Abstract
Background
Peritoneal carcinomatosis (PC) in the setting of mucinous appendiceal neoplasms is characterized by the intraperitoneal accumulation of mucinous ascites and mucin-secreting epithelial cells that leads to progressive compression of intra-abdominal organs, morbidity, and eventual death. We assessed postoperative and oncologic outcomes after aggressive surgical management by experienced surgeons.
Methods
We analyzed clinicopathologic, perioperative, and oncologic outcome data in 282 patients with PC from appendiceal adenocarcinomas between 2001 and 2010 from a prospective database. Kaplan–Meier survival curves and multivariate Cox-regression models were used to identify prognostic factors affecting oncologic outcomes.
Results
Adequate cytoreduction was achieved in 82% of patients (completeness of cytoreduction score (CC)-0: 49%; CC-1: 33%). Median simplified peritoneal cancer index (SPCI), operative time, and estimated blood loss were 14 (range, 0–21), 483.5 min (range, 46–1,402), and 800 ml (range, 0–14,000), respectively. Pathology assessment demonstrated high-grade tumors in 36% of patients and lymph node involvement in 23% of patients. Major postoperative morbidity occurred in 70 (25%) patients. Median overall survival was 6.72 years (95% confidence interval (CI), 4.17 years not reached), with 5 year overall survival probability of 52.7% (95% CI, 42.4, 62%). In a multivariate Cox-regression model, tumor grade, age, preoperative SPCI and chemo-naïve status at surgery were joint significant predictors of overall survival. Tumor grade, postoperative CC-score, prior chemotherapy, and preoperative SPCI were joint significant predictors of time to progression.
Conclusions
Aggressive management of PC from mucinous appendiceal neoplasms, by experienced surgeons, to achieve complete cytoreduction provides long-term survival with low major morbidity.
Mucinous appendiceal neoplasms frequently lead to peritoneal carcinomatosis.1 Organ dysfunction from progressive accumulation of mucinous tumor deposits leads to morbidity and mortality. Histopathologic features play a dominant role in the subsequent clinical behavior of these tumors; Ronnett and colleagues originally described diffuse peritoneal adenomucinosis (DPAM; median survival 112 months) and the more aggressive peritoneal mucinous carcinomatosis (PMCA, median survival 24 months).2 Prognostic and interobserver variability within this classification system led to the use of low- and high-grade categorization of these tumors.3,4 Similarly, Bartlett and colleagues used K-ras mutations and loss of heterozygosity (LOH) markers to classify and predict oncologic outcomes in mucinous appendiceal neoplasms.5
This malignancy lends itself to locoregional therapies, because most patients present with low-grade tumors that are predominantly noninvasive and rarely involve lymph nodes or metastasize to viscera or extra-abdominal sites. Complete cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemoperfusion (HIPEC) is considered the standard of care for this locally recurrent disease.6,7 Retrospective series have demonstrated markedly improved patient survival, decreased tumor recurrence, and less frequent reoperative interventions with this radical approach compared with historical serial debulking procedures or palliative systemic chemotherapy.8–14
This study was designed to provide clinicopathologic and oncologic outcome data and to assess prognostic factors that influence long-term survival in patients treated uniformly at a single institution. We demonstrate that the ability to perform complete CRS with HIPEC confers long-term survival in patients who suffer from peritoneal carcinomatosis of appendiceal origin.
MATERIALS AND METHODS
We analyzed 282 consecutive patients with peritoneal carcinomatosis of appendiceal origin, undergoing CRS with HIPEC between May 2001 and July 2010, from a prospective database. Exclusion criteria included patients with nonappendiceal primary tumors. The study was approved by the University of Pittsburgh institutional review board, and all procedures were performed by surgeons with extensive experience in regional therapies.
Preoperatively, patients were evaluated in a dedicated peritoneal surface malignancy clinic. Intraoperatively, volume of disease was quantified by the Dutch simplified peritoneal cancer index (SPCI); combining lesion size and tumor distribution in specific abdominopelvic regions.15 CRS was performed in accordance with techniques, described by Bao and Bartlett, to achieve complete cytoreduction.16 CC score assessed the extent of residual disease at the end of surgical resection: CC-0: no visible residual disease; CC-1: residual tumors ≤2.5 mm; CC-2: residual tumors 2.5 mm to 2.5 cm; CC-3: residual tumors ≥2.5 cm.17 A standard institutional protocol for HIPEC was initiated after CRS as described by Gusani et al.18 Using the closed technique, a roller-pump heat exchanger perfusion machine (ThermoChem HT-100, ThermaSolutions, Melbourne, FL) allowed adequate saline flow (>800 ml/minute) and a target intraperitoneal tissue temperature of 42°C. Most patients received mitomycin C with 30 mg added to the perfusate initially for 60 min followed by an additional 10 mg of mitomycin C added for a further 40 min.
A subgroup of our study population underwent genetic analysis for K-ras mutations and LOH for tumor suppressor genes to calculate fractional mutation rate (FMR) as described by Bartlett and colleagues.5 Postoperative morbidity was classified according to the Dindo-Clavien grading system.19 For the purposes of analysis, grades 3–5 were considered major complications.
Statistical Analysis
Statistical analysis was performed using SAS 9.2 (Cary, NC). P values < 0.05 were considered significant. Overall survival was calculated from the date of surgery to the date of death. If a patient did not experience death, they were censored at the time of their last follow-up. Time to progression was calculated from the date of surgery to the date of tumor recurrence. If a patient did not experience progression or recurrence, they were censored on the date of their last follow-up or death. Survival times were estimated using the Kaplan–Meier method. Proportional hazards regression was used to examine both univariate and multivariate associations with overall survival and progression-free survival. In univariate analyses, Bonferroni adjustments were made to P values to account for multiple comparisons. All factors that were examined in univariate analysis were considered for entry into the model for multivariate analysis (grade, lymph node involvement, disease type (primary versus recurrent), prior chemotherapy, postoperative CC-score, preoperative albumin, preoperative SPCI, age, operating room time, and time interval between first diagnosis and surgery). Variables were selected for the final multivariate model based on a step-wise selection method. Fisher’s exact tests were used to examine relationships between FMR and tumor grade and Ronnett’s classification system. To assess which covariate best correlated with overall survival, overall survival was modeled as a function of FMR, grade, and Ronnett in three separate models.
RESULTS
Patient Characteristics and Clinical Presentation
Data were available for 282 patients with peritoneal carcinomatosis from primary appendiceal neoplasms (Table 1). Median time interval from initial disease diagnosis until index surgical resection at our institution was 7.2 months (range, 0 days–23 years). Mean age was 55 years with equal gender distribution. Median preoperative CA19-9, carcinoembryonic antigen (CEA), and CA-125 levels were 26.9, 5.9, and 29.6 U/ml, respectively. Half of the patients with known disease status presented with recurrent disease (50.1%). Abdominal pain (51.8%) and distention (35.8%) were the most common presenting symptoms. Before the index surgical resection at our institution, 109 (38.7%) patients received chemotherapy: 67 (61.5%) received chemotherapy after a prior surgical resection and 31 (28.4%) received definitive or neoadjuvant chemotherapy immediately before resection at our institution; 11 patients (10%) had unknown type of prior chemotherapy. Patients with recurrent disease status were comparable to those with primary disease status at the time of presentation in all pre- and perioperative characteristics, except for a higher likelihood of having received prior chemotherapy (54 vs. 23.1%, P = 0.008) and/or CRS (71.1 vs. 33.8%, P = 0.008).
TABLE 1.
Preoperative patient characteristics and presentation (n = 282)
| Age (mean ± SD) | 54.9 (±11.5) |
| BMI (median, range) (n = 234) | 27 (16–59) |
| Preoperative albumin (median, range) (n = 210) | 3.9 (0.7–5.8) |
| Preoperative CA19-9 (median, range) (n = 111) | 26.9 (0.9–22797) |
| Preoperative CEA (median, range) (n = 157) | 5.9 (0.4–901.9) |
| Preoperative CA-125 (median, range) (n = 106) | 29.6 (2.9–381.7) |
| Gender (n, %) | |
| Male | 141 (50) |
| Female | 141 (50) |
| Race (n, %) | |
| White | 259 (91.8) |
| Not white | 10 (3.6) |
| Unknown | 13 (4.6) |
| Disease status (n, %) | |
| Primary | 134 (47.5) |
| Recurrent | 137 (48.6) |
| Unknown | 11 (3.9) |
| ASA (n, %) | |
| 1 | 2 (0.7) |
| 2 | 35 (12.4) |
| 3 | 124 (44) |
| 4 | 28 (9.9) |
| Unknown | 93 (33) |
| Prior surgical therapy (n, %) | |
| Cytoreductive surgery (CRS) (N = 270) | 92 (34.1) |
| Chemoperfusion (N = 276) | 21 (7.6) |
| Clinical parameters (n, %) | |
| Abdominal pain | 146 (51.8) |
| Ascites | 101 (35.8) |
| Bowel obstruction | 35 (12.4) |
| Gastrointestinal bleeding | 9 (3.2) |
| Intestinal perforation | 23 (8.2) |
| Extra abdominal disease | 29 (10.3) |
| Prior chemotherapy (n = 109) | |
| Prior adjuvant | 67 (61.5) |
| Prior neoadjuvant or definitive | 31 (28.4) |
| Unknown | 11 (10.1) |
BMI body mass index, ASA American society of anesthesiology
Operative Characteristics and Pathology
Adequate cytoreduction was achieved in 82% of patients despite a median SPCI of 14. The majority of patients underwent peritoneal stripping (75.5%) and omentectomy (93.3%), with splenectomy being the most common visceral resection (46.8%). Median operative time and estimated blood loss were 483.5 minutes (range, 46–1,402) and 800 ml (range, 0–14,000). A total of 251 (89%) patients received HIPEC with mitomycin C as the drug of choice (98%). Pathologic assessment revealed DPAM in 71 patients (25%) and PMCA in 210 patients (75%); low-grade tumors in 180 patients (64%) and high-grade tumors in 100 (36%) patients. Among patients with available data, positive lymph nodes, K-ras mutations, and FMR ≥ 25% were found in 23.4, 61.3, and 71.59% of patients respectively. We found no correlation between FMR and tumor grade or Ronnett’s classification. Only tumor grade was predictive of survival (hazards ratio (HR) = 8.93; 95% confidence interval (CI), 1.78, 44.7; P = 0.008; Table 2).
TABLE 2.
Operative characteristics and pathology (n = 282)
| Operative time (min) (Median, range) (n = 228) | 483.5 (46–1,402) |
| Estimated blood loss (ml) (Median, range) (n = 250) | 800 (0–14,000) |
| Simplified peritoneal cancer index (SPCI) (median, range) (n = 279) | 14 (0–21) |
| HIPEC (n, %) | 251 (89) |
| HIPEC temperature (median, range) (n = 191) | 42 (38–43) |
| HIPEC duration (median, range) (n = 233) | 100 (60–143) |
| Completeness of cytoreduction (n, %) | |
| CC-0 | 139 (49.3) |
| CC-1 | 91 (32.3) |
| CC-2 | 28 (9.9) |
| CC-3 | 21 (7.5) |
| Unknown | 3 (1.1) |
| Surgical resection (n, %) | |
| Peritoneal stripping | 213 (75.5) |
| Omentectomy | 263 (93.3) |
| Distal pancreatectomy | 27 (9.6) |
| Splenectomy | 132 (46.8) |
| Diaphragmatic resection | 80 (28.4) |
| Hepatectomy | 25 (8.9) |
| Cholecystectomy | 84 (29.8) |
| Low anterior resection | 49 (17.4) |
| Partial colectomy | 28 (9.9) |
| Total abdominal colectomy | 16 (5.7) |
| Small bowel resection | 82 (29.1) |
| Partial gastrectomy | 26 (9.2) |
| Total gastrectomy | 1 (0.4) |
| Ureterectomy | 5 (1.8) |
| Ureterolysis | 100 (35.5) |
| Hysterectomy | 41 (14.5) |
| Cystectomy | 8 (2.8) |
| Ostomy | 78 (27.7) |
| No. of anastomoses (median, range) (n = 275) | 1 (0–5) |
| Ronnett’s histologic classification (n, %) | |
| DPAM | 71 (25.2) |
| PMCA | 210 (74.5) |
| Unknown | 1 (0.4) |
| Tumor grade (n, %) | |
| Low grade | 180 (63.8) |
| High grade | 100 (35.5) |
| Unknown | 2 (0.7) |
| Signet cells present (n, %) | 37 (13.1) |
| Positive lymph node status (n, %) (n = 201) | 47 (23.4) |
| K-ras mutation present (n, %) (n = 111) | 68 (61.3) |
| Fractional mutation rate (FMR) (n = 88) | |
| <25% | 25 (28.4) |
| ≥25% | 63 (71.6) |
FMR number of mutated markers divided by the total number of informative markers, HIPEC hyperthermic intraperitoneal chemoperfusion
Postoperative Characteristics
Ninety-one percent of patients were admitted to the intensive care unit (ICU) postoperatively, with median ICU and hospital length of stay being 3 days and 12 days respectively. Major postoperative morbidity occurred in 24.8% of patients, most commonly pulmonary and wound complications (Table 3). Of the 27 patients who underwent formal pancreatic resection, 4 (14.8%) developed a pancreatic leak. Enterocutaneous fistulae or anastomotic leaks occurred in 12 (6.7%) of 180 patients who had bowel resections (small bowel resection, colectomy, or low anterior resection). Fourteen (5%) patients underwent reoperation for wound or anastomotic complications, and 26 (9%) patients were subjected to percutaneous drainage procedures to treat pleural effusions or intra-abdominal fluid collections/abscesses. Median postoperative levels of CA19-9, CEA, and CA-125 were 13 U/ml (range, 0.8-137), 1.5 U/ml (range, 0.4–491.4), and 16.1 U/ml (range, 1–107.7), respectively. Of the 159 patients with available data, adjuvant chemotherapy was administered to 39 (32.5%). Following discharge, 39 patients were readmitted to the hospital within 30 days; 13 of the 39 (33.3%) patients had major complications. Postoperative infections (grade 3: 7.7%) and wound complications (grade 3: 2.1%) were the most common reasons for readmission. Three patients died within 60 days of surgery (1.1%)
TABLE 3.
Postoperative complications (n = 282)
| Minor complications (grade 1/2) |
Major complications (grade 3–5) |
|
|---|---|---|
| Overall morbidity (n, %) | 102 (36.2) | 70 (24.8) |
| Wound infection (n, %) (n = 281) | 19 (6.7) | 39 (13.8) |
| Sepsis (n, %) | 38 (13.5) | 7 (2.5) |
| Postoperative bleeding (n, %) | 16 (5.7) | 3 (1.1) |
| Cardiac (n, %) | 37 (13.1) | 4 (1.4) |
| Pulmonary (n, %) | 58 (20.6) | 21 (7.5) |
| Renal (n, %) | 31 (11) | 3 (1.1) |
| Biliary leak (n, %) | 0 | 1 (0.4) |
| Pancreatic leak (n, %) | 9 (3.2) | 1 (0.4) |
| DVT or PE (n, %) | 10 (3.5) | 4 (1.4) |
| Enterocutaneous fistula (n, %) (n = 257) | 5 (1.8) | 1 (0.4) |
| Anastomotic leak (n, %) (n = 271) | 5 (1.8) | 6 (2.1) |
Oncologic Outcomes
Median follow-up time was 1.98 years (range, 0.33 months to 8.3 years). Deaths occurred in 80 patients (28.4%) and tumor progression occurred in 120 patients (61.2%); 68 patients had either an unknown date of progression or unknown progression status. The peritoneum was the most common site of recurrence (32.6%). Median overall survival was 6.72 years, with 3- and 5-year overall survival probability of 67.4% (95% CI, 60.1, 73.6) and 52.7% (95% CI, 42.4, 62; Fig. 1a). Median time to progression was 1.79 years, with 3- and 5-year progression-free survival probability of 45.1% (95% CI, 37.4, 52.8) and 32.1% (95% CI, 24.1, 40.1; Fig. 1b).
FIG. 1.
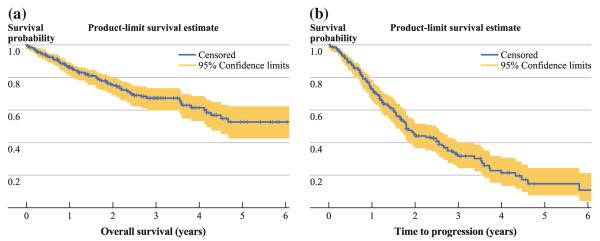
a Kaplan–Meier overall survival curve for all patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (n = 282). Median survival was 6.72 years (95% CI, 4.17 years, not reached); 3- and 5- year overall survival probability was 67.4 and 52.7%, respectively. b Kaplan–Meier curve for time to progression for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (n = 214). Median time to progression was 1.79 years (95% CI, 1.56–2.48); 3- and 5- year overall survival probability was 45.1 and 32.1% respectively
Univariate associations with overall survival were examined for grade, lymph node involvement, prior chemotherapy, postoperative CC-score, preoperative albumin, preoperative SPCI, age, operating room time, and time interval between first diagnosis and surgery. All except for operating room time and time interval between first diagnosis and surgery were significant independent predictors of overall survival. After adjusting for multiple comparisons, age and preoperative SPCI lost significance. Ignoring all other covariates, patients with high-grade disease had a greater risk of death than patients with low-grade disease (HR = 4.79; P = 0.009), patients with positive lymph node involvement had a greater risk of death than those without (HR = 2.87; P = 0.009), and patients with prior chemotherapy had a greater risk of death than those without (HR = 3.11; P = 0.009). The risk of death increased with increasing postoperative CC-score (P = 0.009) and decreased with increasing preoperative albumin (HR = 0.71; P = 0.04).
Grade, age, prior chemotherapy, and preoperative SPCI were joint significant predictors of overall survival. Holding all other factors constant, patients with high-grade tumors had worse overall survival than patients with low-grade tumors (HR = 4.35; 95% CI, 2.57, 7.38) and patients who received prior chemotherapy had a worse overall survival than chemo-naïve patients (HR = 2.06; 95% CI, 1.25, 3.39). The risk of death increased with age (HR = 1.03; P = 0.001) and with higher preoperative SPCI score (HR = 1.1; P = 0.001; Table 4). An additional model was created that examined whether patients who underwent surgical intervention for recurrent disease were at higher risk of death than those with primary disease at presentation, while adjusting for the same covariates. No significant difference was found.
TABLE 4.
Multivariate predictors of overall survival
| Factor | Comparison group | Hazard ratio | 95% CI for HR | P value |
|---|---|---|---|---|
| Grade (baseline = low grade; n = 180) | High grade (n = 97) | 4.35 | (2.57, 7.38) | <0.001 |
| Age (n = 277) | (n = 277) | 1.03 | (1.01, 1.05) | 0.017 |
| Prior CTX (baseline = no; n = 171) | Yes (n = 106) | 2.06 | (1.25, 3.39) | 0.004 |
| Preop SPCI | (n = 277) | 1.10 | (1.04, 1.15) | <0.001 |
Grade, lymph node involvement, prior chemotherapy, postoperative CC-score, preoperative SPCI, and operative time were each independent significant predictors of time to progression. However, after adjusting for multiple comparisons, operative time lost significance. The risk of disease progression was higher among patients with high-grade disease (HR = 3.02; P = 0.01), positive lymph node involvement (HR = 2.53; P = 0.01), and prior chemotherapy (HR = 2.82; P = 0.01). As preoperative SPCI increased, the risk of disease progression increased (HR = 1.08; P = 0.01).
Grade, postoperative CC-score, prior chemotherapy, and preoperative SPCI were joint significant predictors of time to progression. Holding all other factors constant, the risk of disease progression was higher among patients with high-grade disease (HR = 2.9; P = 0.001) and those with prior chemotherapy (HR = 1.9; P = 0.002). The risk increased as preoperative SPCI increased (HR = 1.04; P = 0.05) and as postoperative CC-score increased from CC-0 to CC-2. Disease status at the time of surgical intervention, primary versus recurrent, was not a significant predictor of time to progression (Table 5).
TABLE 5.
Multivariate predictors of time to progression
| Factor | Comparison group | Hazard ratio | 95% CI for HR | P value |
|---|---|---|---|---|
| Grade (baseline = low grade; n = 138) | High grade (n = 74) | 2.92 | (1.91, 4.45) | <0.001 |
| Postop CC SCORE (baseline = CC-0; n = 113) | ||||
| CC-1 (n = 72) | 1.67 | (1.07, 2.62) | 0.026 | |
| CC-2 (n = 18) | 5.31 | (2.86, 9.91) | <0.001 | |
| CC-3 (n = 9) | 3.19 | (1.42, 7.16) | 0.005 | |
| Prior CTX (baseline = no; n = 135) | Yes (n = 76) | 1.92 | (1.27, 2.9) | 0.002 |
| Preop SPCI (n = 212) | (n = 212) | 1.04 | (1.00, 1.09) | 0.049 |
DISCUSSION
The peritoneal based nature of this malignancy makes it an ideal candidate for aggressive locoregional therapies. Historically, these tumors were treated with nonaggressive, “palliative” serial debulking procedures, primarily for symptom management, with selective use of intraperitoneal chemotherapy; tumor recurrence was the norm and long-term cure was uncommon (20% at 10 years).3,20,21 Sugarbaker introduced the concept of radical “curative” CRS to remove all macroscopic tumor deposits, followed by perioperative intraperitoneal chemotherapy to treat residual microscopic disease.1 Based on large retrospective series, improved patient survival has been demonstrated, with less frequent tumor recurrence and less need for potentially morbid reoperative interventions. Current evidence demonstrates median survival of 51–156 months, 10 year overall survival up to 70%, while maintaining overall morbidity of 20–50% and mortality of 1–10%.8–14
In our study, adequate cytoreduction was achieved in 82% of patients, despite a median intraoperative SPCI of 14 and half of the patients had recurrent disease at initial presentation. Cytoreduction of tumor deposits to less than 2.5 mm in diameter has been recommended based on equivalent survival among patients undergoing CC-0 or CC-1 resection. In our univariate analysis, the risk of death and disease progression increased progressively with increasing postoperative CC-score. Every effort should be made to achieve complete microscopic tumor removal (CC-0), because HIPEC has limited ability to penetrate beyond a few millimeters and may not be completely effective for tumors even up to 2.5 mm in size (CC-1). Importantly, major postoperative morbidity occurred in only 24.8% patients and 60 day mortality was 1.1%, despite aggressive surgical management. This underscores the fact that extensive multivisceral resections can be performed even in the setting of recurrent disease, with relatively low major morbidity at experienced centers.
There is significant interobserver variability when classifying these tumors based on Ronnett’s criteria. In addition, there is clinical variability in the behavior of tumors within this classification system; a small percentage of patients with DPAM demonstrate a more aggressive clinical picture, whereas a variable spectrum of biologic behavior may be seen in patients with PMCA. To improve prognostication, Misdraji and Bradley subsequently classified these tumors into high- and low-grade types, whereas Bartlett and colleagues suggested an improved ability to predict survival by mutational profiling of accumulated allelic loss and point mutational damage.3–5 They used LOH in six genes and K-ras mutation that showed a significant association with Ronnett’s tumor histopathology and calculated a FMR, defined as the number of mutated markers divided by the total number of informative markers. In their analysis, an FMR < 25% indicated low-grade disease, an FMR of 25–50% indicated intermediate-grade disease, and an FMR > 50% indicated a high-grade tumor. In our series, K-ras mutations and FMR ≥ 25% were found in 61.3 and 71.6% of patients with available data, respectively. Although we identified no correlation between the three potential classification systems, we found that tumor grade was the best predictor of overall survival in our patient population. Patients with high-grade disease had a greater risk of death and disease progression than patients with low-grade disease.
The role of systemic chemotherapy in mucinous appendiceal neoplasms with peritoneal carcinomatosis is controversial.21,22 Currently, patients may receive neoadjuvant or adjuvant systemic chemotherapy in the setting of unresectable disease or when tumors are deemed at high risk for recurrence secondary to aggressive histopathologic features, including high-grade disease and lymph node involvement. Univariate analysis in our study revealed that patients who receive preoperative chemotherapy at any point since disease diagnosis demonstrated poorer overall survival and time to disease progression; this prognostic factor remained significant in the multivariate model for disease progression. Baratti et al. demonstrated similar findings and may reflect a selection bias for patients with aggressive tumor biology.13 However, in our multivariate model, patients who underwent surgical intervention for recurrent disease did not have worse overall survival, despite a higher frequency of having undergone previous chemotherapy or CRS. This suggests that the negative impact of chemotherapy may be independent of the disease status (primary versus recurrent) at the time of surgery.
In conclusion, appropriate patient selection, optimal cytoreduction, and low morbidity are modifiable factors in this disseminated malignancy and aggressive management by experienced surgeons can lead to long-term survival.
Footnotes
CONFLICT OF INTERESTS None.
REFERENCES
- 1.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 2.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei.”. Am J Surg Pathol. 1995;19(12):1390–408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089–103. doi: 10.1097/00000478-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bradley RF, Stewart JH, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinicopatho-logic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30(5):551–9. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 5.Maheshwari V, Tsung A, Lin Y, Zeh HJ, 3rd, Finkelstein SD, Bartlett DL. Analysis of loss of heterozygosity for tumor-suppressor genes can accurately classify and predict the clinical behavior of mucinous tumors arising from the appendix. Ann Surg Oncol. 2006;13(12):1610–6. doi: 10.1245/s10434-006-9081-1. [DOI] [PubMed] [Google Scholar]
- 6.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14(2):484–92. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 7.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5(4):219–28. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 8.Loungnarath R, Causeret S, Bossard N, Faheez M, Sayag-Beaujard AC, Brigand C, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum. 2005;48(7):1372–9. doi: 10.1007/s10350-005-0045-5. [DOI] [PubMed] [Google Scholar]
- 9.Guner Z, Schmidt U, Dahlke MH, Schlitt HJ, Klempnauer J, Piso P. Cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei. Int J Colorectal Dis. 2005;20(2):155–60. doi: 10.1007/s00384-004-0648-7. [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gush-chin V, Esquivel J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13(5):635–44. doi: 10.1245/ASO.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 11.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245(1):104–9. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart JH, Shen P, Russell GB, Bradley RF, Hundley JC, Loggie BL, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13(5):624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 13.Baratti D, Kusamura S, Nonaka D, Langer M, Andreola S, Favaro M, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15(2):526–34. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 14.Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93(10):1270–6. doi: 10.1002/bjs.5427. [DOI] [PubMed] [Google Scholar]
- 15.Portilla AG, Shigeki K, Dario B, Marcello D. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol. 2008;98(4):228–31. doi: 10.1002/jso.21068. [DOI] [PubMed] [Google Scholar]
- 16.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15(3):204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 17.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 18.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15(3):754–63. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241(2):300–8. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219(2):112–9. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JW, Kemeny N, Caldwell C, Banner P, Sigurdson E, Huvos A. Pseudomyxoma peritonei of appendiceal origin. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1992;70(2):396–401. doi: 10.1002/1097-0142(19920715)70:2<396::aid-cncr2820700205>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


