Abstract
Background
Chronic granulomatous disease (CGD) is a rare inherited disease of the phagocyte NADPH oxidase system that causes defective production of toxic oxygen metabolites, impaired bacterial and fungal killing, and recurrent life-threatening infections, mostly by catalase-producing organisms. We report for the first time, to our knowledge, chronic infections with Actinomyces species in 10 patients with CGD. Actinomycosis is a chronic granulomatous condition that commonly manifests as cervicofacial, pulmonary, or abdominal disease, caused by slowly progressive infection with oral and gastrointestinal commensal Actinomyces species. Treatment of actinomycosis is usually simple in immunocompetent individuals, requiring long-term, high-dose intravenous penicillin, but is more complicated in those with CGD because of delayed diagnosis and an increased risk of chronic invasive or debilitating disease.
Methods
Actinomyces was identified by culture, staining, 16S ribosomal DNA polymerase chain reaction, and/ or a complement fixation test in 10 patients with CGD.
Results
All 10 patients presented with a history of fever and elevated inflammatory signs without evident focus. Diagnosis was delayed and clinical course severe and protracted despite high-dose intravenous antibiotic therapy and/or surgery. These results suggest an unrecognized and unanticipated susceptibility to weakly pathogenic Actinomyces species in patients with CGD because these are catalase-negative organisms previously thought to be nonpathogenic in CGD.
Conclusions
Actinomycosis should be vigorously sought and promptly treated in patients with CGD presenting with uncommon and prolonged clinical signs of infection. Actinomycosis is a catalase-negative infection important to consider in CGD.
Patients with chronic granulomatous disease (CGD) develop recurrent life-threatening infections and tissue granulomata [1, 2]. Bacteria and fungi that commonly cause infection in CGD include Staphylococcus aureus, Serratia marcescens, Burkholderia cepacia complex, Nocardia species, and Aspergillus species. Rare infections with organisms encountered almost exclusively in CGD, such as Paecilomyces species, Trichosporon inkin, and Granulibacter bethesdensis [3], suggest that patients with CGD have a unique susceptibility pattern that has been thought previously to depend on microbial catalase for virulence [1].
The genus Actinomyces consists of a heterogeneous group of gram-positive, non–spore-forming, catalase-negative pleomorphic rods [4] that form a significant component of the commensal microflora of the oral, gastrointestinal, and female genital tracts and that are generally of low pathogenicity [5]. Actinomyces species may invade via damaged mucosa, leading to bacteremia and systemic infections [4] in both healthy individuals [6, 7] and immunocompromised patients [8–10]. Diagnosis of Actinomyces infection is often delayed because of nonspecific and prolonged symptoms, resulting in development of chronic debilitating disease.
Actinomycosis has not been reported previously in CGD, except for 1 patient with pulmonary Actinomyces israelii mentioned in passing [11]. However, in this report we identify 10 severe chronic recurrent infections with Actinomyces species from 3 immunodeficiency centers (National Institutes of Health in Bethesda, Maryland; Paris, France; and Zurich, Switzerland), representing each of the major localizations of human actinomycosis, clearly demonstrating that actinomycosis is a significant and previously unrecognized problem in CGD.
CASE REPORTS
Patient consent
Written informed consent was obtained from all patients and/or parents.
Patient 1
Patient 1 was a 23-year-old Slovenian man with X-linked CGD. He had a history of recurrent, cervical, fistulizing lymphadenitis (only Corynebacterium aquaticum isolated from culture samples) at the age of 19 years and a painless submandibular abscess at the age of 22 years. He developed a painless, tender swelling fixed to adjacent tissues. The lesion was drained and culture yielded Actinomyces naeslundii. Treatment with intravenous ceftriaxone did not lead to complete resolution, despite surgical excision. The patient underwent HLA-genoidentical bone marrow transplantation (BMT) at the age of 23 years in Switzerland. Positron emission tomography– computed tomography (CT) before BMT showed active inflammation in several lymph nodes of the right cervical region, still present 1 month after BMT with full engraftment. Clinically, the lesion resolved completely after 1 month of treatment with intravenous meropenem, when therapy was changed to oral penicillin for 3 months.
Patient 2
Patient 2 was a 6.5-year-old French boy with X-linked CGD. He had a history of Serratia marcescens septicemia at the age of 2 years, with hepatic, splenic, and lung abscesses, pulmonary aspergillosis at the age of 3 years, and undiagnosed severe bilateral pneumonitis despite lung biopsy at the age of 4.5 years. He was identified as having cervical A. naeslundii infection. The patient presented with a tender, cervical fistulizing abscess while receiving cotrimoxazole and itraconazole prophylaxis. Culture from the biopsy yielded A. naeslundii. He was treated orally with amoxicillin, azithromycin, and clindamycin for 1 year and fully recovered.
Patient 3
Patient 3 was a 28-year-old Hispanic woman born and raised in the United States with p22phox-deficient, autosomal-recessive CGD. She had a history significant for multiple staphylococcal liver abscesses and cervical A. naeslundii lymphadenitis requiring adenectomy. She was admitted with fever and a moderate cough. A CT showed multiple new lung, liver, and tubo-ovarian abscesses. Fine-needle aspirations of multiple abscesses yielded Actinomyces gerensceriae. She underwent radiofrequency ablation of her liver abscesses and received intravenous penicillin G (20 million U per day), which caused her multiple abscesses to diminish. She received intravenous antibiotics for 3 months with steady improvement. She continued to take oral azithromycin for the next 21 months without recurrence.
Patient 4
Patient 4 was a 19-year-old white woman from the United States with highly lyonized X-linked CGD and cutaneous lupus. She developed hoarseness and tender, enlarging bilateral cervical lymphadenopathy. The cervical lymphadenopathy was from an infected, necrotizing, cervical lymph node. After minimal response to combined treatment with levofloxacin, vancomycin, and itraconazole, bilateral cervical lymphadenectomy yielded actinomycosis (not further speciated). The patient’s regimen was changed to vancomycin, imipenem, and clindamycin. After the patient developed leukopenia 2 weeks into therapy, her antibiotics were changed from imipenem to intravenous penicillin G (4 million U every 4 h), which she tolerated well. The patient was discharged home with a prescription for oral clindamycin and intravenous penicillin and her condition resolved.
Patient 5
Patient 5 was a 13-year-old African American boy with X-linked CGD diagnosed at 6 weeks of age. He had persistent colitis and recurrent fungal pulmonary infections, requiring multiple pulmonary resections. He developed new cervical lymphadenopathy while he had right lower-lobe pneumonia. The cervical adenopathy had CT attenuation characteristics consistent with necrosis. Lymphadenectomy cultures yielded Burkholderia gladioli, Streptococcus mitis, and Actinomyces species. Dental evaluation found a right upper tooth with significant decay, which was removed. He was subsequently stable and continued to take meropenem for 3 weeks until discharge. The patient’s cervical lymphadenopathy was resolved 1 month later.
Patient 6
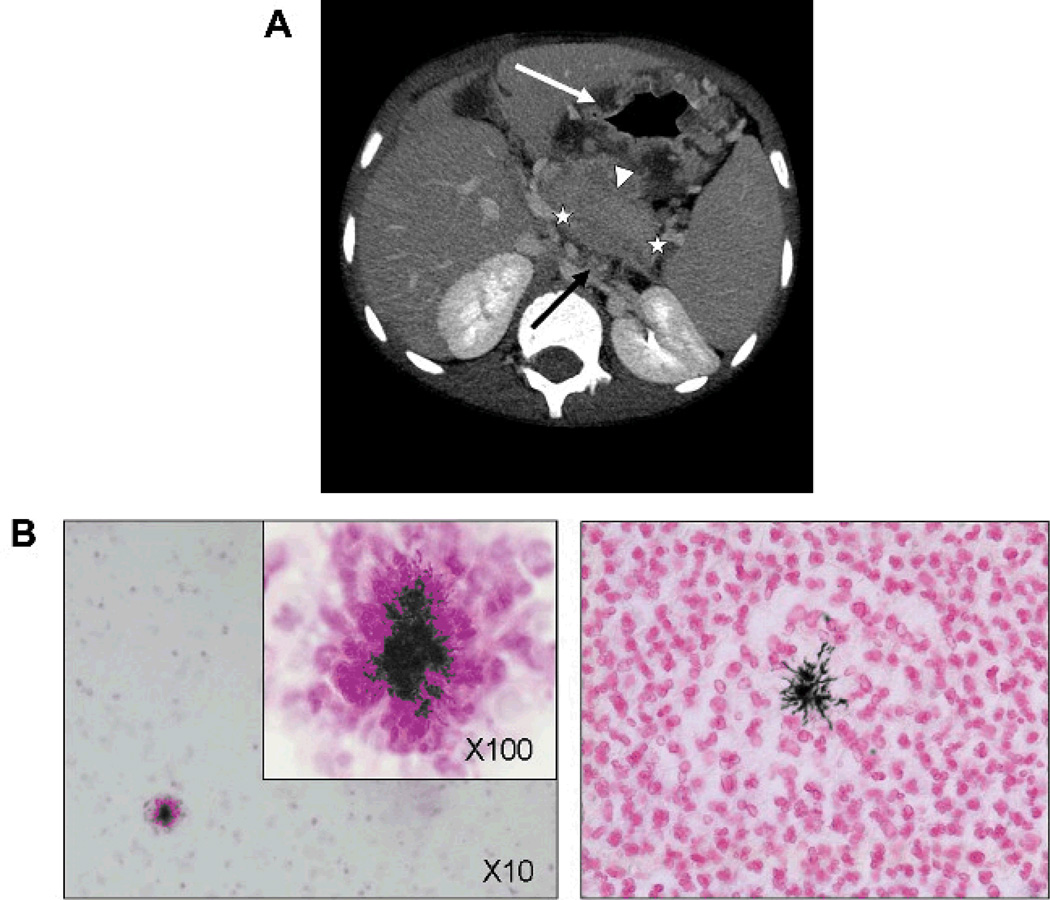
Patient 6 was a 7-year-old boy of French-Algerian origin with X-linked CGD. He was diagnosed as having a hepatic abscess (Figure 1A) and a retrocardiac infiltration of the left lung. The results of ultrasonography-guided liver puncture were positive for A. naeslundii by culture and 16S ribosomal DNA (rDNA) sequencing with typical histologic findings (Figure 1B). Despite intravenous amoxicillin, amikacin, and ciprofloxacin guided by in vitro susceptibility, hepatic lesions and fever persisted. Treatment was changed to intravenous ciprofloxacin, imipenem, and vancomycin for 70 days, leading to partial improvement. For a persistent hepatic mass, he received repeated granulocyte transfusions and surgical removal of the abscess. He gradually recovered while taking oral amoxicillin and clarithromycin for 6 months. At the age of 8 years, he was admitted to the hospital with vomiting, epigastric pain, and weight loss. Abdominal ultrasonography and CT showed typical signs of actinomycosis (Figure 1A), but culture from a biopsy of the gastric wall and 16S rDNA polymerase chain reaction (PCR) results were negative. Treatment with corticosteroids (1 mg/kg daily), intravenous clindamycin (45 mg/kg daily), and penicillin G (470,000 IU/kg daily) for 15 days, followed by oral clindamycin and amoxicillin, led to clinical improvement. Eight months later, the disease recurred and gradually improved with treatment with intravenous amoxicillin and clindamycin for 33 days and monitored plasma levels of oral azithromycin. The boy is now well while receiving oral prophylaxis with azithromycin, amoxicillin, and clindamycin.
Figure 1.
A, Radiologic signs of abdominal actinomycosis on a computed tomographic (CT) scan (patient 6). Abdominal ultrasonography revealed segmentary portal hypertension and perigastric and esophageal varices (not shown); CT scan showed splenomegaly and thrombosis of the splenic vein (extension delimited by white stars), multiple mesenteric lymph nodes (black arrow), gastric wall thickening (white arrow), and a peripancreatic mass (white arrowhead). B, Histologic analysis of abdominal actinomycosis (patient 6). Liver histologic analysis after liver puncture in patient 6 showed extensive parenchymal necrosis and multiple abscesses with filamentous gram-positive bacteria within the granule surrounded by neutrophil “clubbing” cells typical for actinomycosis (original magnification, x10 and x100; Gram stain on the left and Grocott stain on the right).
Patient 7
Patient 7 was a 16-year-old Slovenian boy with X-linked CGD who developed fever, chills, and increasing pain in the right upper abdomen while under irregular treatment with interferon-g. Ultrasonography revealed a solitary lesion in the right liver lobe; a histologic specimen taken during surgery showed intense neutrophil infiltration and congestion of the adjacent sinusoids. Cefotaxime, cloxacillin, and metronidazole for 2 weeks did not lead to resolution. Ultrasonography-guided drainage of the abscess yielded Actinomyces meyeri. Treatment with intravenous penicillin G was given for 2 months, leading to complete regression of the lesion. The patient was then transitioned to oral penicillin V for 1 year, without evidence of recurrence.
Patient 8
Patient 8 was a 38-year-old, white, American man with X-linked CGD diagnosed at 6 years of age. He had had multiple liver abscesses, pneumonias, and severe chronic obstructive pulmonary disease. Multiple liver abscesses were found to harbor A. naeslundii. Six months of amoxicillin therapy led to resolution. However, when hepatic abscesses recurred, he was treated with multiple intravenous regimens (including piperacillin-tazobactam, rifampicin, linezolid, penicillin) with minimal benefit. Additional liver biopsy specimens revealed only degenerating gram-positive debris. Radiofrequency ablation of the persistent abscesses was tolerated well. Six months later he underwent radiofrequency ablation of a different deep hepatic abscess, after which his lesions remained stable for a year. During that time his oral regimen consisted primarily of double-strength trimethoprim-sulfamethoxazole twice per day and voriconazole. He has remained free of recurrence for 3 years.
Patient 9
Patient 9 was a 10-year-old Saudi Arabian boy with p22phox-deficient, autosomal-recessive CGD. He had had severe recurrent lymphadenitis, pneumonia and septicemia, osteomyelitis, and meningitis with cerebral aneurysm and brain infarction due to Aspergillus fumigatus. During evaluation for hematopoietic stem cell transplantation, a CT scan showed erosion of the fifth left posterior rib and pulmonary lesions suggestive of aspergillosis. Examination of an open-lung biopsy specimen demonstrated granulomatous lesions with microabscesses and a polymorphic inflammatory infiltrate with clustered histiocytes and multinucleated giant cells. No pathogen was identified by staining, culture, or PCR of the abscess fluid. Treatment with voriconazole and caspofungin was ineffective. With the appearance of new inflammatory lesions in the left upper lobe and cervical lymphadenitis, additional complement fixation tests (CFTs) for A. naeslundii produced positive results (titers for patients, 1:80–1:160; for controls, 1:20–1:40). 5-Flucytosine, interferon-g, and granulocyte transfusions led to slow resolution of the lesions. The patient underwent HLA-genoidentical hematopoietic stem cell transplantation at the age of 10 years and has remained healthy since.
Patient 10
Patient 10 was a 3-year-old Swiss boy with X-linked CGD diagnosed at 8 months of age who had had recurrent cervical lymph node abscesses and pneumonias. He was diagnosed as having A. naeslundii lymphadenitis by culture from a lymph node biopsy. Actinomyces species had been isolated before by culture from bronchoalveolar lavage fluid at the age of 2 years, but both results were interpreted as contamination. A. naeslundii was isolated again at the age of 7 years by culture from a transthoracic biopsy specimen taken because of persistent pneumonia. After 3 weeks treatment with intravenous clindamycin, minocycline, and ciprofloxacin, followed by oral minocycline and ciprofloxacin, he stabilized, but an open lung biopsy specimen at the age of 7 years showed persistent infection. After 3 months of intravenous ceftriaxone and teicoplanin, followed by 9 months of oral penicillin and trovafloxacin, A. naeslundii has never been reisolated, neither by culture from a mediastinal lymph node biopsy specimen taken at the age of 8 years nor by culture from pleural effusion punctures performed when the patient was 9 years old. Persisting actinomycosis is supported by positive A. naeslundii CFT results in serum from the ages of 4 years through 10 years (titers for patient, 1:320–1:640; for controls, 1:20–1:40). Chest x-ray films and high-resolution CT scans showed typical signs of actinomycosis (Figure 2A); positron emission tomography confirmed active inflammation (Figure 2B). The patient died of chronic renal insufficiency, likely due to prolonged treatment with amphotericin B until the age of 6 years for suspected chronic invasive A. fumigatus pneumonia, and cardiorespiratory failure at 15 years. No autopsy was performed.
Figure 2.
Radiologic signs of pulmonary actinomycosis (patient 10). A, High-resolution computed tomographic (CT) scan showing (1) focal consolidation, mediastinal and hilar lymph node enlargement, pleural effusion, and granulomatous lesions with partial calcification and (2) diffuse interstitial infiltration from the age of 7 years on. B, Representative positron emission tomographic–CT scan confirming infectious foci with active inflammation (encircled).
METHODS
Culture of Actinomyces
Actinomyces grows best anaerobically, although many strains are microaerophilic. Specimens were obtained, transported, and cultured anaerobically on semiselective media. All strains were grown at 37°C on sheep blood agar plates with carbon dioxide added. Hematoxylin-eosin stain was performed according to standard procedures.
16S rDNA PCR
16S rDNA PCR was performed as previously described [12, 13].
Actinomyces serologic testing (CFTs)
CFTs were performed at University Hospital Zurich for patients 9 and 10. The bacterial isolate was identified by conventional methods [14]. A crude antigen of the A. naeslundii isolate from the patient was prepared as described [15] and used to determine the serologic response by complement fixation. A serum sample from a patient with infection of a hip prosthesis by A. naeslundii was used as a positive control [16]. Serum samples from the virology routine laboratory were used as negative controls.
RESULTS
Clinical and therapeutic features of the patients are summarized in Table 1. Survival was good overall, with 9 of the 10 patients successfully treated for their infections. Although 3 of the 9 had apparent recurrences of infection, they were successfully treated again. The 2 patients who underwent BMT had neither flare nor progression of their Actinomyces infections. Therefore, actinomycosis is largely survivable in CGD and controllable during BMT. The patient who died had end-organ damage from long-term use of amphotericin B, given to treat the radiographic appearance of fungal infection.
Table 1.
Patient Characteristics and Treatment of Actinomycosis
| Patient | Age, years |
Sex | Genotype | Age at diagnosis |
Site of actinomycosis | Therapya | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 23 | M | gp91phox | 7 years | Cervical/submandibular lymph nodes | Intravenous ceftriaxone-meropenem, penicillin V, BMT | Yes | Survived, no recurrence |
| 2 | 6 | M | gp91phox | 2 years | Cervical lymph node | Oral amoxicillin-azithromycin-clindamycin | Yes | Survived, no recurrence |
| 3 | 28 | F | p22phox | 6 months | Cervical lymph node, lung/liver abscess | Intravenous penicillin G, oral azithromycin | RFA | Survived, no recurrence |
| 4 | 19 | F | gp91phox | 12 years | Cervical lymph nodes | Intravenous vancomycin-imipenem-clindamycin, oral clindamy- cinintravenous penicillin G | Yes | Survived, no recurrence |
| 5 | 13 | M | gp91phox | 6 weeks | Cervical lymph node | Intravenous meropenem | Yes | Survived, no recurrence |
| 6 | 10 | M | gp91phox | 7 years | Liver | Granulocyte transfusions, oral amoxi-cillin-clarithromycin, corticosteroids, intravenous amoxicillin-clindamy-cin, azithromycin | Yes | Survived, recurrence |
| 7 | 16 | M | gp91phox | 1 year | Liver | Ultrasonography-guided drainage, in- terferon-g, intravenous penicillin G, penicillin V | Yes | Survived, no recurrence |
| 8 | 38 | M | gp91phox | 6 years | Liver | Oral amoxicillin, intravenous tri-methoprim-sulfamethoxazole, intravenous voriconazole | RFA | Survived, recurrence |
| 9 | 10 | M | p22phox | 1 year | Lung, cervical lymph node | BMT, granulocyte transfusions, 5-flucytosine, interferon gamma | No | Survived, no recurrence |
| 10 | 2 | M | gp91phox | 8 months | Lung, cervical lymph node | Intravenous clindamy cin-minocycline-ciprofloxacin, oral minocycline-cipro-floxacin, intravenous ceftriaxone-teicoplanin, oral penicillin-trovafloxacin | Yes | Recurrence, died |
NOTE. BMT, bone marrow transplantation; RFA, radiofrequency ablation.
Items in boldface type indicate that the antibiotic therapy was apparently effective against infection with Actinomyces species.
All of our actinomycosis patients from the United States, Europe, and the Middle East have defects in the cytochrome B component of the NADPH oxidase. The X-linked form of CGD accounts for <65%–70% of cases, whereas the p22phox-deficient form accounts for less than 5% [1, 2]. The absence of any cases of p47phox deficiency, the most common form of autosomal-recessive CGD, suggests an important role for cytochrome B function in Actinomyces infections.
Surgery was associated with successful treatment in this series, as suggested in previous series [17–19]. In several patients (patients 3, 4, and 8), extensive medical management failed before definitive surgery or infection ablation.
DISCUSSION
Actinomyces species belong to the oral commensal flora and become pathogenic only under certain conditions. They colonize the human oral cavity in early childhood, and the prevalence rates of total Actinomycetes flora increase from 31% to 97% within the first 2 years of life [20]. Actinomyces species reside in periodontal pockets, carious teeth, dental plaque, and tonsillar crypts [21] and take advantage of infection, trauma, or surgical injury to penetrate mucosal barriers [22]. Diagnosis of actinomycosis is especially challenging in CGD because patients frequently do not have the typical symptoms and signs of infection. In addition, most clinical signs of Actinomyces infection are nonspecific. Actinomyces is fastidious, requiring ideal growth conditions; only <50% of reported cases have positive cultures [23]. Infection is frequently polymicrobial in otherwise healthy individuals, with Actinobacillus actinomyce-temcomitans being the most common associated pathogen. Other copathogens include Fusobacterium, Bacteroides, Haemophilus, Eikenella, Staphylococcus, Streptococcus, Gemella, Neisseria, and Eubacterium species [24]. Definitive diagnosis rests on isolating the organism from tissue or pus from a normally sterile body site.
Most infections in immunocompetent individuals are cervicofacial [18, 25]. Intrathoracic or abdominal disease is rare [24, 26–31]. Cervicofacial infection may follow any injury to the oral mucosa, even the simple eruption of a tooth [22], and characteristically spreads by direct invasion of adjacent tissues. Symptoms range from painless, slowly enlarging, fluctuant lesions (patients 1–3) to acute painful induration and widespread infection. Nodular lesions complicated by draining sinus tracts may extend through overlying skin or to adjacent bones or tissues.
Hepatic disease has been reported in 15% of immunocompetent patients with abdominal actinomycosis, but only <7% of primary hepatic actinomycosis cases are children [32, 33]. Hematogenous spread may occur through the portal vein from a mucosal lesion or injury or from another abdominal focus of infection [18]. In patients 3 and 6, fever and coughing associated with pulmonary infiltrates preceded the detection of the hepatic abscess; pulmonary infiltrates suggest hematogenous spread from these lesions. The most common presenting manifestations of abdominal actinomycosis are fever, abdominal pain, nausea, vomiting, and weight loss (patients 6–8). Hepatic lesions may extend by direct contiguity to penetrate the abdominal wall, the diaphragm, adjacent viscera, or retroperitoneal tissues [22]. Jaundice is rare, and liver function test results may be variable. Intrahepatic masses may mimic neoplasm, liver abscesses, or miliary disease. Radiofrequency ablation of the infected hepatic abscesses was performed in patients 3 and 8 because of their high risk for further hepatic surgery.
Pulmonary actinomycosis is extremely rare in childhood, with only 47 cases reported in the past 25 years in the English-language literature [24]. It usually occurs after inhalation or aspiration of endogenous microorganisms of the oropharynx in immunocompetent persons with poor oral hygiene or from cervicofacial extension [34]. The usual presentation is a mass in the mediastinum or lung, but it can also manifest as diffuse or local pneumonia, pleural empyema, or an endobronchial mass. Rib involvement is common, with Actinomyces species being able to induce bone resorption [18]. Most individuals present with cough, chest wall pain, weight loss, and fever (patients 3 and 10). Less commonly patients may have hemoptysis [35] or superior vena cava syndrome [18]. Draining sinuses have been reported in up to 11% of cases [24] but were absent in our patients. Pulmonary actinomycosis has no specific clinical or radiologic findings and closely mimics tuberculosis, lung abscess, tumor, fungal infections, and pulmonary infarctions [36]. Patients with CGD may also have pulmonary granulomatous lesions or fungal infections, as suspected and empirically treated in patient 10. The most common CT findings include central necrosis, cavities, mediastinal or hilar lymphadenopathy, endobronchial calcified nodules, and pleural effusion, all of which were present in patient 10. He also showed localized bronchiectasis, irregular bronchial wall thickening, and irregular peribronchial consolidation with abscess formation.
The gold standard for diagnosis of actinomycosis is isolation and culture from a usually sterile body site. Molecular identification by 16S PCR gives another level of certainty and allows definitive speciation [12, 13, 37, 38]. Unfortunately, in patient 9, only serologic testing revealed the presence of Actinomyces species. Because Actinomyces species colonize skin and mucosa even in healthy individuals, isolation from nonsterile body sites is not sufficient for proof of infection. Therefore, detection of Actinomyces species in bronchoalveolar lavage was confirmed by open-lung biopsy in patient 10.
Two principles of actinomycosis treatment have evolved during the past 50 years. The disease should be treated with high doses of antimicrobials for a prolonged period [18, 39] because of the avascularity and induration of infected areas. We use intravenous treatment for 2–6 weeks followed by oral therapy for at least 6–12 months. The antibiotic of choice is penicillin. In cases of allergy or nonresponse, alternatives include erythromycin, tetracycline, clindamycin, cephalosporins, meropenem, and chloramphenicol [18, 40]. Frequent coinfections with other microbes make combination therapy advisable.
The susceptibility of patients with CGD to infection with catalase-negative Actinomyces species confirms that catalase production is neither necessary nor sufficient for microbial virulence in CGD [41–43]. The recent recognition of the central role of superoxide in potassium flux into the phagolysosome and potassium’s role in activating killing explain the lack of importance of microbial hydrogen peroxide and, therefore, catalase in the pathogenesis of infections in CGD [44]. Infections in CGD are caused by a subset of catalase-producing pathogens, which are thought to deprive the CGD phagocyte of the opportunity to use microbial-derived oxidative metabolites [1] by scavenging their own hydrogen peroxide via the production of catalase. However, most catalase-positive organisms do not cause disease in CGD [44]. The addition of this previously recognized uncommon human pathogen to the CGD infection list will allow better characterization of infection susceptibility in CGD. To prevent the chronic debilitating effects of inadequately treated infection and the unnecessary toxic effects of improper anti-infectious treatment, a high level of vigilance for Actinomyces infection is necessary in patients with CGD. The clinical manifestations, sites, and outcome of infection with Actinomyces species in other patients with CGD are important subjects for future investigation.
Acknowledgments
We thank the patients and their families and deeply respect how they cope with severe or fatal disease.
Financial support. This work was supported in part by a grant from the Chronic Granulomatous Disorder Research Trust of the United Kingdom and a Forschungskredit der Universität Zürich 2006 grant (J.R.) and in part by the Division of Intramural Research, National Institute for Allergy and Infectious Diseases, National Institutes of Health (U.L., S.M.H.).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Winkelstein JA, Marino MC, Johnston RB, Jr, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg DE, Ding L, Zelazny AM, et al. A novel bacterium associated with lymphadenitis in a patient with chronic granulomatous disease. PLoS Pathog. 2006;2:e28. doi: 10.1371/journal.ppat.0020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarridge JE, 3rd, Zhang Q. Genotypic diversity of clinical Actinomyces species: phenotype, source, and disease correlation among genospecies. J Clin Microbiol. 2002;40:3442–3448. doi: 10.1128/JCM.40.9.3442-3448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brailsford SR, Lynch E, Beighton D. The isolation of Actinomyces naeslundii from sound root surfaces and root carious lesions. Caries Res. 1998;32:100–106. doi: 10.1159/000016438. [DOI] [PubMed] [Google Scholar]
- 6.Colmegna I, Rodriguez-Barradas M, Rauch R, Clarridge J, Young EJ. Disseminated Actinomyces meyeri infection resembling lung cancer with brain metastases. Am J Med Sci. 2003;326:152–155. doi: 10.1097/00000441-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Dobson SR, Edwards MS. Extensive Actinomyces naeslundii infection in a child. J Clin Microbiol. 1987;25:1327–1329. doi: 10.1128/jcm.25.7.1327-1329.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora AK, Nord J, Olofinlade O, Javors B. Esophageal actinomycosis: a case report and review of the literature. Dysphagia. 2003;18:27–31. doi: 10.1007/s00455-002-0080-5. [DOI] [PubMed] [Google Scholar]
- 9.Nadal HM, Munoz M. Actinomycosis of the liver. Bol Asoc Med P R. 1965;57:225–229. [PubMed] [Google Scholar]
- 10.Roesler PJ, Jr, Wills JS. Hepatic actinomycosis: CT features. J Comput Assist Tomogr. 1986;10:335–337. doi: 10.1097/00004728-198603000-00035. [DOI] [PubMed] [Google Scholar]
- 11.von Goessel H, Hossle JP, Seger R, Gungor T. Characterization of 17 new cases of X-linked chronic granulomatous disease with seven novel mutations in the CYBB gene. Exp Hematol. 2006;34:528–535. doi: 10.1016/j.exphem.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Dalwai F, Spratt DA, Pratten J. Use of quantitative PCR and culture methods to characterize ecological flux in bacterial biofilms. J Clin Microbiol. 2007;45:3072–3076. doi: 10.1128/JCM.01131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, England: Wiley and Sons; 1996. pp. 115–175. [Google Scholar]
- 14.von Graevenitz A, Funke G. An identification scheme for rapidly and aerobically growing gram-positive rods. Zentralbl Bakteriol. 1996;284:246–254. doi: 10.1016/s0934-8840(96)80100-9. [DOI] [PubMed] [Google Scholar]
- 15.Zbinden R, Hany A, Luthy R, Conen D, Heinzer I. Antibody response in six HACEK endocarditis cases under therapy. Apmis. 1998;106:547–552. doi: 10.1111/j.1699-0463.1998.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 16.Wust J, Steiger U, Vuong H, Zbinden R. Infection of a hip prosthesis by Actinomyces naeslundii. J Clin Microbiol. 2000;38:929–930. doi: 10.1128/jcm.38.2.929-930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J. 2003;21:545–551. doi: 10.1183/09031936.03.00089103. [DOI] [PubMed] [Google Scholar]
- 18.Smego RA, Jr, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255–1261. doi: 10.1086/516337. [DOI] [PubMed] [Google Scholar]
- 19.Yildiz O, Doganay M. Actinomycoses and Nocardia pulmonary infections. Curr Opin Pulm Med. 2006;12:228–234. doi: 10.1097/01.mcp.0000219273.57933.48. [DOI] [PubMed] [Google Scholar]
- 20.Sarkonen N, Kononen E, Summanen P, Kanervo A, Takala A, Jousimies-Somer H. Oral colonization with Actinomyces species in infants by two years of age. J Dent Res. 2000;79:864–867. doi: 10.1177/00220345000790031301. [DOI] [PubMed] [Google Scholar]
- 21.Bowden GH, Nolette N, Ryding H, Cleghorn BM. The diversity and distribution of the predominant ribotypes of Actinomyces naeslundii genospecies 1 and 2 in samples from enamel and from healthy and carious root surfaces of teeth. J Dent Res. 1999;78:1800–1809. doi: 10.1177/00220345990780120601. [DOI] [PubMed] [Google Scholar]
- 22.Lerner PI. Actinomyces and Arachnia species. In: Mandell LA, Douglas SD, Bennett BL, editors. Principles and practice of infectious diseases. 3rd ed. Vol. 1. New York, NY: Churchill Livingston; 1990. pp. 1932–1942. [Google Scholar]
- 23.Burden P. Actinomycosis. J Infect. 1989;19:95–99. doi: 10.1016/s0163-4453(89)91739-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee JP, Rudoy R. Pediatric thoracic actinomycosis. Hawaii Med J. 2003;62:30–32. [PubMed] [Google Scholar]
- 25.Pulverer G, Schutt-Gerowitt H, Schaal KP. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis. 2003;37:490–497. doi: 10.1086/376621. [DOI] [PubMed] [Google Scholar]
- 26.Alborzi A, Pasyar N, Nasiri J. Actinomycosis as a neglected diagnosis of mediastinal mass. Jpn J Infect Dis. 2006;59:52–53. [PubMed] [Google Scholar]
- 27.Buyukavci M, Caner I, Eren S, Aktas O, Akdag R. A childhood case of primary hepatic actinomycosis presenting with cutaneous fistula. Scand J Infect Dis. 2004;36:62–63. doi: 10.1080/00365540310017492. [DOI] [PubMed] [Google Scholar]
- 28.Latawiec-Mazurkiewicz I, Juszkiewicz P, Pacanowski J, et al. Tumourlike inflammatory abdominal conditions in children. Eur J Pediatr Surg. 2005;15:38–43. doi: 10.1055/s-2004-830544. [DOI] [PubMed] [Google Scholar]
- 29.Lin TP, Fu LS, Peng HC, Lee T, Chen JT, Chi CS. Intra-abdominal actinomycosis with hepatic pseudotumor and xanthogranulomatous pyelonephritis in a 6-y-old boy. Scand J Infect Dis. 2001;33(7):551–553. doi: 10.1080/00365540110026647. [DOI] [PubMed] [Google Scholar]
- 30.Pinarli FG, Mutlu B, Celenk C, et al. Pulmonary actinomycosis mimicking chest wall tumor in a child. Jpn J Infect Dis. 2005;58:247–249. [PubMed] [Google Scholar]
- 31.Wilson DC, Redmond AO. An unusual cause of thoracic mass. Arch Dis Child. 1990;65:991–992. doi: 10.1136/adc.65.9.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma M, Briski LE, Khatib R. Hepatic actinomycosis: an overview of salient features and outcome of therapy. Scand J Infect Dis. 2002;34:386–391. doi: 10.1080/00365540110080304. [DOI] [PubMed] [Google Scholar]
- 33.Christodoulou N, Papadakis I, Velegrakis M. Actinomycotic liver abscess: case report and review of the literature. Chir Ital. 2004;56:141–146. [PubMed] [Google Scholar]
- 34.Kim TS, Han J, Koh WJ, et al. Thoracic actinomycosis: CT features with histopathologic correlation. AJR Am J Roentgenol. 2006;186:225–231. doi: 10.2214/AJR.04.1749. [DOI] [PubMed] [Google Scholar]
- 35.Lu MS, Liu HP, Yeh CH, et al. The role of surgery in hemoptysis caused by thoracic actinomycosis: a forgotten disease. Eur J Cardiothorac Surg. 2003;24:694–698. doi: 10.1016/s1010-7940(03)00515-3. [DOI] [PubMed] [Google Scholar]
- 36.Alves dos Santos JW, Torres A, Michel GT, et al. Non-infectious and unusual infectious mimics of community-acquired pneumonia. Respir Med. 2004;98:488–494. doi: 10.1016/j.rmed.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Custal-Teixidor M, Trull-Gimbernat JM, Garijo-Lopez G, Valldosera-Rosello M. Fine-needle aspiration cytology in the diagnosis of cervicofacial actinomycosis: report of 15 cases. Med Oral Patol Oral Cir Bucal. 2004;9:467–470. [PubMed] [Google Scholar]
- 38.Oostman O, Smego RA. Cervicofacial actinomycosis: diagnosis and management. Curr Infect Dis Rep. 2005;7:170–174. doi: 10.1007/s11908-005-0030-0. [DOI] [PubMed] [Google Scholar]
- 39.Sudhakar SS, Ross JJ. Short-term treatment of actinomycosis: two cases and a review. Clin Infect Dis. 2004;38:444–447. doi: 10.1086/381099. [DOI] [PubMed] [Google Scholar]
- 40.Choi J, Koh WJ, Kim TS, et al. Optimal duration of IV and oral antibiotics in the treatment of thoracic actinomycosis. Chest. 2005;128:2211–2217. doi: 10.1378/chest.128.4.2211. [DOI] [PubMed] [Google Scholar]
- 41.Chang YC, Segal BH, Holland SM, Miller GF, Kwon-Chung KJ. Virulence of catalase-deficient aspergillus nidulans in p47(phox)-/- mice: implications for fungal pathogenicity and host defense in chronic granulomatous disease. J Clin Invest. 1998;101:1843–1850. doi: 10.1172/JCI2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messina CG, Reeves EP, Roes J, Segal AW. Catalase negative Staphylococcus aureus retain virulence in mouse model of chronic granulomatous disease. FEBS Lett. 2002;518:107–110. doi: 10.1016/s0014-5793(02)02658-3. [DOI] [PubMed] [Google Scholar]
- 43.Dorman SE, Guide SV, Conville PS, et al. Nocardia infection in chronic granulomatous disease. Clin Infect Dis. 2002;35:390–394. doi: 10.1086/341416. [DOI] [PubMed] [Google Scholar]
- 44.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]