults with ADHD were less able to inhibit saccadic responses than participants in the control group. Furthermore, children with ADHD have been shown to commit more inhibitory failures and have longer SSRTs than controls on an oculomotor countermanding task, in contrast to comparable performance between groups on tasks measuring reflexive eye movements without inhibitory demands (Hanisch et al., 2006). These results corroborate earlier findings from the traditional hand-motor stop task studies (Lijffijt et al., 2005; Oosterlaan et al., 1998) whereby deficits in response inhibition uniquely characterize ADHD groups relative to control groups.
Only one direct comparison between hand and eye movement responses on the stop-signal task has been reported. In that study, the investigators were interested in clarifying whether inhibition under each response requirement was achieved via common or separate physiological mechanisms (Logan & Irwin, 2000). As noted previously, the neural signals that initiate eye and hand movements follow a common path up to a certain point, from which these signals diverge and are completed by separate pathways. The authors tested inhibitory performance in healthy adults and found that like the manual stop task, eye movements also show that stopping is faster than going (i.e., SSRT < RTGo). However, overall, the RTGo and SSRT of eye movements is faster than hand responses (Logan & Irwin, 2000). With respect to SSRT, participants had significantly faster SSRTs for eye movements than for hand movements. The investigators interpreted overall findings as consistent with the notion that eye and hand movements are likely regulated by separate inhibitory processes operating under similar principles specified by the race model (Logan, 1994; Logan & Cowan, 1984).
The primary goal of the present study was to explore performance on visual and manual versions of the stop task among children with ADHD and comparison peers. In keeping with previous findings (Armstrong & Munoz, 2003; Hanisch et al., 2006; Logan & Irwin, 2000), faster reaction times were anticipated for both go (RTGo) and stop processes (SSRT) in the oculomotor countermanding task relative to a button-press manual version of the stop task. We also anticipated shorter SSRTs than RTGo values for both versions of the stop task for all participants. Finally, children with ADHD were expected to demonstrate greater variability in reaction times to “go” trials, consistent with the notion that RT variability indexes inattention (Castellanos & Tannock, 2002).
Subtype differences
While problems in behavioral inhibition are believed to play an important role in the development of ADHD, it is unclear how well these findings generalize across ADHD subtypes. Indeed, neither of the meta-analytic reviews on stop task performance addressed differences among ADHD subtypes (Lijffijt et al., 2005; Oosterlaan et al., 1998). Contemporary ADHD research is largely focused on ADHD/C (Nigg, 2006), but researchers are increasingly incorporating subtype level analyses into their study designs and discussing explicitly the relations between ADHD/I and ADHD/C. Unfortunately, however, the general body of research on subtype differences is limited in size and scope. Further, many studies in this area are marked by methodological flaws (e.g., low power, atheoretical designs, inconsistent sampling procedures) that impede our understanding of subtype differences (Adams et al., 2008; Milich et al., 2001). Perhaps the most important concern is that ADHD/I groups are often contaminated by the inclusion of subthreshold ADHD/C participants, thus rendering subsequent results and interpretations ambiguous with respect to subtype differences (Milich et al., 2001). Although such a classification strategy is consistent with DSM-IV diagnostic criteria (APA, 1994), this system does not necessarily reflect the most informative framework for understanding these disorders.
Preliminary studies suggest that interesting patterns of similarities and differences emerge when subtype groups are clearly delineated such that participants with “pure” ADHD/I (i.e., few, if any, hyperactive/impulsive symptoms) are compared to those with ADHD/C on theoretically relevant tasks measuring various aspects of attention and inhibitory functioning (Adams et al., 2008, Derefinko et al., 2008, Castellanos et al., 2006; Fillmore et al., 2008). For example, work in the domain of inhibitory functioning has highlighted areas of discontinuity between the subtypes. Derefinko and colleagues (2008) found that ADHD subtype groups exhibit unique patterns of performance on tasks measuring sensitivity to environmental factors either preceding or following signals to respond or inhibit behavior. Specifically, children with ADHD/I consistently demonstrated longer reaction times and more errors of omission, suggesting a slow, perhaps cautious response style (Derefinko et al., 2008). Fillmore and colleagues (2008) studied reflexive oculomotor inhibition among ADHD subtypes and found that reflexive inhibition was absent in the ADHD/C group, but only partially reduced in ADHD/I relative to comparison children. Together, these findings suggest that interesting differences emerge among ADHD subtypes when carefully defined groups are compared on tasks measuring various components of inhibitory functioning.
Other studies have demonstrated areas where the ADHD subtype groups share similar performance profiles that distinguish them from comparison groups but not from each other. For instance, consistent with arguments regarding a primary importance of deficits in sustaining attention to the construct of ADHD, both the ADHD/C and ADHD/I groups have reliably demonstrated significantly greater reaction time variability than comparison children (Castellanos & Tannock, 2002; Castellanos et al., 2006; Derefinko et al., 2008; Fillmore et al., 2008; Nigg, 2006). Interestingly, similarities also have been observed between the subtypes with regard to inhibitory performance. Comparisons between ADHD subtype groups using the stop task have found few or no differences with regard to SSRT or performance errors on the task (Guerts, Verte, Oosterlaan, Roeyers, & Sergeant, 2005; Huang-Pollack, Mikami, Pfiffner, & McBurnett, 2007). These studies suggest that the ADHD subtypes may share common deficits in the areas of inattention and basic forms of disinhibition measured by the stop task.
Thus, as a second aim, the current study sought to examine whether ADHD subtypes differ with respect to performance on manual and visual versions of the stop task. It was hypothesized that both ADHD groups would demonstrate impairment relative to the comparison group on both versions of the stop task (Guerts et al., 2005; Huang-Pollock et al., 2007), that children with ADHD/I would demonstrate slower overall reaction times relative to children with ADHD/C and comparison children, consistent with previous work showing a slow, cautious response style in a “pure” ADHD/I group (Derefinko et al., 2008). With regard to a possible interaction between tasks and subtypes, we were also interested in whether the visual stop task is a more sensitive measure of the deficits associated with ADHD/I compared to ADHD/C given that the visual task may more directly tap attentional processes than the manual task.
Method
Participants
A sample of 60 children with ADHD and 20 comparison children participated in this study. The children in each diagnostic group were between the ages of 9 and 12 years (M age = 10.85 years, SD = 1.11). Approximately 85% of the children were Caucasian, 11% were African American, and 4% identified themselves as other. The children with ADHD were recruited from the Hyperactive Children’s Clinic in the School of Medicine at the University of Kentucky. A three-step process was developed to ensure that each child had an appropriate diagnosis of ADHD. First, children were only considered for participation if they had been assessed at the psychiatric clinic and received a diagnosis of ADHD inattentive or combined type based on the DSM-IV criteria (APA, 1994). The diagnosticians were a team consisting of a child psychiatrist and another mental health professional. The diagnosis was made by the team using standard measures, including child and parent interviews, child observations, Conners (1997) Parent and Teacher Rating Scales, and when possible, other psychological test results. The clinic diagnosed the children based on a convergence of evidence from these data, ensuring that the child was significantly impaired in at least two settings.
From the clinic diagnoses, a pool of potential participants was identified. In the second step of the diagnostic process, parents of identified children were asked for consent for the research team to review their children’s files. If consent was granted, clinic files were examined by one of the authors to assess eligibility for participation in the study. This process allowed the research team to obtain additional information concerning potential exclusionary criteria, such as children who had an IQ below 80, significant sensory impairment, epilepsy or neurological disorders, or a psychotic disorder. Children who were prescribed a medication that could not be suspended for testing sessions were excluded from the study but children being treated with a psychostimulant medication were eligible to participate. Children who were prescribed a medication that could not be suspended for testing sessions were excluded from the study but children being treated with a psychostimulant medication were eligible to participate. Children were not excluded if they had comorbid psychological disorders or learning disability. Further, children who were diagnosed by the Hyperactive Children’s Clinic with ADHD primarily hyperactive/impulsive subtype were excluded from the study.
Finally, if the above criteria were met, then a parent of the child with ADHD was contacted and invited to participate in the study. The third step in the diagnostic process was an on-site standardized interview with the parent to confirm the diagnosis of ADHD. The interview was similar to the Children’s Interview for Psychiatric Syndromes- Parent Version (P-ChIPS; Weller, Weller, Rooney, & Fristad, 1999), but was limited to verbatim DSM-IV criteria for ADHD and ODD. In the interviews, which were conducted by trained clinical psychology graduate students, parents were asked to respond whether each DSM-IV criterion was true for their child, give behavioral examples, and indicate how the symptom affected their child’s academic and social functioning. Questions were also asked about the frequency and intensity of the behavior to determine whether it was age-appropriate. A symptom was considered present if the parent’s response indicated both age-inappropriate behavior and impairment in functioning criteria. This interview procedure has been used successfully by this research group previously and has achieved inter-rater reliabilities above 95% for symptom counts endorsed by the parent (e.g. Lorch, Sanchez et al., 1999).
In addition to the information from the structured psychiatric interview, parents completed the Child Behavior Checklist (CBCL; Achenbach, 1991) and the Conners Parent Rating Scales—Revised: Short Forms (CPRS-R:S; Conners, 1997). The CBCL is a widely used parent-report questionnaire designed to assess a wide range of behaviors and competencies in children, with high test-retest reliability (r = .90) and internal consistency (Cronbach’s alpha = .84) (Achenbach, 1991). A recent study utilizing receiver-operating characteristic analysis demonstrated that the CBCL provides good diagnostic accuracy in predicting DSM-IV ADHD and ODD/CD in children ages 6 to 18 years using the cut scores specified below (Hudziak, Copeland, Sanger, & Wadsworth, 2004). The CPRS-R:S includes items from the long form of the CPRS—R on each of three subscales: oppositional, cognitive problems/inattention, and hyperactivity. The measure also includes a fourth subscale, the ADHD index, which includes ADHD symptoms derived from DSM-IV diagnostic criteria. This measure is widely used in clinical and research settings as an indicator of the various behavioral symptoms of ADHD as observed by a parent or caregiver (Conners, 1997). The CPRS-R:S has internal reliability across scales (Cronbach’s alpha = .83 - .94) (Conners, Sitarenios, Parker, & Epstein, 1998). Only children whose parental interviews and questionnaire data supported a diagnosis of ADHD were retained and contributed data for this study. Based on these criteria, data from 60 of 74 participants (81%) recruited for the ADHD groups from the Hyperactive Children’s Clinic were used in this study.
Diagnostic Subgroups
Children with ADHD were assigned to one of the two subgroups under investigation (ADHD/C; ADHD/I) based on the history obtained from the psychiatric chart review as well as the more systematic data obtained from the structured interview and the CBCL and CPRS-R:S. Consistent with the exclusionary criteria utilized at the first stage of recruitment, no children with ADHD hyperactive/impulsive subtype were identified within this group. To be placed in the ADHD/C group (n = 43), children must have met criteria for this diagnosis on the structured interview, have T scores above 60 on the Conners Hyperactivity scale and the ADHD Index. Consistent with the literature suggesting a common comorbidity between ADHD/C and oppositional behavior (Weiss, Worling, & Wasdell, 2003; Nigg, 2000), children demonstrating clinically significant conduct problems were not excluded from the ADHD/C group. In forming the ADHD/I group (n = 17), recommendations made by Milich et al. (2001) were followed to ensure that this group did not include subthreshold ADHD/combined children. Specifically, children in the ADHD/I group were required to meet criteria for attention problems on the structured interview and had 3 or fewer symptoms on the hyperactive/impulsive dimension. In addition, these children had T scores above 60 on the Conners Cognitive Problems/Inattention scale and T scores less than 60 on the Hyperactivity scale. Finally, the children in the ADHD/I group had T scores below 60 on the CBCL Aggression and Delinquency scales.
The comparison group of children without ADHD (n = 20) was recruited through newspaper advertisements, posted advertisements in the community, and by word-of-mouth. They were screened during a recruitment phone call in which parents were asked if their children had ever been referred for any behavioral or learning problems. The comparison children were not required to be symptom free, but had to have two or fewer symptoms in a diagnostic category. These children were significantly less symptomatic than the children with ADHD in terms of the DSM-IV criteria for inattention symptoms and hyperactive symptoms, and moreover did not meet diagnostic criteria for any ADHD subtype. In addition, the children in the comparison group were required to have T scores below 60 on all of the relevant rating scales. As indicated in Table 1, the diagnostic interview and rating scale data successfully differentiated between the comparison, ADHD/I, and ADHD/C groups.
Among the children with ADHD, roughly two-thirds were being treated with psychostimulant medication. The remaining children were not taking any prescribed medication at the time of the study. No child received any psychostimulant medication on the day of the study until after the session was completed. This provided a sufficient time period (approximately 24 hours) for clearance of any medication administered on the day before the session. Participants who were receiving other medications that could not be easily withdrawn for testing (e.g., clonidine) were excluded at the time of enrollment. All children received two small toys and $30.00 for their participation in the study.
Groups were not significantly different on the basis of age, gender, racial composition, grade level, maternal education, paternal education, or KBIT vocabulary scores (see Table 2). A significant difference between groups was observed, however, for KBIT matrices scores, F(2, 77) = 3.33, p = .041, with comparison children receiving higher scores than both ADHD groups. Diagnostic group comparisons (ADHD/I vs. ADHD/C) did not evidence any significant differences between these two groups in terms of age, gender, racial composition, grade level, maternal education, paternal education, KBIT vocabulary scores, or KBIT matrices scores.
Table 2.
Demographic Information by Group
| Comparison | ADHD/I | ADHD/C | ||||
|---|---|---|---|---|---|---|
| Variable | (n = 20) | (n = 17) | (n = 43) | F | X2 | p |
| Age (months) | 132.3 (10.4) | 125.1 (13.6) | 131.8 (14.0) | 1.83 | .167 | |
| Gender (% male) | 65 | 64.7 | 83.7 | 3.77 | .152 | |
| Race (% white) | 85.0 | 76.5 | 88.4 | 4.84 | .565 | |
| Grade level | 5.6 (0.9) | 4.7 (1.2) | 5.1 (1.2) | 2.79 | .067 | |
| Mother’s education | 16.3 (1.7) | 15.3 (2.0) | 15.6 (2.6) | 0.90 | .412 | |
| Father’s education | 16.0 (2.1) | 16.0 (3.3) | 14.3 (3.4) | 2.75 | .070 | |
| KBIT vocabulary | 107.5 (12.0) | 102.9 (13.4) | 102.0 (14.3) | 1.16 | .319 | |
| KBIT matrices | 119.4 (13.2) | 109.7 (12.4) | 106.6 (21.8) | 3.33 | .041* |
Note. ADHD/I = ADHD inattentive. ADHD/C = ADHD combined. KBIT = Kaufman Brief Intelligence Test. Parental education is provided in years.
For KBIT matrices, both ADHD subgroups obtained lower scores than healthy comparison children. No differences were observed among ADHD subgroups, however.
Procedure
All procedures were approved by the Institutional Review Board at the sponsoring institution. The study took place at the Behavioral Pharmacology and Neurocognition Research Laboratory in the Department of Psychology at the University of Kentucky. All children were tested individually on non-school days between the hours of 9 am and 5 pm. Upon arrival at the lab the child and parent were greeted by two experimenters who described the general details of the study and the basic testing procedures. Written consent was then obtained from the parent, as well as verbal assent from the child. After obtaining consent, one of the experimenters accompanied the parent to an interview room to complete the semi-structured interview and questionnaires. The other experimenter accompanied the child to a nearby testing room to complete the testing. The administration of these tasks was part of a larger testing battery involving neuropsychological tests and other measures of cognitive functioning that took roughly three hours to complete. Prior to each test, the experimenter provided the task instructions and children performed a brief, 2-3 min familiarization test to ensure that the child understood the task requirements. Children were given a 15-minute break between each testing component. At the conclusion of the session, the child and parent were debriefed and paid for their participation. Experimenters rotated parent interviewing and child testing responsibilities and were blind to participants’ clinical diagnostic status to reduce the risk of experimenter effects on responding and performance.
Measures
Manual Stop Task
The manual stop task was used as a measure of behavioral inhibition. The MS task requires participants to press a button when a stimulus (go signal) appears on the screen, but to withhold responding when a stop signal tone is presented. The go signals—white circles measuring 8 mm in diameter—were presented individually. Each trial began with a 1000 msec presentation of a plus sign (+) in the middle of the computer display. This served both as a location for participants to fixate their attention and as an indication that a trial was about to begin. As soon as the plus sign disappeared, a circle appeared in one of four positions: far right (12 cm from center), middle right (6.5 cm from center), far left (12 cm from center), middle left (6.5 cm from center). Participants were required to press the forward slash key (/) on a standard computer keyboard as soon as they detected a circle on the right or the period key (.) if the circle is on the left, using their middle and index fingers, respectively. The circle was present on the screen for 1 second. A blank screen appeared for 1500 ms before the start of the next trial. The complete task involved 128 trials, with each of the four stimulus positions presented an equal number of times (32 times). A stop signal tone occurred on 32 trials (i.e., 25% of the time), equally distributed among circle positions. The stop signal was a 500 ms 900 Hz tone generated by the computer at a comfortable listening level. Participants were instructed to withhold (i.e., inhibit) their response when a stop signal is presented. Stop signals were presented eight times at each of four stimulus onset asynchronies (SOAs; i.e., delays: 50, 150, 250, and 350 ms) with respect to the onset of the circle presentation. The order of circle locations, stop signal presentation, and delays was random. A test required approximately 8 minutes to complete.
The primary dependent variable for the stop task is the SSRT, which represents an estimate of the time it takes an individual to withhold a response. SSRT is calculated here according to Logan (1994) and consistent with Oosterlaan et al. (1998). Another indicator of inhibitory functioning as measured by the stop task is the proportion of inhibitory failures (i.e., responding following a stop signal) committed by the participant over the course of the task. The intrasubject variability on go trials (GoRTSD) was evaluated as a measure of inattention (Klein, Wendling, Huettner, Ruder, & Peper, 2006). Omission errors were also considered as an indicator of inattention, as greater variability in responding can result in missed responses on go-trials. Reaction time for go trials was measured as an indicator of overall response speed. This variable was also used to calculate the potential trade-off between speed and accuracy (i.e., successful inhibitions) for each group. Finally, choice errors (i.e., hitting the incorrect button on a go trial) were also measured.
Visual Stop Task
The visual stop task was similar to the manual task, but rather than press a button, participants moved their eyes from the fixation to the location of the go signal when it was presented. Participants were instructed not to look at the go signal when a stop signal was presented. With the exception of response requirement, all other task characteristics were the same as those described for the manual task. To stabilize head movements participants’ chins were placed in a chin rest at 27 cm from the computer display.
Response inhibition was measured on a test by the number of times that an individual successfully inhibited an eye movement on all 32 stop-signal trials (i.e., eye movement that was less than half the distance to the target was a successful inhibition). Response activation was measured by the mean RT to the go trials (i.e., the average time from the onset of a circle presentation until the first visual fixation point, indicating the completion of the primary saccade). Eye movements were recorded using a Model 504 Eye Tracking System (Applied Science–Laboratory, Boston MA). The eye tracker was calibrated to detect each participant’s pupils at the start of each testing session using a calibration stimulus. Eye locations were sampled at 60 Hz and given an X/Y coordinate. These coordinates were used to define fixations and saccades. The distance of a saccade and its duration were then calculated using fixation onset and offset times. Onsets of fixations were defined as periods of at least 100 msec in which the line of gaze had a standard deviation of less than 0.5 degrees of visual angle. Offsets of fixations were determined by periods of at least 50 msec in which the gaze position was at least 1 degree of visual angle away from the initial fixation position. The final fixation position was the average of all data sampled between the beginning and end of the fixation.
Statistical analysis
Initial descriptive analyses were performed to explore equivalence across groups with respect to demographic variables. Specifically, between-groups comparisons (i.e., one-way ANOVAs) for sex, age, race, parental education, and estimated IQ were performed to ensure that any observed group differences on response inhibition variables were not due to non-equivalence on these demographic characteristics.
To evaluate inhibitory performance, SSRTs were calculated for each subject and were subjected to planned contrasts based on a priori hypotheses to determine whether comparison children differed significantly from children diagnosed with ADHD, and whether children with ADHD/I differed from children with ADHD/C. Similar analyses were performed to compare the groups with respect to proportion of inhibitory failures, RTGo, variability in responding, and omission errors. Additionally, mixed repeated measures ANOVAs were performed to examine whether stop task performance differed as a function of response requirement (manual, visual) or group (comparison, ADHD/I, ADHD/C). These analyses also included a factor for measure (SSRT, RTGo) to compare speed of inhibiting to the speed of executing a response.
Results
Response inhibition: SSRT and inhibitory failures
Results for the manual and visual versions of the stop task can be found in Table 3.1 Comparisons of SSRT between the visual and manual tasks indicated that children were slower to inhibit (i.e., longer SSRTs) on the visual task than on the manual task, F(1, 65) = 12.31, p < .001, η2 = .16 (see Table 3). For the manual task, planned contrasts revealed significant differences between the comparison group and ADHD groups for SSRT, such that children with ADHD were significantly slower to inhibit responses than were comparison children, t(74) = 2.40, p < .05, d =.58. No subtype difference was observed for SSRT, however, t(74) = .85, ns. A similar pattern was observed on the visual task for SSRT whereby both ADHD groups were significantly slower in inhibiting responses than comparison children, t(68) = 3.01, p < .01, d = .70. Again, the subtype groups were not significantly different from each other, t(68) = .21, ns.
Table 3.
Stop Task Performance by Group
| ADHD vs. Comp |
ADHD/C vs. ADHD/I |
||||
|---|---|---|---|---|---|
| Variable | Comparison | ADHD/I | ADHD/C | t | t |
| Manual | (n = 20) | (n = 14) | (n = 37) | ||
| SSRT | 285.5 (67.2) | 358.6 (125.8) | 335.4 (87.3) | 2.40* | .85 |
| Inhibitory failures (%) | 44.4 (18.4) | 50.2 (26.5) | 54.2 (23.8) | 1.21 | .59 |
| RTGo | 509.8 (58.6) | 564.2S (86.2) | 521.3 (80.4) | 1.54 | 1.90 |
| RTGoSD | 126.7 (22.2) | 141.1 (27.5) | 145.1 (18.9) | 2.73** | .63 |
| Omission errors | 3.6 (5.6) | 7.3 (5.7) | 8.4 (12.7) | 1.51 | .35 |
| Visual | (n = 18) | (n = 16) | (n = 43) | ||
| SSRT | 316.1 (73.4) | 432.6 (159.6) | 423.5 (152.0) | 3.01** | .21 |
| Inhibitory failures (%) | 59.8 (22.2) | 71.4 (28.0) | 69.6 (25.2) | 1.57 | .24 |
| RTGo | 370.0 (65.7) | 380.0 (66.5) | 389.9 (58.6) | .88 | .51 |
| RTGoSD | 141.9 (37.2) | 178.5 (41.8) | 177.1 (40.8) | 3.28** | .12 |
| Omission errors | 8.0 (9.0) | 9.9 (6.8) | 11.5 (14.2) | .84 | .44 |
Note. p< .05,
p< .01.
ADHD/I = ADHD inattentive. ADHD/C = ADHD combined. Comp = comparison.
Although SSRT is derived partially from the proportion of inhibitory failures a participant commits, it is important to examine this variable as a component of the SSRT estimate. Children committed more inhibitory failures on the visual task than the manual task, F(1, 65) = 15.99, p < .001, η2= .20. Children with ADHD did not commit more inhibitory failures than comparison children on either the manual task, t(74) = 1.21, p = ns, or the visual task, t(68) = 1.57, p = ns. Additionally, planned contrasts revealed there were no subtype group differences for this variable on either the manual task, t(74) = .59, ns, or the visual task, t(68) = .24, ns.
Response Speed
With respect to RTGo, children in all three groups had faster responses on the visual task than the manual task, F(1, 65) = 150.63, p < .001, η2 = .70. On the manual task, although children with ADHD/I responded significantly more slowly to go trials than did children in the comparison group, t(74) = 2.05, p < .05, d = .48, children with ADHD/I did not differ significantly from children in the ADHD/C group, t(74) = 1.90, p = .06. Children in the comparison and ADHD/C groups did not significantly differ from each other with respect to go-trial reaction time, t(74) = .53, ns. No group differences were observed for RTGo on the visual task.
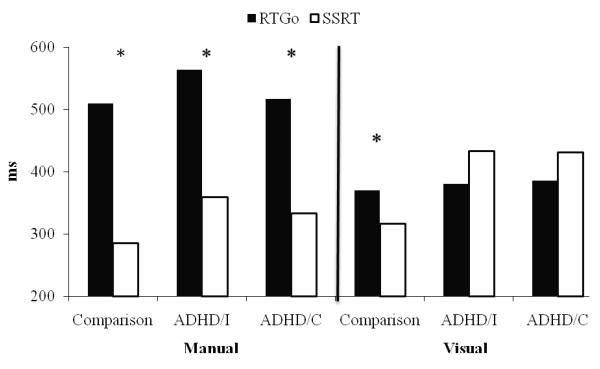
The difference between the RTGo and SSRT was examined for each group across the two tasks. Consistent with previous work, SSRT was faster than RTGo on the manual version of the stop task for all groups (see Figure 1). The speed advantage of stopping was also observed in the visual task, but only in the comparison group, F(1, 19) = 7.13, p < .05, η2 = .27. Surprisingly, children in the two ADHD groups did not demonstrate this disparity. Instead, RTGo and SSRT values were similar on the visual stop task in both the ADHD/I group, F(1, 13) = 1.13, ns, and ADHD/C group, F(1, 36) = 1.32, ns.
Figure 1.
Comparison of reaction time for go trials (RTGo) and stop signal reaction time (SSRT) by group on manual and visual versions of the stop task. Asterisks denote significant differences between RTGo and SSRT, p< .05.
Attention
In the current study, the intrasubject cross-trial variability on go trials (GoRTSD) was evaluated as a measure of inattention. Both ADHD groups were significantly more variable than the comparison group on both the manual, t(74) = 2.73, p < .01, d = .63, and visual stop tasks, t(68) = 3.28, p < .01, d = .80. However, children with ADHD/I did not differ from those with ADHD/C on either the manual, t(74) = .63, ns, or visual t(68) = .12, ns, tasks. Task differences were observed for GoRTSD, whereby children were more variable on the visual task than the manual task, F(1, 65) = 42.37, p < .001, η2 = .40. With respect to omission errors, no significant group differences were observed for the manual, F(2, 74) = 1.41, ns, or visual versions of the stop task, F(2, 68) = .59, ns. However, more omission errors were committed by all groups on the visual task than on the manual task, F(1, 65) = 7.61, p < .01, η2= .11.
Covariates: Gender and KBIT Matrices
When gender was included as a covariate in the analyses reported above, the same pattern of group similarities and differences emerged for all stop task dependent variables. This suggests that differences observed among groups were not accounted for by gender. When KBIT Matrices scores were included as a covariate in a separate analysis, the same pattern of group similarities and differences again emerged for all stop task dependent variables. This suggests that the observed differences between groups on stop task measures were not accounted for by group differences in KBIT matrices performance.
Discussion
The purpose of the current study was to compare performance patterns on two versions of the stop task—manual and visual—among children diagnosed with ADHD and their peers. A second goal was to explore whether there were differences in performance between clearly defined ADHD subtype groups relative to their peers. Results indicated that children in both ADHD groups demonstrated longer SSRTs relative to the comparison group, which supports previous work implicating impaired response inhibition in ADHD. Furthermore, children with ADHD were more variable in their reaction times than comparison children on both version of the stop task. This replicates previous work supporting the importance of problems in sustained attention in the presentation of ADHD (Castellanos & Tannock, 2002).
Although SSRT values were faster than those for RTGo in the comparison group on both versions of the stop task, consistent with the broad body of previous research on stop task performance (Lijffijt et al., 2005; Oosterlaan et al., 1998), this pattern was only observed on the manual task for the ADHD groups. On the visual task, children with ADHD took as long to stop as they did to go (i.e., no difference between RTGo and SSRT). Thus, the slowed inhibition associated with ADHD was most pronounced in the visual domain. Finally, except for RTGo for the manual task, the two ADHD subtype groups did not differ, suggesting that the stop task may not be an effective tool for differentiating the subtypes.
Task comparisons
To our knowledge, this is the first study to evaluate performance on both manual and visual versions of the stop task in a sample of children with ADHD compared to children without the disorder. Although the traditional, button-press stop task has been studied extensively, less is known about the relative utility of using the visual task to understand the cognitive deficits underlying ADHD. Given that eye movements are involved in basic attention processes, studying the ability to withhold saccades may afford a clearer understanding of the interface between attention and inhibition, two processes implicated in the impairments associated with ADHD. In comparing manual and visual versions of the stop task, similar patterns of impairment were observed across both tasks in the ADHD groups relative to the comparison group. Children with ADHD required more time to successfully inhibit responses than did children in the comparison group. There were no differences between the ADHD subtype groups with respect to measures of inhibition, however. Thus, it may be that the capacity to inhibit responding, particularly eye movements, is a measure of the inattentive symptoms that characterize both subtypes rather than the behavioral symptoms that are more intuitively linked to disinhibition.
One of the most notable findings from the current study concerns the differences in the time required to inhibit versus execute responses in each task. Previous studies of stop task performance in ADHD suggest that the typically observed pattern of faster SSRT relative to RTGo is stable and robust (e.g., Oosterlaan et al., 1998; Lijffijt et al., 2005). All children showed this pattern on the manual task. However, on the visual task, children with ADHD showed no difference between RTGo and SSRT. Given that response activation processes are initiated prior to inhibitory processes, this means that for children with ADHD, executing saccades took place at a more rapid pace than the ability to stop them. This unexpected similarity between rates of going and stopping could be due to faster response activation (shorter RTgo) or slower response inhibition (longer SSRT). Figure 1 shows that RTGo values were similar across all three groups on the visual task. However, SSRT values were slower in both ADHD groups, indicating that the unique relation between RTGo and SSRT on the visual task in ADHD is a function of some component involved in slowing response inhibition.
In understanding this finding, it is important to consider the value of being able to inhibit a behavior more quickly than activating a behavior. Put simply, quick inhibitory processes improve the flexibility of one’s behavioral repertoire and increase the likelihood that one will be able to act in accordance with changing demands in the environment. When stopping is slower than going, or even if stopping takes place at the same rate on average as going, it becomes difficult for individuals to regulate their behavior in the face of dynamic environmental conditions. That the unique relation between RTGo and SSRT among children with ADHD was specific to the visual task also carries implications for our understanding of the disorder. For instance, children with ADHD could experience impaired control over selective attention processes and exhibit increased distraction because they have difficulty ignoring irrelevant stimuli in the environment (i.e., problems inhibiting reflexive looks). Taken together, these findings suggest that the visual stop task may be a more sensitive measure of inhibitory impairment in ADHD because it examines the inhibition of eye movements, which reflects an interface between attentional processes and inhibition.
Subtype comparisons
A second purpose of the current study was to examine subtype differences on manual and visual versions of the stop task with well-defined subtype samples. Previous work from this research group has suggested important differences between the two subtypes, including slow response speed and increased omissions in ADHD/I group relative to ADHD/C (Derefinko et al., 2008; Fillmore et al., 2009). In the present study, performance of the two ADHD subtypes was quite comparable across both tasks, especially for measures of inhibition. The only exception was for RTGo, whereby children with ADHD/I, but not children with ADHD/C, were slower than comparison children to execute a response on the manual task. This finding is consistent with a slower response style observed on other tasks for the ADHD/I group (Derefinko et al., 2008; Fillmore et al., 2009). In some contexts, slower responding confers greater accuracy in responding or withholding a response. However, the slower responding observed in the ADHD/I group in the current study did not afford protection from inhibitory failures. Indeed, the ADHD/I group had the slowest RTGo values on the manual task but still had many more inhibitory failures than the comparison group, which had relatively faster RTGo values.
Although some measures yield distinct patterns of performance between the ADHD subtypes, others yield quite similar results (Adams et al., 2008). That the ADHD subtype groups in the current study did not differ with respect to inhibitory performance on the stop task is consistent with other studies examining response inhibition among ADHD subtypes (e.g., Geurts et al., 2006; Huang-Pollock et al., 2007). Beyond simply replicating these previous findings, however, the use of clearly defined subtype groups in the current study extends our understanding of the mechanisms underlying “pure” ADHD/I, where the children exhibit few to no hyperactive/impulsive symptoms.
The similarity between the ADHD/C and ADHD/I groups on the stop task warrants further consideration, given conflicting evidence that the subtype groups are quite distinct in other areas of cognitive and behavioral functioning (i.e., Adams et al., 2008; Diamond, 2005; Milich et al., 2001). One possible explanation as to why the stop task did not differentiate the subtype groups is that ADHD/I is a disorder of disinhibition, sharing this “core” deficit with ADHD/C. This would support the notion that ADHD/I is best conceptualized as a phenotypic variant of ADHD but not necessarily as a distinct disorder. This view is complicated, however, by findings that show inhibitory deficits for the ADHD/C group on some tasks that are not present for the ADHD/I group. Further, on some tasks where children with ADHD/I show significant differences from a comparison group, the direction of the effect is in the opposite direction than might be expected based on performance in the ADHD/C group (Derefinko et al., 2008). That is, children with ADHD/I at times appear overly inhibited rather than disinhibited. Thus, it is difficult to reconcile the current findings with those of previous work examining inhibitory performance in the same sample using different tasks.
An alternate interpretation is that the observed similarity between the subtype groups reflects unique deficits that result in longer SSRT values relative to the comparison group. The stop task is clearly capturing a deficit in both subtype groups, but it is possible that the similar outcomes observed derive from separate developmental pathologies. In this case, ADHD/I may not be an inhibitory disorder, but rather the result of some other kind of information processing deficit. To illustrate, a child who is generally slow to process environmental stimuli or other information necessary to guide behavior would be expected to appear slow in activating a response as well as slow to inhibit a response. This is consistent with the pattern we observe in the ADHD/I group on the manual task. Thus, the stop task may be sensitive to other kinds of deficits beyond gross disinhibition, such as slow information processing. Interestingly, the difference between the ADHD/I and ADHD/C groups for RTGo was not observed on the visual stop task. This finding, in tandem with the aforementioned unique relation between RTGo and SSRT in the ADHD groups, suggests that the visual stop task measures some unique component of responding differently from the manual stop task. This difference should be examined further in future research.
Limitations and Future Directions
The present study attempted to address a number of methodological concerns from the existing literature on ADHD subtype differences, particularly with respect to diagnostic procedures for the ADHD subtype groups (Milich et al, 2001). However, some aspects of the present study may limit the generalizability of the results. For example, the majority of participants in the study were males. Although the proportion of males to females in the current sample was consistent with the estimated gender distribution of ADHD in the general population, including more female participants in the sample would have allowed for evaluation of possible gender effects of the results. The question of whether gender moderates the relation between ADHD subtype and performance characteristics on neurocognitive tasks is a particularly relevant one given that some investigators predict a greater prevalence of ADHD/I than ADHD/C in females (Diamond, 2005).
Although the stop task was effective in differentiating ADHD groups from comparison children, there seems to be limited utility in using these tasks to differentiate the ADHD subtype groups. Additionally, we were unable to determine what underlying mechanisms may account for the slow response style observed in the inattentive group for the manual task. Other areas of impairment may prove more fruitful in explaining the behavioral differences observed between these groups. Indeed, several other clinically relevant variables beyond inhibitory functioning have been proposed as important to the neurocognitive profiles of ADHD subtypes. Among the most likely candidates for the deficits observed in ADHD/I are working memory (Diamond, 2005), attention (Huang-Pollock et al., 2006), motivation (Quay, 1997), various executive functions (Nigg et al., 2002; Pennington & Ozonoff, 1996), slow information processing (Carlson & Mann, 2002; Derefinko et al., 2008, Fillmore et al., 2008) and the complicated interplay among these processes. Future work should focus on untangling the possible explanations by studying the ADHD subtypes across multiple levels of analysis beyond basic neurocognitive tasks by including physiological measures, genetic markers, and long-term outcomes, etc.). This could allow better understanding of the factors that account for areas of continuity and discontinuity between the subtypes, toward the goal of developing a more integrative model of ADHD/I. A substantial amount of work along these lines has already been conducted with the ADHD/C group, but relatively little has been done to evaluate the inattentive subtype and its relation to other disorders, including ADHD/C.
Additionally, as with much of the literature examining subtype differences, the sample sizes in the current study were smaller than would be desirable. One of the problems created by the relatively small sample size is the decision of how to balance the probability of Type I and Type II errors. Stochastically, the number of comparisons made in the current study could produce a family-wise error rate as high as .65. This estimate is simply based on the number of comparisons conducted at a set alpha of .05. However, there are reasons to believe that this overestimates the error rate in the current study. First, many of the comparisons were specified a priori and reflected directional hypotheses. By examining a pre-determined subset of the whole set of possible comparisons, the chance of capitalizing on spurious significant findings is reduced. Relatedly, despite relatively small sample size, we were able to replicate findings from previous studies concerning differences between ADHD and comparison groups and observed some interesting, hypothesized differences between visual and manual tasks. Finally, planned contrasts were only conducted if the omnibus F test was significant. This step requires reliable variation in the group means before evaluating specific effects, again reducing the likelihood of capitalizing on spuriously significant results. Nevertheless, the aforementioned limitations underscore the importance of future replications.
In summary, the current results indicate that while an oculomotor version of the stop task may be more sensitive to the inhibitory deficits observed in ADHD relative to children without ADHD, neither the visual nor the manual stop task were effective in differentiating the ADHD subtypes. This reflects an interesting area of behavioral overlap between two groups of children who have demonstrated unique patterns of continuity and discontinuity on other measures, including other tasks designed to measure disinhibition. This suggests that further study of neurocongitive functioning among ADHD subtypes is a potentially fruitful avenue for better understanding how these diagnostic constructs relate.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA021027 and DA005312.
Footnotes
The number of participants for each analysis changed slightly due to equipment problems on either the manual or visual task. In Table 3, the number of participants with data for each task within each group is listed.
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. University of Vermont, Department of Psychology; Burlington, VT: 1991. [Google Scholar]
- Adams ZW, Derefinko KJ, Milich R, Fillmore MT. Inhibitory functioning across ADHD subtypes: Recent findings, clinical implications, and future directions. Developmental Disabilities Research Reviews. 2008;14:268–275. doi: 10.1002/ddrr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Armstrong IT, Munoz DP. Inhibitory control of eye movements during oculomotor countermanding in adults with attention-deficit hyperactivity disorder. Experimental Brain Research. 2003;152:444–452. doi: 10.1007/s00221-003-1569-3. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berlin L, Bohlin G, Rydell A. Relations between inhibition, executive functioning, and ADHD symptoms: A longitudinal study from age 5 to 8 ½ years. Child Neuropsychology. 2003;9:255–266. doi: 10.1076/chin.9.4.255.23519. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychological Review. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, primarily inattentive type. Journal of Clinical Child and Adolescent Psychology. 2002;31:123–129. doi: 10.1207/S15374424JCCP3101_14. [DOI] [PubMed] [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with attention deficit/hyperactivity disorder. Neuropsychology. 2006;4:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews: Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales—Revised. Multi-Health Systems Inc.; Toronto, ON: 1997. [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Derefinko KJ, Adams ZW, Milich R, Fillmore MT. Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:745–758. doi: 10.1007/s10802-007-9207-3. [DOI] [PubMed] [Google Scholar]
- Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): a neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity) Development and Psychopathology. 2005;17:807–825. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Milich R, Lorch EP, Lynam D. Inhibitory deficits in children with attention-deficit/hyperactivity disorder: Intentional versus automatic mechanisms of attention. Development and Psychopathology. 2009;21:539–554. doi: 10.1017/S0954579409000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory K, Milich R, Lorch EP. Online story comprehension among children with ADHD: Which core deficits are involved? Journal of Abnormal Child Psychology. 2006;34:853–865. doi: 10.1007/s10802-006-9070-7. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. ADHD subtypes: Do they differ in their executive functioning profile? Archives of Clinical Neuropsychology. 2005;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Hanisch C, Radach R, Holtkamp K, Herpertz-Dahlmann B, Konrad K. Oculomotor inhibition in children with and without attention-deficit hyperactivity disorder (ADHD) Journal of Neural Transmission. 2006;113:671–684. doi: 10.1007/s00702-005-0344-y. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Mikami AY, Pfiffner L, McBurnett K. ADHD subtype differences in motivational responsivity but not inhibitory control: Evidence from a reward-based variation of the stop signal paradigm. Journal of Clinical Child & Adolescent Psychology. 2007;36:127–136. doi: 10.1080/15374410701274124. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT, Halperin JM. Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology. 2006;20:420–429. doi: 10.1037/0894-4105.20.4.420. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. Journal of Child Psychology and Psychiatry. 2004;45:1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; Toronto: 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Irwin DE. Don’t look! Don’t touch! Inhibitory control of eye and hand movements. Psychonomic Bulletin & Review. 2000;7:107–112. doi: 10.3758/bf03210728. [DOI] [PubMed] [Google Scholar]
- Lorch EP, Sanchez RP, van den Broek P, Milich R, Murphy EL, Lorch RF, et al. The relation of story structure properties to recall of television stories in young children with attention-deficit hyperactivity disorder and nonreferred peers. Journal of Abnormal Child Psychology. 1999;27:293–309. doi: 10.1023/a:1022658625678. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice. 2001;8:463–488. [Google Scholar]
- Nichols SL, Waschbusch DA. A review of the validity of laboratory cognitive tasks used to assess symptoms of ADHD. Child Psychiatry and Human Development. 2004;34:297–315. doi: 10.1023/B:CHUD.0000020681.06865.97. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:200–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient changes for the coming decade. Biological Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg J. What causes ADHD?: Understanding what goes wrong and why. Guilford Press; 2006. [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD+CD, anxious and control children: a meta-analysis of studies with the stop task. Journal of Child Psychology and Psychiatry. 1998;39:411–425. [PubMed] [Google Scholar]
- Osman A, Kornblum S, Meyer DE. The point of no return in choice reaction time: Controlled and ballistic stages of response preparation. Journal of Experimental Psychology: Human Perception and Performance. 1986;12:243–258. doi: 10.1037//0096-1523.12.3.243. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Quay HC. Inhibition and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1997;25:7–13. doi: 10.1023/a:1025799122529. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Tannock R, Logan G. Inhibitory control, impulsiveness, and attention deficit hyperactivity disorder. Clinical Psychology Review. 1993;13:721–739. [Google Scholar]
- Schall JD, Hanes DP, Taylor TL. Neural control of behavior: countermanding eye movements. Psychological Research. 2000;63:299–307. doi: 10.1007/s004269900008. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Worling DE, Wasdell MB. A chart review study of the Inattentive and Combined Types of ADHD. Journal of Attention Disorders. 2003;7:1–9. doi: 10.1177/108705470300700101. [DOI] [PubMed] [Google Scholar]
- Weller EB, Weller RA, Rooney MT, Fristad MA. Children’s Interview for Psychiatric Syndromes—Parent Version (P-ChIPS) American Psychiatric Association; 1999. [Google Scholar]