Abstract
Background
Most patients with malignant peritoneal mesothelioma (MPM) present with late-stage, unresectable disease that responds poorly to systemic chemotherapy while, at the same time, effective targeted therapies are lacking. We assessed the efficacy of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC) in MPM.
Methods
We prospectively analyzed 65 patients with MPM undergoing CRS/HIPEC between 2001 and 2010. Kaplan–Meier survival curves and multivariate Cox-regression models identified prognostic factors affecting oncologic outcomes.
Results
Adequate CRS was achieved in 56 patients (CC-0 = 35; CC-1 = 21), and median simplified peritoneal cancer index (SPCI) was 12. Pathologic assessment revealed predominantly epithelioid histology (81 %) and biphasic histology (8 %), while lymph node involvement was uncommon (8 %). Major postoperative morbidity (grade III/IV) occurred in 23 patients (35 %), and 60-day mortality rate was 6 %. With median follow-up of 37 months, median overall survival was 46.2 months, with 1-, 2-, and 5-year overall survival probability of 77, 57, and 39 %, respectively. Median progression-free survival was 13.9 months, with 1-, 2-, and 5-year disease failure probability of 47, 68, and 83 %, respectively. In a multivariate Cox-regression model, age at surgery, SPCI >15, incomplete cytoreduction (CC-2/3), aggressive histology (epithelioid, biphasic), and postoperative sepsis were joint significant predictors of poor survival (chi square = 42.8; p = 0.00001), while age at surgery, SPCI >15, incomplete cytoreduction (CC-2/3), and aggressive histology (epithelioid, biphasic) were joint significant predictors of disease progression (Chi square = 30.6; p = 0.00001).
Conclusions
Tumor histology, disease burden, and the ability to achieve adequate surgical cytoreduction are essential prognostic factors in MPM patients undergoing CRS/HIPEC.
Malignant peritoneal mesothelioma (MPM) is an aggressive primary malignancy arising from the serosal lining of the peritoneal cavity and accounts for 250–500 cases annually in the United States. Malignant ascites and locoregional invasion cause significant morbidity and mortality, while lymph node involvement (5–10 %) or extra-abdominal metastases (3–5 %) are uncommon. A majority of patients present with late-stage unresectable disease that responds poorly to systemic chemotherapy, while, at the same time, effective targeted therapies are lacking.1,2
The locoregional nature of the disease lends itself to aggressive locoregional therapies, including cytoreductive surgery (CRS) and perioperative intraperitoneal chemotherapy (PIC) in select patients. To date, no randomized trials or comparative studies involving CRS/PIC for the treatment of MPM have been published. However, a recent systematic review of multiple retrospective institutional studies, using CRS/PIC to treat MPM, demonstrated median survival ranging from 34 to 92 months, with five survival rates up to 59 %.3 In contrast, modern systemic chemotherapy regimens combining cisplatin or gemcitabine with pemetrexed or raltitrexed have demonstrated response rates of 15–40 % and median survival of 12–27 months in randomized trials for pleural mesothelioma and small nonrandomized prospective phase II trials for peritoneal mesothelioma.4–8
The aim of our study is to provide clinic-pathologic and oncologic outcome data in patients treated uniformly with CRS and hyperthermic intraperitoneal chemoperfusion (HIPEC) at a single high-volume institution. We also provide prognostic factors influencing disease progression and long-term survival in order to improve patient selection for these complex surgical procedures.
MATERIALS AND METHODS
We analyzed 65 consecutive patients with MPM, undergoing CRS with HIPEC between March 2001 and August 2010, from a prospective database. The study was approved by the University of Pittsburgh institutional review board, and all procedures were performed by surgeons with extensive experience in regional therapies.
Preoperatively, patients were evaluated in a dedicated peritoneal surface malignancy clinic. Intraoperatively, volume of disease was quantified by the Dutch simplified peritoneal cancer index (SPCI), by which a score is allocated by measuring the maximum thickness of the largest tumor nodule (no tumor = 0; <2 cm = 1; 2–5 cm = 2; >5 cm = 3) in each of seven abdominopelvic regions (pelvis, right lower abdomen, omentum-transverse colon, small bowel-mesentery, subhepatic space-stomach, right subphrenic space, and left subphrenic space). The SPCI adds up to a maximum score of 21.9,10 CRS was performed in accordance with techniques, described by Bao and Bartlett. Completeness of cytoreduction (CC) score assessed the extent of residual disease at the end of surgical resection: CC-0, no visible residual disease; CC-1, residual tumors ≤2.5 mm; CC-2, residual tumors 2.5–2.5 cm; CC-3, residual tumors ≥2.5 cm.11 A standard institutional protocol for HIPEC was initiated after CRS as described by Gusani et al.12 Using the closed technique, a roller-pump heat exchanger perfusion machine (ThermoChem HT-100, ThermaSolutions, Melbourne, FL, USA) allowed adequate saline flow (>800 ml/min) and a target intraperitoneal tissue temperature of 42 °C. Mitomycin C dosing included 30 mg added to the perfusate initially for 60 min followed by an additional 10 mg for 40 min, while cisplatin was added to the perfusion circuit at a dose of 50 mg/m2/l of perfusate. Postoperative morbidity was classified according to the Dindo–Clavien grading system.13 For the purposes of analysis, grades 3–4 were considered major complications.
Statistical Analysis
Statistical analysis was performed using STATA 10 (College Station, TX, USA). p values <0.05 were considered significant. Overall survival was calculated from the date of surgery to the date of death. If a patient did not experience death, they were censored at the time of their last follow-up. Time to progression was calculated from the date of surgery to the date of tumor recurrence. If a patient did not experience progression or recurrence, they were censored on the date of their last follow-up or death. Survival times were estimated using the Kaplan–Meier method. Proportional hazards regression was used to examine both univariate and multivariate associations with overall survival and progression-free survival. In univariate analyses, Bonferonni adjustments were made to p values to account for multiple comparisons. All clinic-pathologic factors that were examined in univariate analysis were considered for entry into the model for multivariate analysis. Variables were selected for the final multivariate model based on a stepwise selection method.
RESULTS
Patient Characteristics and Clinical Presentation
Data were available for 65 patients with malignant peritoneal mesothelioma (Table 1). Disease was isolated to the peritoneal cavity in 56 patients (86 %), while nine patients (14 %) had combined peritoneal and pleural disease at presentation. Mean age was 54 years, with majority (74 %) being males. A history of exposure to asbestos was documented in 18 patients (38 %). Majority of patients were symptomatic at presentation, and the most common complaint was abdominal pain (n = 34, 52 %). Prior to the index surgical resection at our institution, 15 patients (24 %) had received chemotherapy, 14 patients (22 %) had undergone cytoreductive surgery, and five patients (8 %) had received intraperitoneal chemotherapy.
TABLE 1.
Preoperative patient characteristics and presentation (n = 65)
| Preoperative characteristics and presentation | |
|---|---|
| Age (mean ± SD) | 54.4 (± 16.1) |
| BMI (mean ± SD) (n = 39) | 26.8 (± 6.7) |
| Asbestos exposure (n, %) (n = 47) | 18 (38.3) |
| Preoperative albumin (mean ± SD) (n = 45) | 3.4 (± 0.8) |
| Gender (n, %) | |
| Male | 48 (73.8) |
| Female | 17 (26.2) |
| ASA (n, %) (n = 38) | |
| 1 | 1 (2.6) |
| 2 | 11 (28.9) |
| 3 | 21 (55.3) |
| 4 | 5 (13.2) |
| Site of disease at presentation (n, %) | |
| Peritoneal cavity | 56 (86.2) |
| Peritoneal + pleural cavities | 9 (13.8) |
| Prior therapy (n, %) | |
| Systemic chemotherapy (n = 62) | 15 (24.2) |
| Cytoreductive surgery (CRS) (n = 64) | 14 (21.9) |
| Chemoperfusion | 5 (7.5) |
| Clinical parameters (n, %), abdominal pain | 34 (52.3) |
BMI body mass index, ASA American Society of Anesthesiology
Operative Characteristics and Pathology
Adequate cytoreduction (CC-0/1) was achieved in 86 % of patients despite a median SPCI of 12. Median operative time and estimated blood loss were 438 min and 600 ml, respectively. A majority of patients underwent omentectomy (92 %), with splenectomy being the most common visceral resection (48 %). All patients received HIPEC, with the drug of choice being mitomycin C (94 %). Pathologic assessment revealed predominantly epithelioid histology (51 patients, 81 %) and biphasic histology (five patients, 8 %), with a majority (51 %) having high-grade tumors. Lymph node involvement was uncommon (five patients, 8 %) (Table 2).
TABLE 2.
Operative characteristics and pathology (n = 65)
| Operative characteristics and pathology | |
|---|---|
| Operative time (min) (mean ± SD) (n = 60) | 438.4 (± 110.7) |
| Estimated blood loss (ml) (median, IQR) (n = 51) |
600 (400–1,500) |
| Simplified peritoneal cancer index (SPCI) (median, IQR) |
12 (8–16) |
| HIPEC (n, %) | 65 (100) |
| HIPEC drug (n, %) | |
| Mitomycin C | 61 (93.8) |
| Cisplatin | 4 (6.2) |
| Completeness of cytoreduction (n, %) | |
| CC-0 | 35 (53.8) |
| CC-1 | 21 (32.3) |
| CC-2 | 5 (7.7) |
| CC-3 | 4 (6.2) |
| Surgical resection (n, %) | |
| Lysis of adhesions | 31 (47.7) |
| Omentectomy | 60 (92.3) |
| Splenectomy | 31 (47.7) |
| Diaphragmatic stripping or resection | 27 (41.5) |
| Hepatectomy or RFA | 6 (9.2) |
| Cholecystectomy | 12 (18.5) |
| Low anterior resection | 6 (9.2) |
| Total abdominal colectomy | 1 (1.5) |
| Small bowel resection | 6 (9.2) |
| Partial gastrectomy | 2 (3.1) |
| Ureterolysis | 11 (16.9) |
| Hysterectomy | 1 (1.5) |
| Ostomy | 6 (9.2) |
| No. of anastamoses (n, %) | |
| None | 46 (70.8) |
| 1 anastamosis | 14 (21.5) |
| 2 anastamoses | 4 (6.2) |
| 3 anastamoses | 1 (1.5) |
| Histologic classification (n, %) (n = 63) | |
| Epithelioid | 51 (81) |
| Sarcomatous | 3 (4.8) |
| Biphasic/mixed | 5 (7.9) |
| Well-differentiated papillary | 2 (3.2) |
| Multicystic | 2 (3.2) |
| Tumor grade (n, %) (n = 41) | |
| Low grade | 20 (48.8) |
| High grade | 21 (51.2) |
| Positive lymph node status (n, %) (n = 61) | 5 (8.2) |
HIPEC hyperthermic intraperitoneal chemoperfusion
Postoperative Characteristics
Of the 65 patients, 91 % were admitted to the intensive care unit (ICU) postoperatively, with median ICU and hospital length of stay being 2 and 12 days, respectively. A total of 12 patients (18 %) underwent reoperation: four patients (33 %) for enteric leak, 1 patient (8 %) for an intra-abdominal abscess, and seven patients (58 %) for other reasons. Major postoperative morbidity (grade III/IV) occurred in 23 patients (35 %); most commonly pulmonary (25 patients, 39 %), cardiac (15 patients, 23 %), and wound complications (16 patients, 25 %). The 60-day mortality rate was 6 % (four patients). Of the 51 patients with available data, adjuvant chemotherapy was administered to 7 (14 %) (Table 3).
TABLE 3.
Postoperative complications (n = 65)
| Complications | |
|---|---|
| Morbidity (n, %) | |
| None | 18 (27.7) |
| Minor morbidity (grade I/II) | 20 (30.8) |
| Major morbidity (grade III/IV) | 23 (35.4) |
| 60-day mortality (n, %) | 4 (6.2) |
| Wound infection (n, %) | 16 (24.6) |
| Sepsis (n, %) | 10 (15.4) |
| Postoperative bleeding (n, %) | 2 (3.1) |
| Cardiac (n, %) | 15 (23.1) |
| Pulmonary (n, %) | 25 (38.5) |
| Pancreatic leak (n, %) | 3 (4.6) |
| Enterocutaneous fistula (n, %) | 6 (9.2) |
| Reason for return to OR (n, %) (n = 12) | |
| Anastamotic leak | 4 (33.3) |
| Intra-abdominal abscess | 1 (8.3) |
| Other | 7 (58.3) |
| Reason for return to ICU (n, %) (n = 11) | |
| Cardiac | 2 (18.2) |
| Postoperative bleed | 1 (9.1) |
| Hypoxia | 8 (72.2) |
| Hospital length of stay (days) (median, IQR) | 12 (9–18) |
| ICU length of stay (days) (median, IQR) (n = 64) | 2 (1–5) |
| 30-day hospital readmission (n, %) (n = 62) | 8 (12.9) |
| Adjuvant chemotherapy (n, %) (n = 50) | 7 (14) |
ICU intensive care unit
Oncologic Outcomes
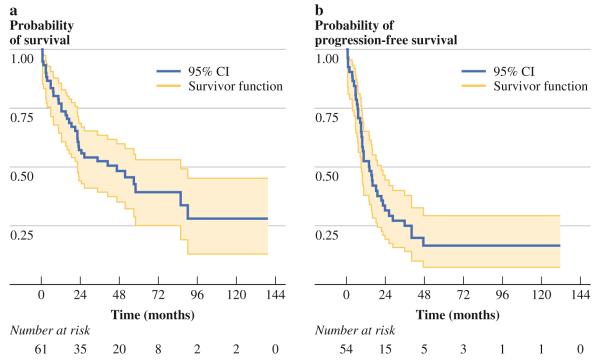
Median follow-up time was 36.8 months (IQR, 12.2– 53.1 months). Death occurred in 37 patients (57 %), while tumor progression occurred within the peritoneal cavity alone in 21 patients (70 %) and within both peritoneal and pleural cavities in nine patients (27 %). A total of 13 patients had unknown disease progression status at last follow-up. Median overall survival was 46.2 months, with 1-, 2-, and 5-year overall survival probability of 77, 57, and 39 %, respectively (Fig. 1a). Median progression-free survival was 13.9 months, with 1-, 2-, and 5-year disease failure probability of 47, 68, and 83 %, respectively (Fig. 1b).
FIG. 1.
a Kaplan–Meier overall survival curve for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (n = 65). Median overall survival was 46.2 months, with 1-, 2-, and 5-year overall survival probability of 77, 57, and 39 %, respectively. b Kaplan–Meier curve for time to progression for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (n = 54). Median progression-free survival was 13.9 months, with 1-, 2-, and 5-year disease failure probability of 47, 68, and 83 %, respectively
Univariate associations with poor survival were examined for age, gender, BMI, ASA, preoperative albumin level, asbestos exposure, symptoms, completeness of cytoreduction, SPCI, blood loss, operative time, specific visceral resection, number of anastomoses, tumor histology/grade, lymph node involvement, and postoperative morbidity. On univariate analysis, significant predictors of poor survival included older age at surgery (p = 0.02), lower preoperative albumin level (p = 0.0001), higher SPCI (p = 0.04), higher CC-score (p = 0.01), incomplete cytoreduction CC-2/3 (p = 0.001), higher EBL (p = 0.003), need for intraoperative blood transfusion (p = 0.05), high-grade tumor histology (p = 0.004), aggressive histology (i.e. sarcomatoid, biphasic), (p = 0.003), major wound infection (p = 0.004), postoperative sepsis (p = 0.04), prolonged ileus >3 weeks (p = 0.002), low albumin level at discharge (p = 0.04), and reoperation for complications (p = 0.04). There was a trend toward poor survival in patients with asbestos exposure (p = 0.06) and those with cardiac complications (p = 0.06). In a multivariate Cox-regression model, age at surgery, SPCI >15, incomplete cytoreduction (CC-2/3), aggressive histology (sarcomatoid, biphasic), and postoperative sepsis were joint significant predictors of poor survival (chi square = 42.8; p = 0.00001) (Table 4).
TABLE 4.
Multivariate predictors of mortality and poor progression
| Factor | Hazard ratio | Standard error | 95 % CI for HR | p value |
|---|---|---|---|---|
| Predictors of mortality (χ2 = 42.8; p = 0.00001) | ||||
| Age at surgery (baseline <60 years) | 1.1 | 0.02 | (1.02–1.1) | 0.001 |
| SPCI (baseline <15) | 2.6 | 1.2 | (1.1–6.2) | 0.04 |
| CC score (baseline = CC-0/1) | 2.6 | 1.3 | (1.0–6.9) | 0.05 |
| Histology (baseline = epithelioid, well-differentiated papillary, multicystic) | 5.5 | 3.0 | (1.9–16.1) | 0.002 |
| Sepsis | 2.1 | 0.9 | (0.9–4.9) | 0.08 |
| Predictors of progression (chi square = 30.6; p = 0.00001) | ||||
| Age at surgery (baseline <60 years) | 1.03 | 0.01 | (1.0–1.05) | 0.02 |
| SPCI (baseline <15) | 3.4 | 1.3 | (1.5–7.3) | 0.002 |
| CC score (baseline = CC-0/1) | 6.4 | 4.6 | (1.5–26.3) | 0.01 |
| Histology (baseline = epithelioid, well-differentiated papillary, multicystic) | 5.4 | 2.6 | (2.1–14.0) | 0.001 |
Significant predictors of poor progression on univariate analysis included older age at surgery (p = 0.02), low preoperative albumin level (p = 0.0001), higher SPCI (p = 0.04), higher CC-score (p = 0.01), higher EBL (p = 0.003), need for intraoperative blood transfusion (p = 0.05), high-grade tumor histology (p = 0.004), incomplete cytoreduction CC-2/3 (p = 0.001), aggressive histology (i.e. sarcomatoid, biphasic), (p = 0.003), major wound infection (p = 0.004), prolonged ileus >3 weeks (p = 0.002), and low albumin level at discharge (p = 0.04). In a multivariate Cox-regression model, age at surgery, SPCI >15, incomplete cytoreduction (CC-2/3), and aggressive histology (sarcomatoid, biphasic) were joint significant predictors of disease progression (Chi square = 30.6; p = 0.00001) (Table 4).
DISCUSSION
Recent success with CRS/PIC in peritoneal surface malignancies, such as pseudomyxoma peritonei and ovarian cancer, has led to a number of centers publishing their experience with this treatment strategy in MPM, demonstrating median survival up to 92 months and 5-year survival rates up to 59 % in select patients.14–17 These oncologic outcomes are far superior to the 12–27 months median survival with systemic chemotherapy and best supportive care strategies.4,6,14 However, no randomized or comparative trials of CRS/HIPEC have been published, and oncologic outcomes vary significantly, based on a number of important prognostic factors identified in various small single-institution studies.
The prognostic effect of tumor histology on survival and progression has been consistently demonstrated in a number of published series.18–20 Our data also demonstrates that patients with aggressive histologies, including sarcomatoid and biphasic subtypes, derive minimal benefit from CRS/HIPEC with short survival (median survival, 51.5 months [95 % CI 23–90.2 months] for epithelioid/well-differentiated papillary/multicystic versus 10.5 months [95 % CI 0.5–17 months] for sarcomatoid/biphasic histology) and early progression (median PFS, 16 months [95 % CI 10.1–26.1 months] for epithelioid/well-differentiated papillary/multicystic versus 6.3 months [95 % CI 0.5–10.6 months] for sarcomatoid/biphasic histology). Although neoadjuvant and adjuvant chemotherapy are often used in these patients, there is little data to support the efficacy of this approach. A lack of understanding of MPM carcinogenic and resistance mechanisms has hampered the development of effective systemic therapies. The highly chemoresistant nature of MPM infers activity of multiple tumor resistance and survival mechanisms.21,22 Molecular targeted therapies against various aberrantly activated growth factor receptors and cellular pathways, involved in tumor invasion and metastasis, are currently being tested in preclinical, phase I, and phase II studies.23 Newer technologies such as next-generation sequencing techniques will help identify genes/pathways that are critical to development of MPM, provide reliable prognostic/predictive gene signatures, and facilitate application of effective targeted therapies, based on patient-specific contexts and tumor vulnerabilities.24–26
Consistent with previously published data, the ability to achieve adequate macroscopic resection of MPM to less than 2.5-mm residual deposits (CC-0/1) is an important independent prognostic factor in our multivariate models of survival (median OS, 56.7 months [95 % CI 23–. months] for CC-0/1 versus 7.4 months [95 % CI 0.5–24.7 months] for CC-2/3 resection) and disease progression (median PFS, 15.8 months [95 % CI 9.6–23.8 months] for CC-0/1 versus 5.4 months [95 % CI 0.5–. months] for CC-2/3 resection).18,19,27,28 In 1978, Dedrick and colleagues demonstrated limited depth of penetration of intraperitoneal chemotherapy to <3 mm, providing a rationale for adequate macroscopic tumor resection prior to HIPEC. The addition of hyperthermia increases the depth of penetration of chemotherapy, has direct toxic effects on tumor cells, and has a synergistic effect with specific cytotoxic drugs.29,30 Yan and colleagues published a CT-based algorithm for preoperatively predicting successful complete cytoreduction. According to their analysis, large tumor volume in the epigastrium and along the small bowel and its mesentery is especially difficult to cytoreduce adequately.31
We found that intraoperative SPCI at the time of CRS was a significant predictor of disease progression and survival. Our data suggest that patients with SPCI >15 are unlikely to benefit from aggressive CRS/HIPEC (median OS, 85.6 months [95 % CI 22.6–. months] for SPCI ≤15 versus 12.2 months [95 % CI 0.5–46.2 months] for SPCI >15; median PFS, 18.1 months [95 % CI 9.6–35.6 months] for SPCI ≤15 versus 6.3 months [95 % CI 1–13.5 months] for SPCI >15). In 2010, Yan and colleagues proposed a TNM-based clinicopathologic staging system for survival in MPM and used PCI as a surrogate for T-stage stratification.32 Similarly, SPCI-based stratification of our patient population into T1 (SPCI 0–5), T2 (SPCI 6–10), T3 (SPCI 11–15), and T4 (SPCI 16–21) was used to validate their proposed TNM staging system: stage 1 (T1N0M0), stage II (T2–3N0M0), and stage III (T1–4N1M1). Our data demonstrate a significant trend toward stratification of survival by stage that is consistent with the proposed TNM staging system, with 5-year probability of survival for patients with stage I, II, and III disease being 100, 48 % (95 % CI 26.8–66.4 %) and 19 % (95 % CI 4.1–42.8 %), respectively. With this TNM staging system, the 5-year probability of PFS for our patients with stage I, II, and III disease was 50 % (95 % CI 0.6–91.0 %), 21 % (95 % CI 6.8–41.4 %), and 10 % (95 % CI 1.8–27.7 %), respectively. The accuracy of preoperative SPCI assessment will improve with emerging MR imaging techniques, as demonstrated by Barone et al.,33 and will help formulate therapeutic plans based on accurate preoperative stage stratification.
We demonstrate a significant independent effect of patient age on survival and disease progression consistent with data from Feldman and colleagues at the NIH.28 Patients older than 65 years of age had poor survival (median OS, 85.6 months [95 % CI 40.7–. months] for age B65 years versus 17 months [95 % CI 3–22.2 months] for age >65 years) and early disease progression (median PFS, 23.8 months [95 % CI, 9.6–. months] for age ≤65 years versus 9.1 months [95 % CI 6.8–13.9 months] for age >65 years), without evidence for increased treatment-related morbidity within this subgroup.
A total of nine patients (14 %) in our study had combined peritoneal and pleural disease. Two patients who developed small, stable, asymptomatic pleural plaques without abdominal disease recurrence on follow-up imaging 10 months and 3 years after CRS+HIPEC for MPM; the first patient was lost to follow-up, while the other remains under surveillance. One patient developed a persistent postoperative pleural effusion after CRS/HIPEC and underwent pleurodesis for positive cytology, without recurrence. Two patients died within 1 year of CRS/HIPEC from rapid peritoneal and pleural disease progression. One patient had previously undergone bilateral pleurectomy procedures and hyperthermic intrathoracic chemoperfusion (HITEC) a year prior to developing MPM. This patient then underwent CRS/HIPEC and remains disease-free. Another patient had also undergone CRS/HITEC 3 years previously and underwent palliative CRS/HIPEC for severe, debilitating ascites and pain from MPM. She died of complications in the early postoperative period. One patient underwent palliative resection of mediastinal mesothelioma for SVC syndrome 20 months following CRS/HIPEC and died 26 months following that procedure without abdominal disease recurrence. The last patient underwent CRS/HITEC 6 months following CRS/HIPEC, then recurred in the chest cavity after 13 months and died 15 months later despite systemic chemotherapy. This small subgroup of patients with combined pleural and peritoneal mesothelioma did not demonstrate survival difference from those with MPM alone.
In conclusion, aggressive CRS/HIPEC is an effective therapeutic strategy in select patients with MPM. Disease control rates and long-term survival for patients with aggressive histologies, such as sarcomatoid and biphasic subtypes, are similar to historical controls receiving systemic therapy and best supportive care and are unlikely to benefit from CRS/HIPEC while being subjected to the inherent morbidity and potential mortality associated with this procedure. Similarly, patients with high tumor burden and those with tumors that cannot be adequately cytoreduced are poor candidates for CRS/HIPEC. Therefore, careful patient selection is essential until more effective targeted chemotherapeutic agents are available.
Footnotes
Disclosure None.
REFERENCES
- 1.Munkholm-Larsen S, Cao CQ, Yan TD. Malignant peritoneal mesothelioma. World J Gastrointest Surg. 2009;1:38–48. doi: 10.4240/wjgs.v1.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol. 2004;159:107–12. doi: 10.1093/aje/kwh025. [DOI] [PubMed] [Google Scholar]
- 3.Yan TD, Welch L, Black D, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol. 2007;18:827–34. doi: 10.1093/annonc/mdl428. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 5.van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–9. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 6.Janne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer. 2005;7:40–6. doi: 10.3816/CLC.2005.n.020. [DOI] [PubMed] [Google Scholar]
- 7.Simon GR, Verschraegen CF, Janne PA, Langer CJ, Dowlati A, Gadgeel SM, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol. 2008;26:3567–72. doi: 10.1200/JCO.2007.15.2868. [DOI] [PubMed] [Google Scholar]
- 8.Janne PA, Simon GR, Langer CJ, Taub RN, Dowlati A, Fidias P, et al. Phase II trial of pemetrexed and gemcitabine in chemotherapy-naive malignant pleural mesothelioma. J Clin Oncol. 2008;26:1465–71. doi: 10.1200/JCO.2007.14.7611. [DOI] [PubMed] [Google Scholar]
- 9.Swellengrebel HA, Zoetmulder FA, Smeenk RM, Smeenk RM, Antonini N, Verwaal VJ. Quantitative intra-operative assessment of peritoneal carcinomatosis—a comparison of three prognostic tools. Eur J Surg Oncol. 2009;35:1078–84. doi: 10.1016/j.ejso.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Verwaal VJ, van Tinteren H, van Ruth S, Zoetmulder FA. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2004;91:739–46. doi: 10.1002/bjs.4516. [DOI] [PubMed] [Google Scholar]
- 11.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15:204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 12.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15:754–63. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484–92. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 15.Austin F, Mavanur A, Sathaiah M, Steel J, Lenzner D, Ramalingam L, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19:1386–93. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 17.Rufian S, Munoz-Casares FC, Briceno J, Díaz CJ, Rubio MJ, Ortega R, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol. 2006;94:316–24. doi: 10.1002/jso.20597. [DOI] [PubMed] [Google Scholar]
- 18.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–42. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 19.Baratti D, Kusamura S, Cabras AD, Laterza B, Balestra MR, Deraco M. Lymph node metastases in diffuse malignant peritoneal mesothelioma. Ann Surg Oncol. 2010;17:45–53. doi: 10.1245/s10434-009-0756-2. [DOI] [PubMed] [Google Scholar]
- 20.Schaub NP, Alimchandani M, Quezado M, Kalina P, Eberhardt JS, Hughes MS, et al. A novel nomogram for peritoneal mesothelioma predicts survival. Ann Surg Oncol. 2013;20:555–61. doi: 10.1245/s10434-012-2651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 22.Fennell DA, Gaudino G, O’Byrne KJ, Mutti L, van Meerbeeck J. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol. 2008;5:136–47. doi: 10.1038/ncponc1039. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo C, Bei R, Procopio A, Modesti A. Molecular targets and targeted therapies for malignant mesothelioma. Curr Med Chem. 2008;15:855–67. doi: 10.2174/092986708783955446. [DOI] [PubMed] [Google Scholar]
- 24.Bueno R, De Rienzo A, Dong L, Gordon GJ, Hercus CF, Richards WG, et al. Second generation sequencing of the mesothelioma tumor genome. PloS One. 2010;5:e10612. doi: 10.1371/journal.pone.0010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melaiu O, Cristaudo A, Melissari E, Di Russo M, Bonotti A, Bruno R, et al. A review of transcriptome studies combined with data mining reveals novel potential markers of malignant pleural mesothelioma. Mutat Res. 2012;750:132–40. doi: 10.1016/j.mrrev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Rios F, Chuai S, Flores R, Shimizu S, Ohno T, Wakahara K, et al. Global gene expression profiling of pleural mesotheliomas: overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res. 2006;66:2970–9. doi: 10.1158/0008-5472.CAN-05-3907. [DOI] [PubMed] [Google Scholar]
- 27.Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2006;13:229–37. doi: 10.1245/ASO.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560–7. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 29.Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27:365–74. doi: 10.1053/ctrv.2001.0232. [DOI] [PubMed] [Google Scholar]
- 30.Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98:247–52. doi: 10.1002/jso.21051. [DOI] [PubMed] [Google Scholar]
- 31.Yan TD, Haveric N, Carmignani CP, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005;103:839–49. doi: 10.1002/cncr.20836. [DOI] [PubMed] [Google Scholar]
- 32.Yan TD, Deraco M, Elias D, Glehen O, Levine EA, Moran BJ, et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer. 2011;117:1855–63. doi: 10.1002/cncr.25640. [DOI] [PubMed] [Google Scholar]
- 33.Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2012;19:1394–401. doi: 10.1245/s10434-012-2236-3. [DOI] [PubMed] [Google Scholar]