Summary
Formation of fibrin sleeves around catheter tips is a central factor in catheter failure during chronic implantation, and such tissue growth can occur despite administration of anticoagulants. We developed a novel method for monitoring catheter patency. This method recognizes the progressive nature of catheter occlusion, and tracks this process over time through measurement of changes in catheter resistance to a standardized 1 mL bolus infusion from a pressurized reservoir. Two indirect measures of catheter patency were used: (a) reservoir residual pressure and (b) reservoir discharge time. This method was applied to the study of catheter patency in rats comparing the effect of catheter material (silastic, polyurethane, Microrenathane™), lock solution (heparin, heparin/dexamethasone) and two different cannulation sites (superior vena cava via the external jugular vein, inferior vena cava via the femoral vein). Our findings reveal that application of flexible smaller-size silastic catheters and a dexamethasone lock solution resulted in prolonged catheter patency. Patency could be maintained over nine weeks with the femoral vein catheters, compared with five weeks with the external jugular vein catheters. The current method for measuring catheter patency provides a useful index for the assessment of tissue growth around the catheter tip. The method also provides an objective and quantitative way of comparing changes in catheter patency for different surgical methods and catheter types. Our method improves on the conventional method of assessing catheter occlusion by judging the ability to aspirate from the catheter.
Keywords: Indwelling catheters, catheter patency, fibrosis, steroids
The increased use of small animal models in pharmacological, biomedical and behavioural research has brought about an increased interest in techniques for vascular cannulation and issues surrounding long-term catheter patency. Formation of a fibrin sleeve around the catheter tip is a central factor in catheter failure during chronic implantation. The fibrin sleeve can form despite administration of anticoagulants, either as a catheter lock solution (Foley et al. 2002) or systemically (Ruggiero & Aisenstein 1983, Di Costanzo et al. 1984). Fibrin sleeves begin to form as early as 24 h after catheterization (Hoshal et al. 1971). This inflammatory repair response to the catheter includes acute inflammation, chronic inflammation and foreign body reaction with granulation tissue, and macrophage and foreign body giant cell interaction (Anderson 1988). In man, 42–100% of central venous catheters are surrounded by a ‘fibrin sleeve’ (Xiang et al. 1998). The fibrin sleeve represents a potential source of catheter-related infection, withdrawal occlusion and pulmonary embolism (Lloyd et al. 1993).
Long-term catheter patency is determined by factors that include site of catheter placement, technique of catheter insertion, biocompatibility of the catheter material, diameter and shape of the catheter, as well as the schedule of catheter flushes (Cocchetto & Bjornsson 1983). In rats, a common site of placement of a chronic venous catheter is the external jugular vein (Yoburn et al. 1984, Patsalos et al. 1992, Noble et al. 1994, Burvin et al. 1998), though other sites such as the femoral vein and the inferior vena cava have also been used (Koeslag et al. 1984, Pages et al. 1993, Burvin et al. 1998, de Jong et al. 2001). Review of the literature in which rats have been cannulated via the external jugular vein indicates that patency of the catheter seldom extends beyond 30 days (Cocchetto & Bjornsson 1983).
Our study applied a novel objective method for monitoring catheter patency. This method recognizes the progressive nature of catheter occlusion, and tracks this process over time through measurement of changes in catheter hydraulic resistance to a standardized bolus infusion. This method improves on the conventional method of subjectively assessing catheter patency by judging the ability to aspirate from the catheter, and represents a variation of the inline pressure-monitoring method that has been applied in human subjects (Stokes et al. 1989, Yamataka et al. 1993, Arai et al. 2002). Our application of measuring the discharge time (DT) of a standardized bolus infusion, while measuring changes in catheter pressure provides a useful index for the assessment of the extent of tissue growth around the catheter tip.
We examined different catheter materials, different catheter lock solutions, as well as two different cannulation sites. First, long-term catheter patency was compared between catheters made of silastic, polyurethane and microrenathane™. Second, the biomaterial with the best performance was chosen to compare patency of catheters maintained with a lock solution containing either heparin only or dexamethasone and heparin. Last, the effectiveness of these two lock solutions were compared for two cannulation sites – the external jugular vein and the femoral vein.
Materials and methods
Animals and surgical procedures
Experimental protocols were reviewed and approved by the University of Southern California Institutional Animal Care and Use Committee. Male Sprague–Dawley rats, weighing 350–375g, were used. The animals were obtained from a commercial vendor (Harlan Sprague–Dawley Labs, Indianapolis, IN, USA) that ensures fidelity of the strain through a genetic monitoring programme based on physical characteristics, reproductive indices and the polymerase chain reaction. Animals were certified as pathogen free (adventitious viruses, Mycoplasma sp., respiratory and enteric bacteria, ecto- and endoparasites) according to monthly microbiological monitoring (available from URL: http://www.harlan.com/healthreports/index.asp). The rats were individually housed in polypropylene cages with sawdust used as routine bedding for two weeks prior to the study. Housing conditions were characterized by room temperature maintained at 21.1±1.1°C with 15–20 air changes per hour, humidity between 40 and 70%, and a 12 h light–dark cycle with lights going off at 18:00 h. All rats had free access to standard rodent chow and water. After experiments, euthanasia of rats was conducted in accordance with the Guideline on the Panel of Euthanasia of the American Veterinary Medical Association. Anaesthesia was induced with 2.5% halothane in oxygen in a flow-through chamber, then increased to 5% until cessation of respiration. Thereafter, the animal was decapitated.
Prior to cannulation, rats were anaesthetized with halothane (Halocarbon Labs, River Edge, NJ, USA) 2.5% induction, 1.3% maintenance in a mixture of 30% O2–70% N2O. Body temperature was maintained at 37.0°C with a homoeothermic water blanket connected to a temperature controller (model TCAT–12, Physitemp; Clifton, NJ, USA). Aseptic technique was followed during all aspects of the animal surgery. The catheters (all blunt tipped) and stainless steel plug were sterilized using ethylene oxide.
Rats were cannulated via the external jugular vein as follows. A 2 cm × 4 cm area was shaved on the ventromedian aspect of the neck, and a 1 cm longitudinal skin incision was made ventrally along the midline of the neck. The right external jugular vein was separated from the surrounding neck musculature. The cephalad end of the external jugular vein was tied. The caudal end was occluded with a microaneurysm clip (#B– 1V, ASSI®, Westbury, NY, USA), which was released on insertion of the catheter. The catheter flushed with a 4 U/mL heparin/saline solution (heparin-sodium porcine derived, Baxter, Deerfield, IL, USA) was advanced 3.5 cm caudally into the superior vena cava, close to the right atrium. The catheter was fixed with two 5-0 silk ligatures, tunnelled subcutaneously (s.c.) to the dorsum of the neck and drawn back up through the skin. A 1.0 cm distal end of tubing served as a percutaneous access port, which was sealed with a stainless steel plug. This portion of tubing was sutured to skin and the port served for routine post-surgical catheter flushes, as well as to assess catheter patency. Prior to recovery of the animal, 0.5% bupivacaine-HCl (Marcaine, Abbott Labs, North Chicago, IL, USA) was infused s.c. at the edges of the skin incision. Animals were transferred to a warmed recovery cage until able to ambulate. Flunixin meglumine 2.5 mg/kg, s.c. (Banamine, Fort Dodge Animal Health, Fort Dodge, Iowa, USA) was administered for added postoperative pain control three times at 12-hour intervals. Signs of any local skin inflammation, if present, were treated with topical application of bacitracin ointment.
For cannulation of the inferior vena cava, a similar approach to surgical preparation and catheter insertion was taken. Our approach was a variant of the approach used earlier by Kaufman, who proposed insertion of the catheter into the inferior vena cava by a direct abdominal approach (Kaufman 1980). In our method, the catheter was advanced 8.0 cm from the right femoral vein at the level of the inguinal area until its tip in the inferior vena cava was at the approximate level of the xiphisternum. A percutaneous access port was established as described above. Surgical incisions were closed with silk suture. Both external jugular vein catheter and femoral vein catheter were flushed immediately after surgery and twice weekly through the access port using 0.8 mL of 4U/mL heparin/saline solution, followed by injection of 0.05 mL of a heparin 50U/mL saline lock solution. On days when catheter patency was evaluated, catheter flushes occurred after the patency assessment.
Method for assessment of catheter patency
Patency of the catheters was assessed in a standardized manner during administration of a 1 mL bolus of saline through the percutaneous catheter port. The port was connected to a T-connector with one arm of the ‘T’ connected to a Statham strain-gauge pressure transducer and the other arm connected to an elastomeric infusion reservoir. The reservoir was customized from a commercially available infusion pump (Advanced Neuromodulation Systems, Inc, Plano, TX, USA) by removing the flow restrictor, which normally limits the flow rate for slow infusion applications (Holschneider et al. 2002). The reservoir was filled by needle puncture through its silastic septum with 1 mL of saline. Outflow from the elastomeric reservoir was controlled by an electrically activated stainless steel solenoid valve (The Lee Company; Essex, CT, USA). Two proxy measures of catheter patency were recorded: (a) residual reservoir pressure (RRP) after discharge of its liquid content, and (b) DT until end of fluid discharge from the reservoir. Parameters RRP and DT were derived from in vivo recording of the pressure at the T-connector port with an online signal recording system (Axotape 2.0, Axon Instruments, Foster City, CA, USA, sampling rate 40 Hz). Recordings of catheter patency were stopped after DT exceeded 60s, because catheters were considered to be non-patent at that point. Following the final assessment of catheter patency, the animal was killed and autopsy was performed to confirm the position of the catheter and to allow for visual inspection of the extent of tissue growth around the catheter tip. A total of 15 rats were randomly divided into a 7-, 14- and 21-day group, with five rats in each post-surgical group. Animals were respectively killed on days 7, 14 and 21.
Evaluation of catheter material and size on catheter patency
The first five groups of rats were used to study the effect of catheter material and size. There were five rats in each group. Five combinations of catheter material and diameter were tested: (1) 5 F silastic (0.76/ 1.65mm silicone catheter, Dow Corning Corp, Midland, MI, USA), (2) 3 F silastic (0.64/1.19mm, Dow Corning Corp, Midland, MI, USA), (3) 3.5 F polyurethane (0.69/ 1.20mm, Access Technologies, Skokie, IL, USA), (4) 2 F size polyurethane (0.33/ 0.66mm, Access Technologies, Skokie, IL, USA) and (5) ~3 F microrenathane™ (0.62/ 0.97mm, Braintree Scientific, Inc Braintree, MA, USA). Microrenathane™ is a type of polyurethane tubing with high tensile strength and biocompatible properties. Catheter patency was assessed at the time of surgery and post-surgically twice a week for four weeks.
Use of lock solution with dexamethasone for improving catheter patency
Whereas heparin lock solutions have been commonly used for anticoagulation in catheter maintenance (Kaufman 1980, Xiang et al. 2000), dexamethasone has been reported to minimize formation of the fibrin sleeve by virtue of its anti-inflammatory properties (Chervu et al. 1989, Painter 1991, Villa et al. 1994). The external jugular vein was cannulated with a 3 F silastic catheter and a percutaneous access port was established as described above. Post-surgically, catheter patency was maintained by twice weekly flushes with 0.8 mL of 4U/ mL heparin/saline solution through the access port, followed by administration of a lock solution. Two different lock solutions were compared, each passed through a 0.22 micron syringe fitter: either 0.05 mL of saline containing 50U/mL heparin, or 0.05 mL of saline containing 50U/mL heparin plus 1mg/mL dexamethasone (Sigma, St Louis, MO, USA). In these animals, patency was assessed twice weekly during weeks 1–4, and once a week during weeks 5–9. In each group, five rats were used to test the effects of lock solutions on catheter patency.
Femoral vein cannulation
Cannulation of the inferior vena cava via the femoral vein has been cited by previous authors as prolonging catheter patency, possibly because this approach allows greater blood flow around the catheter tip (Kaufman 1980). Effects on catheter patency of varying the diameter of the silastic catheter, as well as the presence or absence of a dexamethasone lock solution were investigated. Fifteen rats were used in this study and divided into three groups. The three groups were: Group 1: 3 F silastic catheter, heparin lock solution (0.10 mL of 50 U/mL), Group 2: 3 F silastic catheter, dexamethasone/heparin lock solution (0.10 mL, dexamethasone 1mg/mL in 50 U/ mL heparin–saline solution), Group 3: 2 F silastic catheter, dexamethasone/heparin lock solution (0.10 mL, dexamethasone 1 mg/mL in 50U/mL heparin–saline solution). Patency was evaluated twice weekly during weeks 1–4 and once a week during weeks 5–9.
Data analysis
Group size for assessment of DT and RRP from the various experimental groups was five rats in each group. Data were expressed as mean±SEM. ANOVA and Student– Newman–Keuls post hoc tests were used to evaluate significant differences (P<0.05).
Results
Measurements of catheter patency
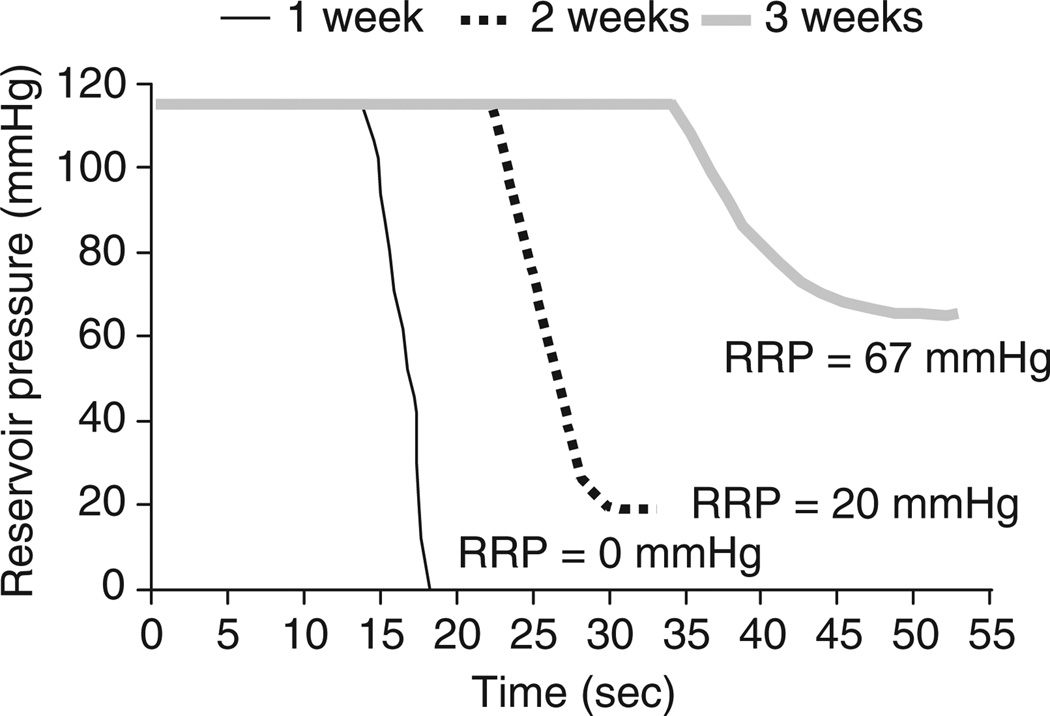
Representative pressure recordings on post-surgical days 7, 14 and 21 showed that the DT was increased markedly with implantation duration (Figure 1). In addition, the RRP, initially 0mmHg, became increasingly positive after 14 and 21 days of implantation.
Figure 1. Changes in reservoir pressure during standardized infusion of a 1 mL bolus of saline in a single animal on post-surgical days 7, 14 and 21.
After implantation of a 3 F silastic catheter in the external superior jugular vein, baseline pressures are shown from 0 to 10 s for each of the three curves. Opening of the valve at the 10th second allowed bolus administration and controlled release of the reservoir content. From weeks 1 to 3, there is a progressive increase in residual reservior pressure (RRP), as well as an increase in discharge times. The duration between opening of the valve (10th second) and the release of the saline solution (pressure change) were increased gradually with prolonged catheter implantations
Autopsy on those animals indicated that after one week of implantation, tissue had grown at the site of initial catheter insertion, but not around the catheter tip. After two weeks of implantation, tissue had begun to grow around the tip of the catheter, but the layer of fibrosis was thin and pierced by many small holes due to routine flushing. By the end of the third week, thick tissue growth encapsulated the tip of catheter completely with the infusate being released only through tiny pores in the fibrotic tissue. The progressive growth of tissue at the catheter tip reflected the increases of DT and RRP.
Effects of catheter material and diameter on external jugular vein catheter patency
For the catheters inserted via the external jugular vein, patency (DT <60s) was maintained the longest (~3 weeks) in the 3 F silastic group (Table 1). Catheters in all other groups were patent for two weeks or less. The patency of the smallest 2 F polyurethane tubing was maintained for only 10 days. There was also no difference with respect to duration of patency between the 3 F Microrenathane™ catheters and the other polyurethane catheters.
Table 1.
Effects of catheter material and diameter on catheter patency after cannulation of the superior vena cava via the external jugular vein
| Catheter material | Diameter | ID/OD (mm) | Patency (days) |
|---|---|---|---|
| Silastic | 5 F | 0.76/1.65 | 13.8±2.8 |
| Silastic | 3 F | 0.64/1.19 | 21.0±1.9** |
| Polyurethane | 3.5 F | 0.69/1.20 | 12.2±2.0 |
| Polyurethane | 2 F | 0.33/0.66 | 10.0±1.2* |
| Microrenathane™ | ~3 F | 0.62/0.97 | 11.0±3.0 |
Values are means±SEM. Each group comprised five rats and a catheter lock solution of 0.05 mL of 4 U/mL heparin saline was used. Catheters were considered to be patent when discharge time DT was <60 s
P<0.05 versus 5 F silastic catheter by ANOVA and Student–Newman–Keuls test
P<0.01 versus 5 F silastic catheter, as well as 2 F and 3.5 F polyurethane catheters, and microrenathane catheter by ANOVA and Student–Newman–Keuls test
Comparison of dexamethasone or heparin lock solutions on external jugular vein catheter patency
In the heparin group, DT increased markedly after two weeks of cannulation (Figure 2a). In contrast, DT remained unchanged for nearly four weeks of cannulation in the dexamethasone/heparin group. Similarly, RRP began to increase after 10 days of cannulation in the heparin group, whereas RRP remained near 0 mmHg in the dexamethasone/heparin group. Patency was maintained on average for 20.9± 1.8 days in the heparin group and for 35.7 ± 2.2 days in the dexamethasone/heparin group (Figure 2b), a difference that was statistically significant (P<0.01).
Figure 2. Effects of dexamethasone/heparin lock solution on discharge time (DT) and residual reservior pressure (RRP) after cannulation of the external jugular vein with a 3 F silastic catheter.
Values are means±SEM. Catheter patency was significantly prolonged in the dexamethasone/heparin lock solution group compared with the heparin group (P<0.01) the dexamethasone/heparin group (Figure 2b), a difference that was statistically significant (P<0.01).
Effects of dexamethasone on catheter patency after cannulation of the femoral vein
In all groups cannulated via the femoral vein, patency was maintained for over nine weeks, with no catheter demonstrating a DT>25s (Figure 3a). Due to their smaller inner diameter, the 2 F silastic catheters exhibited immediately after surgery (day 0) a DT that was nearly twice that of the 3 F catheters. After nine weeks of implantation, DT remained unchanged in the 2 F catheter group. In contrast, DT doubled in both 3 F catheter groups, when compared with its post-surgery value (P<0.05). As a result, there was no difference in DT among the three experimental groups after nine weeks of cannulation.
Figure 3. Effects of dexamethasone (Dex) on (a) discharge time (DT), (b) residual reservior pressure (RRP) of femoral vein cannulas.
Catheters contained a lock solution that was either heparin (Hep) or dexamethasone plus heparin (Dex/Hep). The femoral vein cannulas were kept patent for over nine weeks. The 2 F catheter maintained with dexamethasone demonstrated even less of an increase in DT or RRP during this period than the 3 F catheter. Values are means±SEM
RRP remained lower over the duration of observation in the two groups treated with dexamethasone/heparin when compared with the group that received only a heparin lock solution (Figure 3b). At the end of the nine-week study period, compared with 38.9±4.0 mmHg in the 3 F silastic heparin group, RRP was 20.1±3.4mmHg in the 3 F silastic group treated with dexamethasone/ heparin (P<0.05) and 5.6±1.6 mmHg in the 2 F silastic catheter group maintained with dexamethasone/heparin (P<0.01). In addition to remaining patent for fluid infusion, femoral vein catheters were found to allow for long-term blood sample collection. In the 3 F silastic group treated with heparin, aspiration of blood could be performed for 7–8 weeks. Even by the end of nine weeks of implantation, blood sample aspiration was possible from 2 and 3 F silastic catheters maintained with dexamethasone/heparin.
Rats cannulated via the femoral vein exhibited normal ambulation in their homecages, suggesting that collateral circulation was adequate for leg perfusion under standard conditions.
Discussion
We found that the material, diameter, type of lock solution and site of insertion contributed to the effective long-term maintenance of an intravenous catheter. Use of a flexible 2 F silastic catheter inserted in the inferior vena cava via the femoral vein with concomitant use of a dexamethasone lock solution was optimal in terms of long-term patency, which was maintained for more than two months.
Many investigators have been trying to find new methods that can extend the patency of cannulation in vivo. We modified Kaufman’s method by cannulating the inferior vena cava via the femoral vein, rather than through a direct approach, which requires exposure of the vessel through a midline abdominal incision. Our approach avoided two potential problems in Kaufman’s method. First, opening of the peritoneal cavity may prolong post-surgical recovery and increase the potential for post-surgical infection. Second, intra-abdominal movement of the intestine can push catheters out of the vessel when cannulation occurs by direct puncture of the inferior vena cava. Using our modification of the Kaufman technique, catheter patency was maintained effectively for more than nine weeks, and may have continued further with a longer observation period.
Catheter patency was markedly prolonged when the catheter tip was located in the inferior vena cava when compared with the superior vena cava. Thus, 3 F silastic catheters were patent for more than nine weeks when inserted into the inferior vena cava via the femoral vein, whereas identical catheters inserted in the superior vena cava via the external jugular vein remained patent for only five weeks. It is possible that the larger vessel diameter of the inferior vena cava prolonged catheter patency. A larger vessel diameter improves blood flow around the catheter tip, thereby extending the patency (Kaufman 1980). Tissue growth usually happens at the site of vessel ligation and grows towards the tip of catheter along the passage of cannulas. Deep implantation of the catheter in a vein with high blood flow may retard the growth of reactive tissue.
It has been reported that heparin can inhibit smooth muscle cell proliferation after arterial injury (Lindner et al. 1992, Volker et al. 1995) and that intimal thickening after vascular injury may be modulated in part by heparin-binding growth factors (Bachinsky et al. 1995). Our results show that for long-term catheter implantation via the external jugular vein, heparin alone in the lock solution cannot fully inhibit catheter fibrosis, and complete encapsulation of the catheter tip ensues within three weeks. This is consistent with experimental findings that suggest that though heparin inhibits mechanisms that promote fibrosis, neointimal cellular proliferation and thrombus formation, it does so only at high concentrations (Spears et al. 1994). In vitro studies suggest that concentrations of 1000–50,000 U/mL are required (Spears et al. 1994). Such high concentrations are difficult to maintain chronically at the site of catheter insertion, and pose a potential risk of bleeding (Sasseen et al. 2001).
Dexamethasone has been reported to suppress intimal hyperplasia in vascular injury (Colburn et al. 1992, Petrik et al. 1998). Compared with a lock solution containing only heparin, a dexamethasone/ heparin lock solution allowed the superior vena cava catheter to remain patent for more than five weeks. In the cannulation of the inferior vena cava, the benefit of the dexamethasone was less apparent, primarily because the approach through the femoral vein itself prolonged catheter patency. After nine weeks of observation, DT was the same for catheters used with or without dexamethasone in the lock solution. However, RRP remained significantly less in the dexamethasone group compared with the control group. Such differences may relate to RRP being potentially more sensitive than DT for detecting early changes in catheter patency. The 2 F catheter maintained with dexamethasone also remained patent over nine weeks, and demonstrated even less of an increase in DT or RRP during this period than the 3 F catheter. This may have been due to the smaller diameter of the 2 F catheter, which allowed for greater blood flow around the tip, less irritation of the vessel’s intimal wall, with minimal resultant fibrosis.
Tissue growth at the catheter tip developed faster around the catheter tip for 2 F polyurethane tubing when compared with 3 F silastic tubing (superior vena cava groups). In addition to diameter, the stiffness of the catheter can also substantially influence its biocompatibility. It is possible that, despite a smaller diameter, the polyurethane catheter caused greater irritation to the vessel’s intimal lining than did the larger diameter but softer silastic catheter. The irritation may have served as a focal site for accelerated tissue growth. The superiority of silicone rubber tubing has been previously demonstrated for chronic venous cannulation (Davis 1966, Lipton 1972, Upton 1975).
The chosen method of prospectively recording catheter resistance to infusion of a standardized bolus infusion allowed us to track the progressive nature of catheter occlusion. DT and changes in catheter pressure may provide a useful index for the assessment of the extent of tissue growth around the catheter tip. Our method improves on past methods in which catheter occlusion was evaluated mostly by the ability to withdraw blood or inject saline through the catheter, using ‘patent’ or ‘not patent’ as the outcome measure. It represents a variation of the in-line pressure monitoring method used in human subjects in which detection of catheter obstruction is made during continuous infusion using an external motorized pump (Stokes et al. 1989, Yamataka et al. 1993, Arai et al. 2002). Unlike the in-line pressure monitoring method, in which injection occurs under constant flow conditions, our method uses a flow whose rate is dependent on the existent pressure downstream. This allows for the assessment of an additional variable, ‘total DT’ of a standardized volume that correlates with progressive occlusion.
Our method provides a practical way to prospectively monitor patency in vivo in small animals without the need for pressure guide wires, which have been used in larger animals such as dogs, under guidance of fluoroscopy, to diagnose arterial stenosis (De Bruyne et al. 2000, Pijls et al. 2000, Shalman et al. 2001). Our method also provides a quantitative way of comparing changes in patency for different surgical methods and catheter types in small animals. Though the indices provided by our method do not directly quantify growth of fibrosis around the catheter tip, autopsy suggested that the pressure changes during infusion were directly related to the extent of tissue growth. Future research will clarify the applicability of this method for assessing patency in chronic cannulations of other vessels or ducts.
Acknowledgements
We thank Professor Oscar U Scremin, Department of Physiology, UCLA, for the excellent technical assistance. This work was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (RO1 EB-00300-03), the Whitaker Foundation (RG-99-0331) and the Veterans Administration.
References
- Anderson JM. Inflammatory response to implants. American Society of Artificial Internal Organs Transaction. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- Arai J, Mouri Y, Miyamoto Y. Detection of peripherally inserted central catheter occlusion by in-line pressure monitoring. Paediatric Anaesthesia. 2002;12:621–624. doi: 10.1046/j.1460-9592.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Bachinsky WB, Barnathan ES, Liu H, et al. Sustained inhibition of intimal thickening. In vitro and in vivo effects of polymeric beta-cyclodextrin sulfate. Journal of Clinical Investigation. 1995;96:2583–2592. doi: 10.1172/JCI118322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burvin R, Zloczower M, Karnieli E. Double-vein jugular/inferior vena cava clamp technique for long-term in vivo studies in rats. Physiology & Behavior. 1998;63:511–515. doi: 10.1016/s0031-9384(97)00486-1. [DOI] [PubMed] [Google Scholar]
- Chervu A, Moore WS, Quinones-Baldrich WJ, Henderson T. Efficacy of corticosteroids in suppression of intimal hyperplasia. Journal of Vascular Surgery. 1989;10:129–134. doi: 10.1067/mva.1989.0100129. [DOI] [PubMed] [Google Scholar]
- Cocchetto DM, Bjornsson TD. Methods for vascular access and collection of body fluids from the laboratory rat. Journal of Pharmaceutical Science. 1983;72:465–492. doi: 10.1002/jps.2600720503. [DOI] [PubMed] [Google Scholar]
- Colburn MD, Moore WS, Gelabert HA, Quinones-Baldrich WJ. Dose responsive suppression of myointimal hyperplasia by dexamethasone. Journal of Vascular Surgery. 1992;15:510–518. doi: 10.1067/mva.1992.32078. [DOI] [PubMed] [Google Scholar]
- Davis JD. A method for chronic intravenous infusion in freely moving rats. Journal of the Experimental Analysis of Behavior. 1966;9:385–387. doi: 10.1901/jeab.1966.9-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyne B, Pijls NH, Heyndrickx GR, Hodeige D, Kirkeeide R, Gould KL. Pressure-derived fractional flow reserve to assess serial epicardial stenoses: theoretical basis and animal validation. Circulation. 2000;101:1840–1847. doi: 10.1161/01.cir.101.15.1840. [DOI] [PubMed] [Google Scholar]
- de Jong WH, Timmerman A, van Raaij MT. Long-term cannulation of the vena cava of rats for blood sampling: local and systemic effects observed by histopathology after six weeks of cannulation. Laboratory Animals. 2001;35:243–248. doi: 10.1258/0023677011911697. [DOI] [PubMed] [Google Scholar]
- Di Costanzo J, Sastre B, Choux R, et al. Experimental approach to prevention of catheter-related central venous thrombosis. Journal of Parenteral and Enteral Nutrition. 1984;8:293–297. doi: 10.1177/0148607184008003293. [DOI] [PubMed] [Google Scholar]
- Foley PL, Barthel CH, Brausa HR. Effect of covalently bound heparin coating on patency and biocompatibility of long-term indwelling catheters in the rat jugular vein. Comparative Medicine. 2002;52:243–248. [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM, Harimoto J, Yang J, Scremin OU. An implantable bolus infusion pump for use in freely moving, non-tethered rats. American Journal of Physiology. Heart and Circulatory Physiology. 2002;283:H1713–H1719. doi: 10.1152/ajpheart.00362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshal VL, Jr, Ause RG, Hoskins PA. Fibrin sleeve formation on indwelling subclavian central venous catheters. Archives of Surgery. 1971;102:253–258. doi: 10.1001/archsurg.1971.01350040115023. [DOI] [PubMed] [Google Scholar]
- Kaufman S. Chronic, nonocclusive, and maintenance-free central venous cannula in the rat. American Journal of Physiology. 1980;239:R123–R125. doi: 10.1152/ajpregu.1980.239.1.R123. [DOI] [PubMed] [Google Scholar]
- Koeslag D, Humphreys AS, Russell JC. A technique for long-term venous cannulation in rats. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1984;57:1594–1596. doi: 10.1152/jappl.1984.57.5.1594. [DOI] [PubMed] [Google Scholar]
- Lindner V, Olson NE, Clowes AW, Reidy MA. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. Journal of Clinical Investigation. 1992;90:2044–2049. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JM. Superior sagittal sinus as a chronic venous route in the rat. Journal of Applied Physiology. 1972;32:701–702. doi: 10.1152/jappl.1972.32.5.701. [DOI] [PubMed] [Google Scholar]
- Lloyd DA, Shanbhogue LK, Doherty PJ, Sunderland D, Hart CA, Williams DF. Does the fibrin coat around a central venous catheter influence catheter-related sepsis? Journal of Pediatric Surgery. 1993;28:345–348. doi: 10.1016/0022-3468(93)90229-e. discussion 348–9. [DOI] [PubMed] [Google Scholar]
- Noble F, Coric P, Turcaud S, Fournie-Zaluski MC, Roques BP. Assessment of physical dependence after continuous perfusion into the rat jugular vein of the mixed inhibitor of enkephalin-degrading enzymes, RB 101. European Journal of Pharmacology. 1994;253:283–287. doi: 10.1016/0014-2999(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Pages T, Fernandez JA, Adan C, Gamez A, Viscor G, Palacios L. A method for sampling representative muscular venous blood during exercise in rats. Laboratory Animals. 1993;27:171–175. doi: 10.1258/002367793780810342. [DOI] [PubMed] [Google Scholar]
- Painter TA. Myointimal hyperplasia: patho-genesis and implications. 2. Animal injury models and mechanical factors. Artificial Organs. 1991;15:103–118. doi: 10.1111/j.1525-1594.1991.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Patsalos PN, Alavijeh MS, Semba J, Lolin YI. A freely moving and behaving rat model for the chronic and simultaneous study of drug pharmacokinetics (blood) and neuropharmacokinetics (cerebrospinal fluid): hematological and biochemical characterization and kinetic evaluation using carbamazepine. Journal of Pharmacological and Toxicological Methods. 1992;28:21–28. doi: 10.1016/1056-8719(92)90061-5. [DOI] [PubMed] [Google Scholar]
- Petrik PV, Law MM, Moore WS, Colburn MD, Quinones-Baldrich W, Gelabert HA. Dexa-methasone and enalapril suppress intimal hyper-plasia individually but have no synergistic effect. Annals of Vascular Surgery. 1998;12:216–220. doi: 10.1007/s100169900143. [DOI] [PubMed] [Google Scholar]
- Pijls NH, De Bruyne B, Bech GJ, et al. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: validation in humans. Circulation. 2000;102:2371–2377. doi: 10.1161/01.cir.102.19.2371. [DOI] [PubMed] [Google Scholar]
- Ruggiero RP, Aisenstein TJ. Central catheter fibrin sleeve-heparin effect. Journal of Parenteral and Enteral Nutrition. 1983;7:270–273. doi: 10.1177/0148607183007003270. [DOI] [PubMed] [Google Scholar]
- Sasseen BM, Gray BD, Gal D, et al. Local delivery of a hydrophobic heparin reduces neointi-mal hyperplasia after balloon injury in rat carotid but not pig coronary arteries. Journal of Cardiovascular Pharmacology and Therapeutics. 2001;6:377–383. doi: 10.1177/107424840100600407. [DOI] [PubMed] [Google Scholar]
- Shalman E, Barak C, Dgany E, Noskowitcz H, Einav S, Rosenfeld M. Pressure-based simultaneous CFR and FFR measurements: understanding the physiology of a stenosed vessel. Computers in Biology & Medicine. 2001;31:353–363. doi: 10.1016/s0010-4825(01)00010-5. [DOI] [PubMed] [Google Scholar]
- Spears JR, Yellayi SS, Makkar R, et al. Effects of thermal exposure on binding of heparin in vitro to the arterial wall and to clot and on the chronic angiographic luminal response to local application of a heparin film during angioplasty in an in vivo rabbit model. Lasers in Surgery and Medicine. 1994;14:329–346. doi: 10.1002/lsm.1900140406. [DOI] [PubMed] [Google Scholar]
- Stokes DC, Rao BN, Mirro J, Jr, et al. Early detection and simplified management of obstructed Hickman and Broviac catheters. Journal of Pediatric Surgery. 1989;24:257–262. doi: 10.1016/s0022-3468(89)80007-7. [DOI] [PubMed] [Google Scholar]
- Upton RA. Simple and reliable method for serial sampling of blood from rats. Journal of Pharmaceutical Sciences. 1975;64:112–114. doi: 10.1002/jps.2600640123. [DOI] [PubMed] [Google Scholar]
- Villa AE, Guzman LA, Chen W, Golomb G, Levy RJ, Topol EJ. Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. Journal of Clinical Investigation. 1994;93:1243–1249. doi: 10.1172/JCI117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker W, Bohm A, Schmidt A, et al. Inhibition of smooth muscle cell proliferation and neointimal growth by low-anticoagulant heparin. Arzneimit-telforschung. 1995;45:546–550. [PubMed] [Google Scholar]
- Xiang DZ, Verbeken EK, Van Lommel AT, Stas M, De Wever I. Composition and formation of the sleeve enveloping a central venous catheter. Journal of Vascular Surgery. 1998;28:260–271. doi: 10.1016/s0741-5214(98)70162-4. [DOI] [PubMed] [Google Scholar]
- Xiang DZ, Verbeken EK, Van Lommel AT, Stas M, De Wever I. Intimal hyperplasia after long-term venous catheterization. European Surgical Research. 2000;32:236–245. doi: 10.1159/000008770. [DOI] [PubMed] [Google Scholar]
- Yamataka A, Kawamoto S, Ishikawa M, Lancaster JF, Miyano T, Lynch SV. A new technique for early detection of portal vein and arterial thromboses. Indwelling mesenteric venous catheterization and relevance to small bowel transplantation. Transplantation. 1993;56:509–511. doi: 10.1097/00007890-199309000-00004. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Morales R, Inturrisi CE, et al. Chronic vascular catheterization in the rat: comparison of three techniques. Physiology & Behavior. 1984;33:89–94. doi: 10.1016/0031-9384(84)90018-0. [DOI] [PubMed] [Google Scholar]