Abstract
Purpose
Brain metastases of breast cancer cause neurocognitive damage and are incurable. We evaluated a role for temozolomide in the prevention of brain metastases of breast cancer in experimental brain metastasis models.
Experimental Design
Temozolomide was administered in mice following earlier injection of brain-tropic human epidermal growth factor receptor 2 (HER2)-positive Jimt1-BR3 and triple negative 231-BR-EGFP sublines, the latter with and without expression of 06-methylguanine-DNA methyltransferase (MGMT). Additionally, the percentage of MGMT-positive tumor cells in 62 patient-matched sets of breast cancer primary tumors and resected brain metastases was determined immunohistochemically.
Results
Temozolomide, when dosed at 50, 25, 10 or 5 mg/kg, 5 days/week, beginning 3 days after inoculation, completely prevented the formation of experimental brain metastases from MGMT-negative 231-BR-EGFP cells. At a 1 mg/kg dose, temozolomide prevented 68% of large brain metastases, and was ineffective at a dose of 0.5 mg/kg. When the 50 mg/kg dose was administered beginning on days 18 or 24, temozolomide efficacy was reduced or absent. Temozolomide was ineffective at preventing brain metastases in MGMT-transduced 231-BR-EGFP and MGMT-expressing Jimt-1-BR3 sublines. In 62 patient-matched sets of primary breast tumors and resected brain metastases, 43.5% of the specimens had concordant low MGMT expression, while in another 14.5% of sets high MGMT staining in the primary tumor corresponded with low staining in the brain metastasis.
Conclusions
Temozolomide profoundly prevented the outgrowth of experimental brain metastases of breast cancer in an MGMT-dependent manner. These data provide compelling rationale for investigating the preventive efficacy of temozolomide in a clinical setting.
Keywords: Brain metastasis, metastasis, temozolomide, breast cancer, MGMT
Introduction
Breast cancer is the second most frequent cause of brain metastases (BM), after lung cancer. BM often occur in advanced human epidermal growth factor receptor type 2 (HER2)-positive breast cancer patients including those with stable extracranial disease or while responding to systemic therapy (1, 2). For patients with triple-negative (estrogen receptor and progesterone receptor negative, HER2-normal) advanced breast cancer, a similar percentage develop BM, in a setting of progressive systemic disease (3). Current treatments for BM are palliative, including whole brain radiotherapy, stereotactic radiosurgery, neurosurgery and steroids (4).
Chemotherapy or molecular therapies have played only a limited role in the treatment of BM (5-11), as the brain is protected from most drugs by the blood-brain barrier (BBB). Pharmacokinetic and imaging studies in mouse models indicate that the extent of BBB opening following its disruption by the formation of a BM is limited and heterogeneous (12-14), a conclusion supported by clinical studies (rev. in (15-17)). Using an experimental BM model system, we previously tested multiple drugs for the ability to prevent the formation of BM or to shrink established BM. Six drugs have shown partial efficacy in the prevention setting (rev. in (18)), none was able to shrink established BM.
Temozolomide is an oral, brain-permeable alkylating agent characterized by significant uptake in the central nervous system, and is used in the treatment of primary brain tumors (19-21). This compound induces a number of different DNA lesions and acts in an 06-methylguanine-DNA methyltransferase (MGMT)-dependent manner (rev. in (22)). Knowledge on the efficacy of temozolomide in advanced breast cancer is scarce (23), and the potential of this compound in prevention of BM from breast cancer is unknown. We investigated the preventive effect of temozolomide using an experimental model of breast cancer BM, and determined the functional contribution of MGMT expression in this setting.
We hypothesized that temozolomide would significantly prevent the formation of BM in breast cancer patients whose tumors have low-to-no MGMT activity. The percentage of breast cancer patients representing this category, as well as whether the primary breast tumor is a good indication of MGMT status in tumor cells metastatic to the brain was unknown. To address these questions, we determined MGMT expression immunohistochemically (IHC) in patient-matched sets of primary breast cancers and resected BM.
Materials and Methods
Materials
For in vitro and in vivo experiments, temozolomide was obtained from Sigma (St. Louis, MO) or the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, NCI, respectively.
Cell culture and in vitro experiments in 231-BR-EGFP cells
A brain metastatic derivative of the triple-negative breast cancer cell line MDA-MB-231 was transduced with EGFP (231-BR-EGFP), as previously reported (24). Cells were maintained in high glucose DMEM medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Life Technologies) at 37 °C in 5% CO2.
Cell viability was assessed using MTT (Sigma) as previously described (25). Three separate experiments were performed, with n=4 for each data point within an experiment. For clonogenic assays, cells were plated at a single cell density and treated with vehicle or temozolomide 24 h later and every 3rd day for 10 days. Colonies were fixed and stained with crystal violet for quantification. Three separate experiments were performed with n=3 for each data point within an experiment. Western blot analysis was preformed per standard procedures. Primary antibodies for MGMT (Cell Signaling Technologies, Danvers, MA) and α-Tubulin (Oncogene, Cambridge, MA) were used.
Cell culture and in vitro experiments in Jimt-1-Br3 cells
Derivation of the HER2-positive Jimt-1-Br3 cells is described in the supplemental figure legend. Jimt-1-Br3 were maintained similarly to 231-BR-EGFP cells, but with the addition of 200 μM L-glutamine (Life Technologies, Grand Island, NY).
For shRNA-mediated MGMT knockdown in Jimt-1-BR3 cells, MISSION® VSV-g pseudotyped lentiviral particles expressing shRNA targeting MGMT (Sequence #1 CCGGAGC CTGGCTGAATGCCTATTTCTCGAGAAATAGGCATTCAGCCAGGCTTTTTTTG or #2 CC GGTGAGCGACACACACGTGTAACCTCGAGGTTACACGTGTGTGTCGCTCATTTTTTG) and scrambled non-target control (Sequence CCTAAGGTTAAGTCGCCCTCGCTCTAGCGAG GGCGACTTAACCTTAGG) (Sigma-Aldrich, St. Louis, MO) were used to transduce Jimt-1-BR3 cells at MOI=10, according to the manufacturer’s recommendations. Polyclonal populations were selected for two weeks in the presence of puromycin.
For clonogenic assays in Jimt-1-Br3 cells, 250 cells were plated at single cell density and, after 24h, treated with either vehicle or temozolomide at 10, 50 or 100 μM final concentrations. After 14 days, colonies were fixed and stained with crystal violet for quantification.
Animal experiments
All animal experiments were conducted under an approved Animal Use Agreement with the NCI.
MGMT-negative cell lines: To assess the preventive role of temozolomide, 5-7 week-old female NRC nu/nu mice (Charles River Laboratories, Fredrick, MD) were inoculated with 175,000 231-BR-EGFP cells in 0.1 mL PBS in the left ventricle of the heart (25-27). Three days after tumor cell inoculation, mice were randomized to temozolomide at a dose of 50 mg/kg delivered by oral gavage in saline, 5 days a week for 4 weeks, or vehicle (saline). Subsequent experiments used temozolomide doses of 25, 10, 5, 1 and 0.5 mg/kg. To evaluate the efficacy of temozolomide in treating established BM, mice received temozolomide (50 mg/kg) beginning on either day 18 or day 24 post-injection of 231-BR-EGFP cells, 5 days a week for two and one week, respectively. In all experiments, mice were euthanized under CO2 anesthesia 28 days after tumor cell injection, and the brains were removed at necropsy. Five hematoxylin and eosin (H&E) stained serial sections (10 microns thick), one every 600 microns in a sagittal plane through the right hemisphere of the brain were analyzed at 50x magnification using an ocular grid. Every micro- or large (<300 and >300 microns along the longest axis, respectively) metastasis in each section was tabulated. The left hemisphere of the brain was used for IHC analysis.
To investigate the impact of temozolomide on survival, mice injected with 231-BR-EGFP cells were randomized to vehicle, temozolomide on days 3-14, or temozolomide on days 17-28 post-injection, per the schedule described above. Mice were maintained without further treatment up to 109 days and were sacrificed for signs of metastatic progression (loss of 20% body weight, seizures and paralysis).
MGMT-positive cell lines: To determine the functional contribution of MGMT expression in the 231-BR-EGFP BM prevention model, expression of human MGMT or a control vector was induced in independent polyclonal populations using a lentiviral expression system. Briefly, the human MGMT cDNA was purchased from Origene Technologies (Rockville, MD) and cloned into the pCDH-CMV-Hygro lentiviral vector (Systems BioScience, Mountain View, CA) for lentivirus production and subsequent infection of cells per the manufacturer’s recommended protocol. Post-infection, 231-BR-EGFP cells infected with MGMT or a vector control virus were selected in hygromycin for 2 weeks. Three independent populations of cells expressing MGMT or vector virus were harvested.
Two vector expressing- and two MGMT expressing polyclonal populations were injected into the left ventricle of female nude mice, 16 mice per arm. On day 3 post-injection, each group was randomly divided into vehicle or 50 mg/kg temozolomide by oral gavage arms, 5 days a week for 4 weeks.
A brain tropic derivative of HER2-positive Jimt-1 breast cancer cells (28) was selected. In derivation experiments, 500,000 Jimt-1 cells (28) were injected in the left ventricle of 5-7 week-old female NRC nu/nu mice, and mice were housed until they showed signs of distress. Mice were euthanized if they lost greater than 20% of their starting body weight or they became paralyzed. At necropsy, brains were removed, manually dissociated and placed in tissue culture. Cells that grew out were pooled as Jimt-1-BR1 cells, and the procedure was repeated 2 additional times to establish Jimt-1-BR3 cells which form brain metastases in 100% of mice injected over a 3-6 week period. For temozolomide experiments, 175,000 Jimt-1-BR3 cells were injected and randomized to vehicle or 50 mg/kg temozolomide identically to MGMT-negative 231-BR-EGFP experiments.
Patient matched primary tumors and BM
MGMT status was assessed in primary tumors and in corresponding BM. Two sets of matched primary breast tumors and resected BM were selected from the Polish BM Consortium (n=106) and the University of Kiel, Germany (n= 14) databases, respectively. Formalin-fixed, paraffin embedded samples were used for construction of a tissue microarray (TMA). MGMT IHC expression in the primary tumor and BM was compared for 62 matched sets, clinical parameters were established for 49 of those sets (excluded were 51 samples with no viable tumor tissue and 6 with insufficient clinical information).
Immunohistochemistry
All procedures were performed according to the manufacturer’s instructions (Imgenex, Corp, San Diego, CA and LifeSpan, BioSciences, Inc.). The antigen-antibody complex was visualized using the Visualization System: Novolink™ Polymer Detection System. Tissue microarray sections were deparaffinized in xylene and rehydrated through graded alcohol concentrations (100%, 96%, 80%, and 70%). For antigen retrieval, slides were pre-treated with a low pH target retrieval solution (Dako). Endogenous biotin was blocked with an appropriate kit. Sections were incubated for 1 hour with an antibody against the human MGMT (monoclonal antibody; clone MT3.1; dilution: 1:50, Imgenex, Corp, San Diego, CA and monoclonal antibody from LifeSpan, BioSciences, Inc., dilution 1:50). Only a nuclear staining was considered positive. Tonsil tissue served as a positive control. The immunoreactivity was scored semi-quantitatively as follows: 0:<5% positive tumor cells, 1+: 5-75% positive tumor cells, 2+: 75-95% positive tumor cells, 3+: >95% positive tumor cells (29); data were subsequently combined into a MGMT-negative (<5% positive) and MGMT-positive (>5% positive) categories (Supplemental Table 1). The testing lab was blinded to patient characteristics.
Statistical Analysis
The Wilcoxon rank sum test compared distributions from two-samples. In the rare case the data were normally distributed, a one-way or factorial analysis of variance was performed on the data. Holm’s method was used to adjust for multiple comparisons. Analysis of MGMT IHC data used the following tests as appropriate: Fisher’s exact test for 2×2 tables, Cochran-Armitage trend test for 2×C ordered tables, and the Jonckheere-Terpstra trend test for doubly ordered R×C tables. Actuarial analyses used the Kaplan-Meier method. Overall survival was measured from the date of first primary tumor surgery to date of death or last follow-up. Survival times were censored if the subject was alive as of the last follow-up. The log-rank test was used to test for differences between strata. All reported P-values are two-sided. Considering the large number of tests performed, only P<0.005 were deemed statistically significant, while 0.005 < P < 0.05 were deemed a strong trend.
Results
Temozolomide prevention of triple-negative 231-BR-EGFP experimental BM
A previously characterized model system using a brain-tropic subline of the triple negative human MDA-MB-231 breast carcinoma cell line (231-BR-EGFP) was used to generate experimental brain metastases. Tumor cells were injected into the left cardiac ventricle; on day 3 post-injection, mice were randomized to receive either vehicle or temozolomide. At necropsy on day 28 post-injection, experimental brain metastases were quantified in step sections through one brain hemisphere as micrometastases or large metastases (based on a cutoff of 300 microns along the longest axis, comparable to a several mm lesion in a human brain). Temozolomide, administered at a dose of 50 mg/kg, 5 days a week for 4 weeks, prevented the formation of all large- and micro-BM over two replicate experiments (Table 1); even single tumor cells could not be detected in sections of the brains. Vehicle treated mice developed BM at normal rates. This dose was reported to be consistent with clinically achievable doses of temozolomide (30, 31). Doses of 25, 10 and 5 mg/kg on the same schedule yielded the same results, complete prevention of BM formation in vivo (Table 1). At a dose of 1 mg/kg, 50-fold lower than the starting dose, medians of 0.9 and 1.2 large metastases per section were present in treated brains, as compared to 2.3 and 4.8 large metastases per section in vehicle treated mice, respectively, corresponding to 61 and 75% reductions (P = .80 and .059, respectively) in the two experiments conducted. Similar inhibition of micrometastatic lesions was observed. At a dose of 0.5 mg/kg, two logs lower than a widely reported preclinical regimen, temozolomide did not significantly prevent the formation of large- or micrometastases. The data identify a potent preventive effect of temozolomide on the formation of 231-BR-EGFP experimental brain metastases of breast cancer over a wide dose range.
Table 1.
Temozolomide prevention of experimental breast cancer brain metastases over a two-log dose responsea.
| Temozolomide (mg/kg): | |||||||
|---|---|---|---|---|---|---|---|
| 50 | 25 | 10 | 5 | 1 | 0.5 | ||
|
|
|||||||
| Experiment: | Lesion: | ||||||
| 1 | Large | 0 (2)b | |||||
| P<.0001 | |||||||
| Micro | 0 | ||||||
| (63.3)c | |||||||
| P<.0001 | |||||||
| 2 | Large | 0 (2.6) | 0 (2.6) | ||||
| P<.0001 | P<.0001 | ||||||
| Micro | 0 (86.4) | 0 (86.4) | |||||
| P<.0001 | P<.0001 | ||||||
| 3 | Large | 0 (6.5) | 0 (6.5) | 0 (6.5) | |||
| P<.0001 | P<.0001 | P<.0001 | |||||
| Micro | 0 | 0 | 0 | ||||
| (143.3) | (143.3) | (143.3) | |||||
| P<.0001 | P<.0001 | P<.0001 | |||||
| 4 | Large | 0.9 (2.3) | 1.2 (2.3) | ||||
| P=80 | P=86 | ||||||
| Micro | 40.1(101.1) | 58(101.1) | |||||
| P=22 | P=.63 | ||||||
| 5 | Large | 1.2 (4.8) | 5 (4.8) | ||||
| P=.059 | P=.90 | ||||||
| Micro | 67(178.6) | 115(178.6) | |||||
| P=028 | P=30 | ||||||
Female nude mice were injected in the left cardiac ventricle with 1.75 × 105 tumor cells from a brain tropic triple negative breast carcinoma cell line (231-BR-EGFP). Beginning on day 3 post-injection, mice received temozolomide by oral gavage, 5 days/week for 4 weeks. Mice were sacrificed 28 days post-injection. Experimental brain metastases were quantified in serial sections through one hemisphere as micrometastases (micro) or large metastases (≤300 and ≥300 microns along the longest axis, respectively). The effect of temozolomide was compared to that of a vehicle control in each experiment. Sample size was 3-10 mice per group.
Median number of large metastases
Median number of micrometastases that formed, respectively at the indicated concentration of temozolomide (median number of metastases that formed in vehicle treated mice).
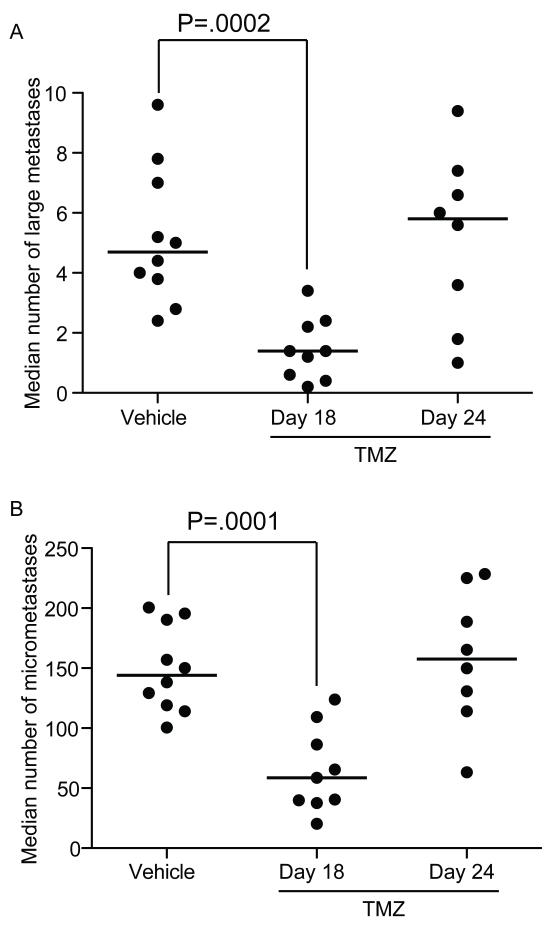
Temozolomide was ineffective in the treatment (i.e., shrinking) of metastatic breast cancer (23). In experiments testing the ability of temozolomide to treat established BM, mice were randomized to receive temozolomide beginning on either day 18 or day 24 post-injection. Temozolomide administered on day 18 post-injection of 231-BR-EGFP cells resulted in a median of 1.4 large metastases per section, compared to 4.7 for the vehicle controls, a 70% reduction (P = .0002; Fig 1A). A 59% reduction was observed in micrometastases (P = .0001; Fig 1B). Day 18 post-injection represents a timepoint where multiple micrometastases and occasional large metastases are present. When treatment was further delayed to day 24 post-injection, a time when greater numbers of large metastases had formed, as demonstrated in imaging studies (32), the efficacy of temozolomide was lost (a median of 5.8 large metastases per section compared to 4.7 in vehicle-treated mice, and similar trends were observed for micrometastases; Fig 1B). Prolongation of the experiment was impossible, as mice administered vehicle or temozolomide on day 24 required euthanasia for paralysis and other indications. The data indicate that the inhibitory effect of temozolomide was reduced by late administration, possibly due to decreased delivery throughout larger tumor masses and/or shorter exposure to the drug.
Figure 1. Delayed administration of temozolomide (TMZ) is less effective on experimental 231-BR-EGFP brain metastasis.
231-BR-EGFP cells were injected into the left cardiac ventricle of female nude mice; the mice randomized to three treatment groups: vehicle on days 3-28 post-injection, or 50 mg/kg temozolomide five days a week, beginning either day 18 or day 24 post-injection. A. Number of large metastases (>300 microns in a single dimension) per brain section, B. Number of micrometastases per brain section (<300 microns). Each dot represents the median per mouse and the line designates the group median. A one-way ANOVA was performed on transformed data and Holm’s method was used to adjust the model based t-test p-values (n=8 to 10).
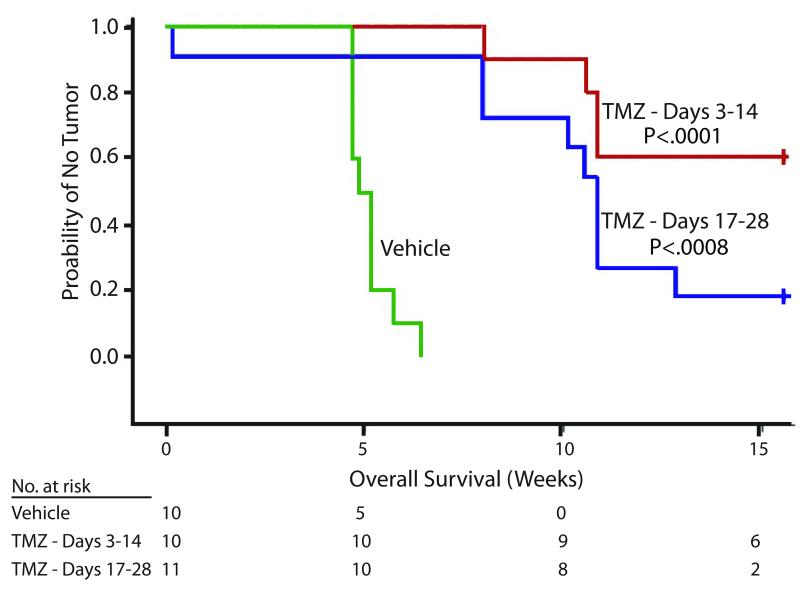
To investigate the impact of preventing experimental BM on mouse survival, mice were injected with 231-BR-EGFP cells in the left cardiac ventricle, and then randomized to three arms: vehicle from days 3-14; temozolomide (50 mg/kg, 5 days a week) from days 3-14 or temozolomide from days 17-28. All mice were then left untreated and monitored for signs of paralysis, weight loss or seizures requiring euthanasia. All vehicle treated mice required euthanasia by day 45 post-injection, with a median survival of 5 weeks post-injection compared to 10.9 weeks for the delayed treatment, (Fig 2; median survival was not reached in the early treatment). The two schedules of two week temozolomide treatment significantly increased survival (P = .0003 by log rank test). Earlier (days 3-14) and delayed (days 17-28) administration of temozolomide resulted in long term survival of 60% (6/10 mice) and 18% (2/11 mice), respectively; a significant difference compared to vehicle (P < .0001 and .0008, respectively). Taken together, the data indicate that temozolomide administration prolonged mouse survival and was associated with cures. This is consistent with data from previous experiments showing no evidence of disease from histopathologic counts. In this experiment the early versus late treatment arms received the same cumulative dose of temozolomide, and the data favored early treatment.
Figure 2. Early administration of temozolomide (TMZ) provides long-term survival in a preclinical model.
231-BR-EGFP cells were injected into the left cardiac ventricle of female nude mice; the mice randomized to three treatment groups: Vehicle, days 3-28 post-injection; 50 mg/kg temozolomide, five days a week, days 3-14 post-injection; 50 mg/kg temozolomide five days a week, days 17-28 post-injection. Mice were housed without further treatment until day 109 post-injection. They were sacrificed upon signs of progression such as 20% body weight loss, paralysis or seizures. Kaplan-Meier analysis of survival is shown. The Log-rank test was used to compare strata and Holm’s method was used to adjust p-values.
Expression of MGMT and sensitivity to temozolomide
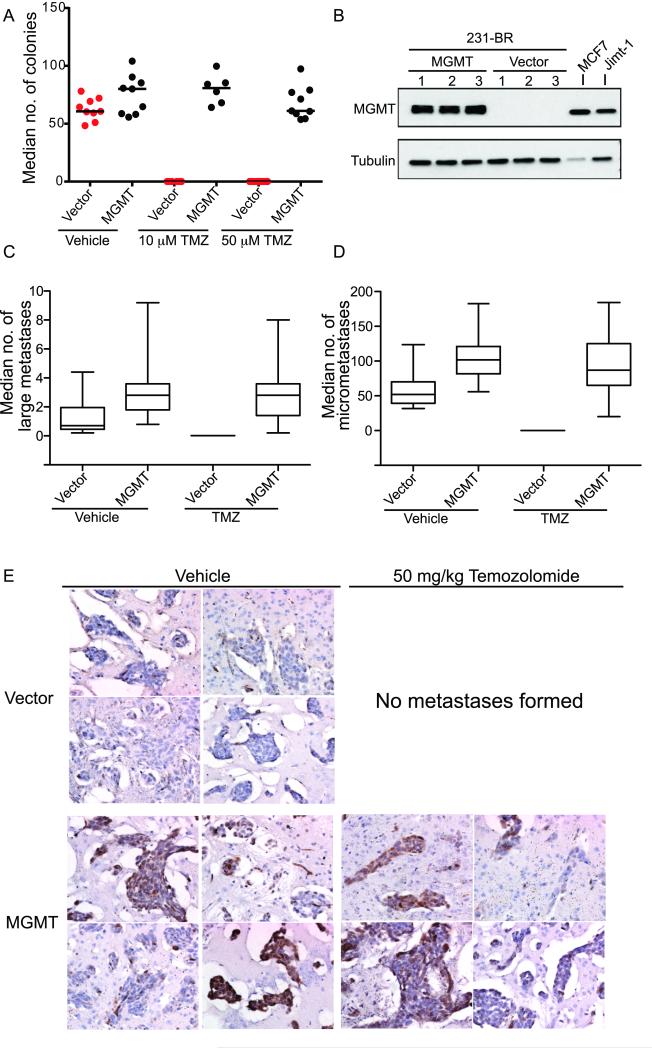
Whereas temozolomide induces a number of DNA lesions, evidence suggests that the formation of the O6-methylguanine DNA lesions are associated with temozolomide activity in primary brain tumors (22). This lesion is repaired by the enzyme O6-methylguanine-DNA-methyltransferase (MGMT). In in vitro experiments, temozolomide completely abolished the ability of single 231-BR-EGFP cells to form colonies (Fig 3A, P = .003 at 10 μM and P = .0004 at 50 μM) but decreased proliferation by only 24% (P = .0002, Supplemental Fig 1). In agreement with the sensitivity of 231-BR-EGFP cells to temozolomide, the cells were MGMT negative by western blot (Fig 3B) and qRT-PCR (data not shown). To determine the functional contribution of MGMT expression in our brain metastasis preventive model, expression of a MGMT cDNA was induced using a lentiviral expression system. Independent polyclonal populations of vector or MGMT expressing 231-BR-EGFP cells were isolated (Fig 3B). When 231-BR-EGFP cells were forced to express MGMT, in vitro colonization was similar to vehicle treated cells at both 10 (P = 1.0) and 50 μM (P = .92) concentrations of temozolomide; proliferation in vitro was unaffected.
Figure 3. MGMT overexpression abrogates temozolomide (TMZ) prevention of experimental brain metastasis formation.
A. Clonogenic assay for the effect of temozolomide on the outgrowth of single cells in vitro. 231-BR-EGFP vector and MGMT transduced populations were plated at single cell densities, treated with the indicated concentration of temozolomide and the number of colonies counted after 10 days. Each dot represents the mean of triplicate data points within an experiment and the line designates the median of the data from 3 experiments (n=6 to 9). No colonies formed in the vector cells in the presence of temozolomide. B. Western blot analysis of 3 independent polyclonal populations of 231-BR-EGFP cells transduced with either an empty vector or human MGMT cDNA. Two other cell lines, MCF-7 and Jimt-1-BR3 are also shown as positive controls. C-E. 231-BR-EGFP cells transduced with vector or MGMT were injected into the left cardiac ventricle of female nude mice, and randomized to vehicle or 50 mg/kg temozolomide, 5 days a week, beginning on day 3 post-injection. (n=8 to 16). C. Median large metastases per brain section. D. Median micrometastases per brain section. E. Expression of MGMT in experimental brain metastases. Formalin-fixed paraffin-embedded sections of mouse brain with metastases were stained for MGMT expression. Representative images are shown from 2 mice per group. 200x magnification.
The impact of MGMT expression on temozolomide prevention of 231-BR-EGFP BM was tested in experimental BM assays. Two vector- and two MGMT-positive polyclonal populations were injected into mice as described, and randomized to vehicle or 50 mg/kg temozolomide on the same schedule as previously described. Data from the two vector and two MGMT expressing polyclonal cell populations were combined as no significant difference was detected between the populations. A representative experiment of two conducted is shown (Fig 3C-D). For the two experiments conducted, vehicle treated mice injected with 231-BR-EGFP-vector cells developed medians of 1.4 and 0.7 large BM per section, while temozolomide again abrogated all large and micrometastasis development. Mice that received 231-BR-EGFP cells expressing MGMT, and were treated with temozolomide, developed a median of 1.7 and 2.8 large BM per section, respectively. These data were similar to that of untreated mice injected with 231-BR-EGFP and represented a strong trend of distinction from the temozolomide treated mice injected with 231-BR-EGFP (P = .066 and P = .0003, respectively). The data suggest that MGMT expression may modulate brain metastatic activity to a limited extent (Fig 3C). In agreement with previous data, temozolomide completely inhibited the formation of BM by 231-BR-EGFP-vector cells (P < .001 for both experiments), bringing to four the number of independent experiments with complete preventive activity (see Table 1). For the MGMT expressing 231-BR-EGFP cells, temozolomide administration resulted in a median of 0.6 and 2.8 large BM per section in the two experiments conducted, as compared to 1.7 and 2.8 large BM per section in vehicle treated mice, respectively (P = .010 and P = .48). Similar trends were observed for micrometastases (Fig 3D). Expression of MGMT in the tumor cells that formed metastases in the brain was heterogeneous at the end of the experiment; however, the majority of lesions maintained some level of expression of the transduced gene (Fig 3E). Thus, temozolomide prevention of BM formation in this model system was MGMT-dependent. We have been unable to identify a second BM model system that is low in MGMT expression for validation purposes.
We tested the BM preventive ability of temozolomide in a second experimental brain metastasis model system. A brain-tropic subline of the HER2-positive breast cancer cell line Jimt-1(28), which is MGMT-positive (Fig 3B) was derived by three rounds of intracardiac injection, formation of experimental brain metastases, sterile harvest and ex vivo culture. Jimt-1 cells are reported to be lapatinib resistant in vitro and may therefore be representative of advanced disease (33). In keeping with its MGMT positive status, there was no significant prevention of BM formation by temozolomide when mice were injected with Jimt-1-BR3 cells and randomized to vehicle or 50 mg/kg temozolomide beginning on day 3 post-injection (5 days per week; Supplemental Fig 2A-B). Vehicle treated mice developed a median of 8.6 large metastases and 64.6 micrometastases per section, compared with 8.5 large metastases and 57.7 micrometastases for mice treated with 50 mg/kg temozolomide (P =0.36 and P=0.63, respectively). Jimt-1-BR3 cells were then transduced with shRNAs, using a scrambled control and two independent MGTMT-targeting constructs. MGMT expression of the resulting polyclonal populations is shown on Supplemental Figure 2C. Colonization assays, which were a faithful indicator of in vivo experimental brain metastasis for 231-BR-EGFP cells (Fig 3A), were performed in the presence and absence of temozolomide. Knockdown of MGMT had no significant effect on the colonization of Jimt-1-BR3 cells in the absence of temozolomide; both shRNA constructs strongly sensitized the cells to temozolomide, at 50 and 100 μM concentrations (p<0.0001 for comparisons with scramble control, Supplemental Figure 2D). The data are consistent with a requirement for low MGMT expression for temozolomide prevention of experimental brain metastasis.
MGMT expression in patient matched primary breast tumors and brain metastases
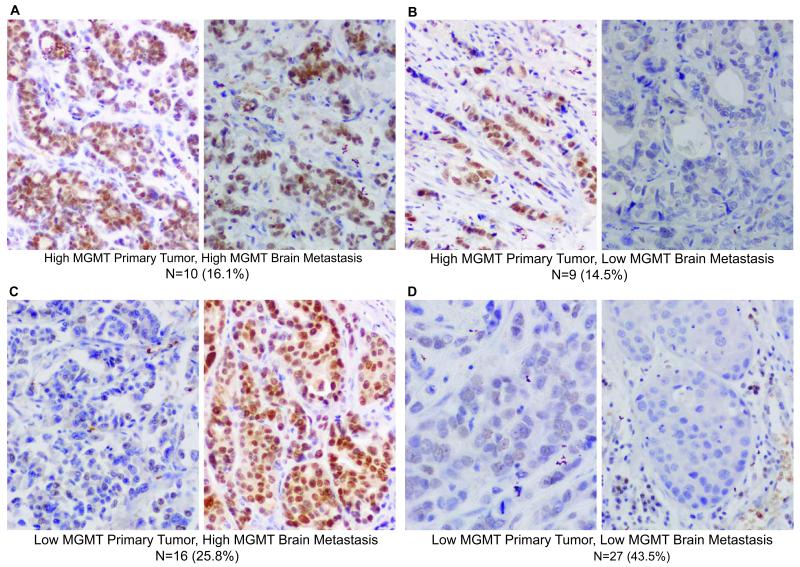
We hypothesize, based on the preclinical data presented, that temozolomide may be effective in preventing the formation of BM from single tumor cells or micrometastases in patients with tumors that have low-to-no MGMT activity. What percentage of breast cancer patients this represents this subset, as well as whether the primary breast tumor is an accurate predictor of MGMT status in BM, remains a question. MGMT has been screened by multiple methodologies, with the methylation status of its promoter used most often. We reasoned that many molecular mechanisms can down-regulate MGMT, besides DNA methylation, and elected to use an immunohistochemical (IHC) assay for overall protein levels. Two tissue microarrays (TMAs) were stained for MGMT and the percentage of MGMT-positive tumor cells scored by a pathologist. A total of 62 matched sets were stained, each consisting of a primary breast tumor and resected BM from the same patient, a cohort of rare specimens. Initially a previously reported set of cutoffs were utilized, including >5%, 6-75%, 76-95% and >95% positive tumor cells (29). Based on the paucity of samples in each of the latter three categories, the data were dichotomized to low (<5% positive tumor cells) and high (>5%) staining categories. All four potential patterns of MGMT staining in the primary tumor and matched resected BM were observed (low MGMT expression in both primary tumor and BM; low expression in the primary tumor, high expression in the BM; high expression in the primary tumor, low expression in the BM; low expression in both the primary tumor and BM; Fig 4). Overall, the concordance of primary tumor and BM MGMT expression was weak: In only 60% of cases dichotomized MGMT status in primary tumor and BM was concordant. Forty-four percent (n=27) contained concordant low MGMT staining in primary tumors and BM and in another 9 cases (15%) high MGMT expression in the primary tumor corresponded with low expression in the BM. Taken together, a majority of BM (36 out of the 62 matched sets, 58%) had low MGMT expressing BM. The data indicate that a majority of BM are low in MGMT expression and therefore potentially preventable by temozolomide.
Figure 4. MGMT expression in matched sets of human breast primary tumors and resected brain metastases.
A-D. Sixty-two patient matched sets were collected from tumor banks in Poland and Germany. TMAs of the specimens were stained for MGMT and evaluated for the percent of positively staining tumor cells (nuclear staining only), dichotomized at 5%. The number and percentage of specimens in each category is given below each representative photomicrograph.
Clinical parameters including hormone receptor status, grade and subtype of primary tumor, as well as treatment, type of first metastatic progression and dominant site of metastatic disease were compared for low versus high MGMT-staining tumors, either the primary tumor or the matched resected BM (Table 2). A strong trend was observed in BM for an association of HER2 overexpression and MGMT negativity (P=0.089), suggesting the eligibility of this subset for potential clinical trials. Nine patients (33%) with low MGMT BM (n=27) experienced brain as the first site of metastatic progression, as compared to 16 patients (76%) with high MGMT expressing BM (P = .004). BM as a first site of progression was associated with overall survival (log-rank p=.008), whereas MGMT staining, either in primary tumor or resected BM was not (Supplemental Table 2, Supplemental Fig 3).
Table 2.
Associations of primary tumor and brain metastasis MGMT expression with patient characteristicsa
| Number with Characteristic/Total (%) |
|||
|---|---|---|---|
| Variable | MGMT-negative (≤5% positive tumor cells) |
MGMT-positive (>5% positive tumor cells) |
P |
|
| |||
| Table 2A, Primary tumor (n=43 and 19, respectively ) | |||
|
| |||
| Breast cancer type | |||
| Ductal | 26/31 (84%) | 13/17 (76%) | 0.52 |
| Lobular | 3/31 (9.7%) | 1/17 (5.9%) | |
| Other | 2/31 (6.5%) | 3/17 (18%) | |
|
| |||
| HR status | |||
| ER positive | 17/31 (55%) | 12/17 (71%) | 0.36 |
| PR positive | 10/31 (32%) | 9/16 (56%) | 0.13 |
| HER2 positive | 16/31 (52%) | 7/17 (41%) | 0.56 |
|
| |||
| Subtypeb | |||
| HR(+)/HER2(−) | 8/31 (26%) | 8/17 (47%) | |
| HR(+)/HER2(+) | 10/31 (32%) | 4/17 (24%) | 0.23 |
| HR(−)/HER2(+) | 6/31 (19%) | 3/17 (18%) | |
| HR(−)/HER2(−) | 7/31 (23%) | 2/17 (12%) | |
|
| |||
| Grade | |||
| 1 | 0/28 (0%) | 2/17 (12%) | |
| 2 | 10/28 (36%) | 6/17 (35%) | 0.19 |
| 3 | 18/28 (64%) | 9/17 (53%) | |
| Unknownc | 2 | 4 | |
|
| |||
| Treatment prior to BM | |||
| Neoadjuvant | 8/31 (26%) | 4/17 (24%) | 1.0 |
| chemotherapy | |||
| 12/31 (39%) | 7/17 41%) | ||
| Adjuvant chemotherapy | 2/31 (6.5%) | 0/17 (0%) | |
| Metastatic chemotherapy | |||
| Adjuvant/neoadjuvant | 0.25 | ||
| and metastatic | 17/31 (55%) | 8/17 (47%) | |
| chemotherapy | 0/31 (0%) | 2/17 (12%) | |
| No | |||
| 13/31 (42%) | 10/17 (59%) | 0.37 | |
| Endocrine therapy | 11/31 (35%) | 5/16 (31%) | 1.0 |
| Trastuzumab | 20/28 (71%) | 16/17 (94%) | 0.12 |
| Radiation therapy | |||
| Site of first progression | |||
| Regional (nodal) | 3/31 (9.7%) | 0/17 (0%) | 0.54 |
| Distant | 28/31 (90.3%) | 17/17 (100%) | |
|
| |||
| Dominant site of metastatic diseased |
|||
| Soft tissuee | 0 | 0 | 0.11 |
| Bone | 0/31 (0%) | 2/16 (13%) | |
| Viscera | 31/31 (100%) | 14/16 (87%) | |
|
| |||
| Table 2B, Brain metastases (BM) (n=36 and 26, respectively) | |||
|
| |||
| Receptor status | |||
| ER positive | 16/27 (59%) | 13/21 (62%) | 1.0 |
| PR positive | 9/26 (35%) | 10/21 (48%) | 0.39 |
| HER2 positive | 16/27 (59%) | 7/21 (33%) | 0.089 |
|
| |||
| Subtypeb | |||
| HR(+)/HER2(−) | 1/27 (3.7%) | 4/19 (21%) | 0.64 |
| HR(+)/HER2(+) | 11/27 (41%) | 7/19 (37%) | |
| HR(−)/HER2(+) | 11/27 (41%) | 2/19 (11%) | |
| HR(−)/HER(−) | 4/27 (15%) | 6/19 (32%) | |
|
| |||
| Brain as 1st metastatic site | 9/27 (33%) | 16/21 (76%) | 0.004 |
|
| |||
| No. of BM at time of surgery | |||
| 1 | 19/27 (70%) | 9/20 (45%) | |
| 2-3 | 6/27 (22%) | 8/20 (40%) | 0.13 |
| >3 | 2/27 (7.4%) | 3/20 (15%) | |
|
| |||
| KPS>70 | 25/27 (93%) | 17/21 (81%) | 0.38 |
|
| |||
| Treatment after BM | |||
| Radiation therapy | 23/25 (92%) | 18/20 (90%) | 1.0 |
| Chemotherapy | 16/24 (67%) | 12/19 (63%) | 1.0 |
| Endocrine therapy | 5/24 (21%) | 3/20 (15%) | 0.71 |
| Trastuzumab | 5/10 (50%) | 2/2 (100%) | 0.47 |
| Lapatinib | 6/10 (60%) | 0/2 (0%) | 0.45 |
Histopathologic and clinical data from a cohort of 49 metastatic breast cancer patients, for which matched primary tumor and resected BM FFPE specimens, and clinical data were available for analysis. MGMT expression was determined IHC and dichotomized into negative (≤5% MGMT-staining tumor cells) and positive (>5% MGMT-staining tumor cells) samples. Tthe following statistical tests were used as appropriate: Fisher’s exact test for 2×2 tables, Cochran-Armitage trend test for 2×C ordered tables, and the Jonckheere-Terpstra trend test for doubly ordered R×C tables.
Subtype was analyzed as an ordered variable.
Unknown was not used in the statistical analysis of the Tumor Grade.
Multiple metastatic sites were assigned into three categories (soft tissue, bones, viscera) and the dominant site classified by the category associated with the worst prognosis in the following order of increasing gravidity: soft tissues, bones, viscera.
Soft tissue was not used in the statistical analysis of the Dominant Site of Disease.
Discussion
Temozolomide shows efficacy in primary brain tumors, but is considered inactive in metastatic breast cancer (23). This compound has also been tested in patients with established BM from a variety of cancer types with limited responses, either as monotherapy (34-37), in combination with other cytotoxic agents ((38-40) as examples), or with radiation therapy ((41-43) as examples). Many of these studies enrolled patients with multiple cancer histologies, were focused on responses rather than on initial development of disease, and did not investigate molecular correlates.
We report the preclinical testing of temozolomide in the 231-BR-EGFP experimental BM model system. The 231-BR-EGFP model system was previously reported to be representative of breast cancer craniotomy specimens in terms of proliferation and apoptosis rates, and a neuroinflammatory response (44). The experimental metastasis model employed histologic counts as opposed to imaging, since imaging signals can be variably diminished by their depth within the brain. This model was extensively tested for the prevention of BM by 18 drugs (18). Partial prevention of BM was noted for several drugs (18), but none was completely effective. In contrast, four experiments at 50 mg/kg, two experiments at 25 mg/kg, and one experiment each at 10 and 5 mg/kg dosing schedules completely prevented the formation of 231-BR-EGFP BM. We attempted dual fluorescent staining of brain sections with antibodies specific to human mitochondria and endoplasmic reticulum to identify residual tumor cells that may have been unseen on H&E staining, but remain uncertain of any positive staining. The complete abrogation of BM demonstrated in this study is unique. The extensive dose response of temozolomide prevention of 231-BR-EGFP BM, over a log of doses, suggests that lower doses of drug may be used in a prevention trial. Indeed, temozolomide efficacy has been studied in lower metronomic regimens with good results (45-47).
Our data also suggest that the inhibitory effect of temozolomide was reduced by late administration. Using histologic counts, a two week administration of temozolomide starting at day 17 post-injection was superior to a one week regimen starting on day 24 post-injection. This experiment contained two variables: the size of the brain lesions at the initiation of treatment and the cumulative dose of temozolomide delivered. However, in a survival analysis, equal cumulative doses of temozolomide were administered. Earlier administration of temozolomide (days 3-14 post-injection) produced long term survival in 60% of mice, superior to later administration (days 17-28 post-injection). It remains possible that even greater numbers of mice were “cured” of BM in the survival experiment, as some mice also develop bone metastases and require euthanasia for similar symptoms, such as paralysis.
In the 231-BR-EGFP model system, the preventive efficacy of temozolomide was MGMT dependent. Expression of MGMT in the MGMT-null 231-BR-EGFP subline abrogated the BM preventive activity of temozolomide. In addition, we developed a new model system for experimental BM of breast cancer based on the Jimt-1 breast cancer cell line (28). Temozolomide was ineffective at preventing BM in the MGMT-positive Jimt-1-BR3 model. This observation raises the question of whether MGMT expression needs to be an enrollment criterion for a temozolomide BM preventive trial. In earlier studies MGMT expression was detected by enzymatic activity, promoter methylation, mRNA level and IHC (48, 49). We reasoned that many events, not just promoter methylation, can down regulate MGMT gene expression and enzymatic activity, therefore we employed an IHC assay. We used two TMAs containing rare matched sets of primary breast tumors and the resected BM from the same patient, were employed. In this series, 59% of patients had low MGMT expressing BM. However, only 60% of resected BM retained concordant MGMT staining with the primary tumor. Thus, primary tumor MGMT status is an unfaithful predictor of the BM status, and therefore should not be used as trial enrollment criterion. In patients undergoing BM excision, MGMT status may be determined in the first metastasis, but its consistency in subsequent metastases remains to be established. Current knowledge indicates that all patients should be enrolled, and primary tumor MGMT expression should be quantified retrospectively. A higher than 40% MGMT-positivity rate of BM might be factored into statistical calculations of a trial size.
Pharmacologic prevention of BM remains an important goal that is rarely addressed in clinical trials. In small cell lung cancer prophylactic WBRT has been prospectively demonstrated to reduce BM incidence (50). Cognitive decline from WBRT occurs in a proportion of patients and is irreversible, leading to hesitation in using this modality in breast cancer where survival times can be relatively long. Thus, the identification of new BM preventive strategies remains an important goal. We advocate for randomized phase II secondary BM prevention trials to provide initial evidence of a preventive effect (51). Patients with limited numbers of BM, treated with surgery or stereotactic radiosurgery are at high risk for the development of subsequent brain lesions. Such patients could be randomized to placebo or the proposed preventive, in addition to standard systemic therapy. The primary endpoint would be freedom from a new BM, distant from radiosurgical or surgical beds, and other endpoints should include time to whole brain radiotherapy and quality of life, rather than shrinkage of an established lesion. The finding that temozolomide more effectively prevents the outgrowth of a few tumor cells, as opposed to shrinking a lesion containing millions of tumor cells, makes intuitive sense. The number of tumor cells that must be impacted varies. Micrometastases may have a more normal vasculature and peritumoral hydrostatic pressure, both of which facilitate drug delivery. A cytostatic agent may be sufficient to prevent brain colonization, while a cytotoxic agent would be required to shrink an established lesion. There are some hints of clinical BM preventive activity of temozolomide. In advanced melanoma patients, 2 out of 20 patients treated with temozolomide developed BM, as compared with 9 out of 21 treated with DTIC (P = .03) (35). Extensive stable disease was reported in early trials of capecitabine/temozolomide and lapatinib/temozolomide for breast cancer patients with BM (7, 40).
In conclusion, our preclinical study suggests that temozolomide may effectively prevent the outgrowth of BM in high-risk advanced breast cancer patients. These data provide a compelling rationale for BM prevention trials using temozolomide in high-risk advanced breast cancer patients.
Supplementary Material
Statement of Translational Relevance.
Brain metastases of breast cancer are prevalent in metastatic patients with HER2-positive and triple-negative tumors, and contribute to patient morbidity and mortality. Chemotherapy to shrink established brain metastases has been generally ineffective. We present extensive preclinical data demonstrating that the brain-permeable drug temozolomide completely prevented experimental brain metastasis formation in the MDA-MB-231-BR model system over a wide range of doses. Temozolomide failed to shrink established brain metastases. Temozolomide prevention of brain metastasis formation was dependent on low MGMT expression. MGMT expression was determined immunohistochemically in matched sets of primary breast tumors and brain metastases; approximately 60% of resected brain metastases were low in MGMT expression. The data provide evidence to support a clinical trial of temozolomide for the prevention of breast cancer brain metastases.
Acknowledgments
Financial Support: Intramural program of the National Cancer Institute and US Department of Defense Breast Cancer Research Program, Grant Number: W81 XWH-062-0033; Intramural grant of the Medical University of Gdańsk, Poland. Grant number ST-51.
Footnotes
Potential conflicts of interest: PSS receives research funding from Sanofi.
REFERENCES
- 1.Brufsky A, Mayer M, Rugo H, Kaufman P, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with Her2-positive metastatic breast cancer: Incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–43. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 2.Bendell J, Domchek S, Burstein H, Harris L, Younger J, Kuter I, et al. Central Nervous System Metastases in Women who Receive Trastuzumab-Based Therapy for Metastatic Breast Carcinoma. Cancer. 2003;97:2972–7. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 3.Lin N, Claus E, Sohl J, Razzak A, Arnout A, Winer E. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer. High incidence of central nervous system metastases. Cancer. 2008;113:2638–45. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steeg P, Camphausen K, Smith Q. Brain metastases as preventive and therapeutic targets. Nature Rev Cancer. 2011;11:352–63. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosner D, Nemoto T, Lane W. al. e. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832–9. doi: 10.1002/1097-0142(19860815)58:4<832::aid-cncr2820580404>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Boogerd W, Dalesio O, Bais E. al. e. Response of brain metastases from breast cancer to systemic chemotherapy. Cancer. 1992;69:972–80. doi: 10.1002/1097-0142(19920215)69:4<972::aid-cncr2820690423>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Rivera E, Meyers C, Groves M, Valero V, Francis D, Arun B, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases of breast cancer. Cancer. 2006;107:1348–54. doi: 10.1002/cncr.22127. [DOI] [PubMed] [Google Scholar]
- 8.Melisko ME, Kunwar S, Prados M, Berger MS, Park JW. Brain metastases of breast cancer. Expert Rev Anticancer Ther. 2005 Apr;5(2):253–68. doi: 10.1586/14737140.5.2.253. PubMed PMID: 15877523. [DOI] [PubMed] [Google Scholar]
- 9.Walbert T, Gilbert M. The role of chemotherapy in the treatment of patients with brain metastases from solid tumors. Int J Clin Oncol. 2009;14:299–306. doi: 10.1007/s10147-009-0916-1. [DOI] [PubMed] [Google Scholar]
- 10.Lin N, Carey L, Liu M, Younger J, Come S, Bullitt E, et al. Phase II trial of lapatinib for brain metastases in patients with HER2+ breast cancer. J Clin Oncol. 2006;24(18S):503. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin N, Carey L, Liu M, Younger J, Come S, Ewend M, et al. Phase II trial of lapatinib for brain metasetases in patients with human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res. 2008;26(12):1993–9. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockman P, Mittapalli R, Taskar K, Rudraruju V, Gril B, Bohn K, et al. Heterogeneous blood-brain barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin Cancer Res. 2010;16:5662–78. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taskar K, Rudraraju V, Mittapali R, Samala R, Thorsheim H, Lockman J, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29:770–81. doi: 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Percy D, Ribot E, Chen Y, McFadden C, Simedrea C, PS PS, et al. In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol. 2011;46:718–25. doi: 10.1097/RLI.0b013e318226c427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muldoon L, Soussain C, Jahnke K, Johanson C, Siegal T, Smith Q, et al. Chemotherapy delivery issues in central nervous system malignancy: A reality check. J Clin Oncol. 2007;25:2295–305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain M. Anticancer therapies and CNS relapse: overcoming blood-brain and blood-cerebrospinal fluid barrier impermeability. Expert Rev Neuother. 2010;10:547–61. doi: 10.1586/ern.10.14. [DOI] [PubMed] [Google Scholar]
- 17.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and trojan horses. Clin Cancer Res. 2007 Mar;15(6):1663–74. doi: 10.1158/1078-0432.CCR-06-2854. PubMed PMID: 17363519. [DOI] [PubMed] [Google Scholar]
- 18.Lin N, Amiri-Kordestani L, Palmieri D, Liewehr D, Steeg P. CNS metastasis: Old challenge, new frontiers. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0790. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brindley C, Antoni W, Newlands E. Plasma and tissue disposition of mitozolomide in mice. Br J Cancer. 1986;53:91. doi: 10.1038/bjc.1986.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Reilly S, Newlands E, Glaser M, Brampton M, Rice-Edwards J, Illingworth R, et al. Temozolomide: A new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29A:940–2. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- 21.Newlands E, Blackledge G, Slack J, Rustin G, Smith D, Stuart N, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856. Br J Cancer. 1992;65:287–91. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newlands E, Stevens M, Wedge S, Wheelhouse R, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Trt Rev. 1997;23:35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 23.Trudeau M, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada-Clinical Trials Group (NCIC-CTG) Ann Oncol. 2006;17:952–6. doi: 10.1093/annonc/mdl056. [DOI] [PubMed] [Google Scholar]
- 24.Yoneda T, Williams P, Hiraga T, Niewolna M, Nishimura R. A bone seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking cloe in vivo and in vitro. J Bone and Mineral Res. 2001;16:1486–95. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 25.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007 May;1(9):4190–8. doi: 10.1158/0008-5472.CAN-06-3316. PubMed PMID: 17483330. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri D, Lockman P, Thomas F, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer. Clin Cancer Res. 2009;15:6148–57. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008 Aug 6;100(15):1092–103. doi: 10.1093/jnci/djn216. PubMed PMID: 18664652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy P, Friedlander E, Tanner M, Kapanen A, Carraway K, Isola J, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–82. [PubMed] [Google Scholar]
- 29.Ingold B, Schraml P, Heppner F, Moch H. Homogeneous MGMT immunoreactivity correlates with an unmethylated MGMT promoter status in brain metastases of various solid tumors. PloS One. 2009;4:e4775. doi: 10.1371/journal.pone.0004775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirst T, Vesterinen H, Sena E, Egan K, Mcleod M, Whittle I. Systematic review and meta-analysis of temozolomide in animal models of glioma: was clinical efficacy predicted? Br J Cancer. 2013;108:64–71. doi: 10.1038/bjc.2012.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donawho C, Luo Y, Luo Y, Penning T, Bauch J, Bouska J, et al. ABT-888, an orally active Poly (ADP-Ribose) Polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 32.Heyn C, Ronald J, MacKenzie L, MacDonald I, Chambers A, Rutt B, et al. In vivo magnetic resonance imaging of single cells in mouse brains with optical validation. Magnetic Resonance in Medicine. 2006;55(1):23–9. doi: 10.1002/mrm.20747. [DOI] [PubMed] [Google Scholar]
- 33.Koninki K, Barok M, Tanner M, Staff S, Pitkanen J, Hemmila P, et al. Multiple molecular mechanisms underlying trastuzumab and lapatinib resistance in JIMT-1 breast cancer cells. Cancer Lett. 2010;294:211–9. doi: 10.1016/j.canlet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Dziadzusko R, Ardizzoni A, Postmus P, Smit E, Price A, Debruyne C, et al. Temozolomide in patients with advanced non-small cell lung cancer with and without brain metastases: a phase II study of the EORTC LUng Cancer Group (08965) Eur J Cancer. 2003;39:1271–6. doi: 10.1016/s0959-8049(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 35.Paul M, Summers Y, Calvert A, Rustin G, Brampton M, Thatcher N, et al. Effect of temozolomide on central nervous system relapse in patients with advanced melanoma. Melanoma Res. 2002;12:175–8. doi: 10.1097/00008390-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Siena S, Crino L, Danova M, DelPrete S, Cascinu S, Salvagni S, et al. Dose-dense temozolomide regimen for the treatment of brain metastases of melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol. 2010;21:655–61. doi: 10.1093/annonc/mdp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrey L, Olson J, Raizer J, Mack M, Rodavirch A, Boutros D, et al. A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol. 2001;53:259–65. doi: 10.1023/a:1012226718323. [DOI] [PubMed] [Google Scholar]
- 38.Larkin J, Hughes S, Beirne D, Patel P, Gibbens I, Bate S, et al. A phase I/II study of lumustine and temozolomide in aptients with cerebral metastases from malignant melanoma. Br J Cancer. 2007;96:44–8. doi: 10.1038/sj.bjc.6603503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christodoulou C, Bafaloukos D, Linardou H, Aravantinos G, Bamias A, Carina M, et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastses of solid tumors: A Hellenic Cooperative Oncology Group (HeCOG) phase II study, J Neurooncol. 2005;71:61–5. doi: 10.1007/s11060-004-9176-0. [DOI] [PubMed] [Google Scholar]
- 40.Azambuja Ed, Zardavas D, Lemort M, Rossari J, Moulin C, Buttice A, et al. Phase I trial combining temozolomide plus lapatinib for the treatment of brain metastases in patients with HER2-positive metastatic breast cancer: the LAPTEM trial. Ann Oncol. 2013;24:1–5. doi: 10.1093/annonc/mdt359. [DOI] [PubMed] [Google Scholar]
- 41.Verger E, Gil M, Yaya R, Vinolas N, Villa S, Pujol T, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Rad Oncol Biol Physics. 2005;61:185–91. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 42.Chua D, Krzakowski M, Chouaid C, Pallotta M, Martinez J, Gottfried M, et al. Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from Non-small-cell lung cancer: A randomized, open-label phase II study. Clin Lung Cancer. 2010;11:176–81. doi: 10.3816/CLC.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 43.Addeo R, Rosa CD, Faiola V, Leo L, Cennamo G, MOntella L, et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer. 2008;113:2524–31. doi: 10.1002/cncr.23859. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald D, Palmieri D, Hua E, Hargrave E, Herring J, Qian Y, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metast. 2008;25(7):799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp J, Bouffet E, Stempak D, Gammon J, Stephens D, Johnston D, et al. A multi-centre Canadian pilot study of metronomic temozolomide combined with radiotherapy for newly diagnosed paediatric brainstem glioma. Eur J Cancer. 2010;46:3271–9. doi: 10.1016/j.ejca.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 46.Pouratian N, Gasco J, Sherman J, Shaffrey M, Schiff D. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol. 2007;82:281–8. doi: 10.1007/s11060-006-9280-4. [DOI] [PubMed] [Google Scholar]
- 47.Lashkari H, Saso S, Moreno L, Athanasiou T, Zacharoulis S. Using different schedules of temozolomide to treat low grade gliomas: systematic review of their efficacy and toxicity. J Neurooncol. 2011;105:135–47. doi: 10.1007/s11060-011-0657-7. [DOI] [PubMed] [Google Scholar]
- 48.Karayan-Tapon L, VQuillien, Guilhot J, Wagner M, Fromont G, Saikali S, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J, Neurooncol. 2010;97:311–22. doi: 10.1007/s11060-009-0031-1. [DOI] [PubMed] [Google Scholar]
- 49.Christmann M, Nagel G, Horn S, Krahn U, Wiewrodt D, Sommer C, et al. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int J Cancer. 2010;127:2106–18. doi: 10.1002/ijc.25229. [DOI] [PubMed] [Google Scholar]
- 50.Slotman B, Faive-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. New Engl J Med. 2007;357:664–72. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 51.Steeg P. The right trials. Nature. 2012;485:S58–9. doi: 10.1038/485S58a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.