Abstract
Dominant mutations in sarcomere proteins such as the myosin heavy chains (MHC) are the leading genetic causes of human hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). Here we demonstrate that expression of the HCM cardiac MHC gene (Myh6) R403Q mutation in mice can be selectively silenced by an RNAi cassette delivered by an adeno-associated virus vector. RNAi-transduced MHC403/+ mice had neither hypertrophy or myocardial fibrosis, the pathologic manifestations of HCM for at least 6 months. As inhibition of HCM was achieved by only a 25% reduction in the levels of the mutant transcripts, we suggest that the variable clinical phenotype in HCM patients reflects allelic-specific expression and that partial silencing of mutant transcripts may have therapeutic benefit.
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease characterized by an increase in left ventricular wall thickness (LVWT), disorganization of cardiomyocytes and expansion of myocardial fibrosis that occurs in the absence of systemic disease (1–3). HCM is the leading cause of non-violent sudden death in young adults and the most common cause of sudden death on the athletic field(4). HCM is caused by mutations in genes that encode protein constituents of the cardiac sarcomere, the contractile unit of muscle (5, 6) More than 1000 distinct pathogenic mutations have been identified, and over half of these occur in MYH7 (encoding β myosin heavy chain) and MYBPC (encoding myosin binding protein-C)(7). Most HCM mutations, and all that occur in MYH7, are missense mutations, producing amino acid substitutions in myosin that perturb the sarcomere’s contractile function.
The human HCM mutation MYH7 R403Q causes particularly severe disease that is characterized by early-onset and progressive myocardial dysfunction, and a high incidence of sudden cardiac death (8). Heterozygous MHC403/+ mice express the R403Q mutation in Myh6, under the control of the endogenous Myh locus. Myh6 and MYH7 are highly homologous in sequence and encode the predominant myosin isoforms in the adult hearts. MHC403/+ mice recapitulate human HCM and develop hypertrophy, myocyte disarray and increased myocardial fibrosis (9). Analyses of mutant myosins isolated from MHC403/+ mice showed that HCM mutations cause fundamental changes in sarcomere functions, including increased acto-myosin sliding velocity, force generation, and ATP hydrolysis (10). These changes in turn alter calcium cycling and gene transcription in myocytes and ultimately induce pathologic remodeling of the heart in vivo (11, 12,13). Understanding this pathogenic cascade has led to the identification of secondary signaling molecules as potential therapeutic targets (13,14), but no strategies have been defined that correct the primary biophysical and biochemical abnormalities of sarcomeres with HCM mutations.
Selective reduction in the expression of the mutant protein would be the most direct approach for correcting sarcomere dysfunction. As a first step in pursuing this strategy, we determined whether in vivo allele-specific repression of Myh6 R403Q was feasible. Because mice hemizygous for a normal Myh6 gene are viable, fertile, and have essentially normal cardiac function (15), we reasoned that inactivation of the mutant sarcomere protein allele is unlikely to have adverse cardiovascular effects. We used an RNA interference (RNAi) construct because this powerful tool has successfully reduced gene expression in many systems and can distinguish between genes that differ by one single nucleotide (16). We selected adeno-associated virus packed with serotype 9 capsid (AAV-9) as a delivery vehicle because this vector has strong tropism for cardiac tissues (17,18). To enhance the cardiac tropism we engineered the vector so that AAV-9 expression was under the control of the cardiac specific troponin T (cTnT) promoter.
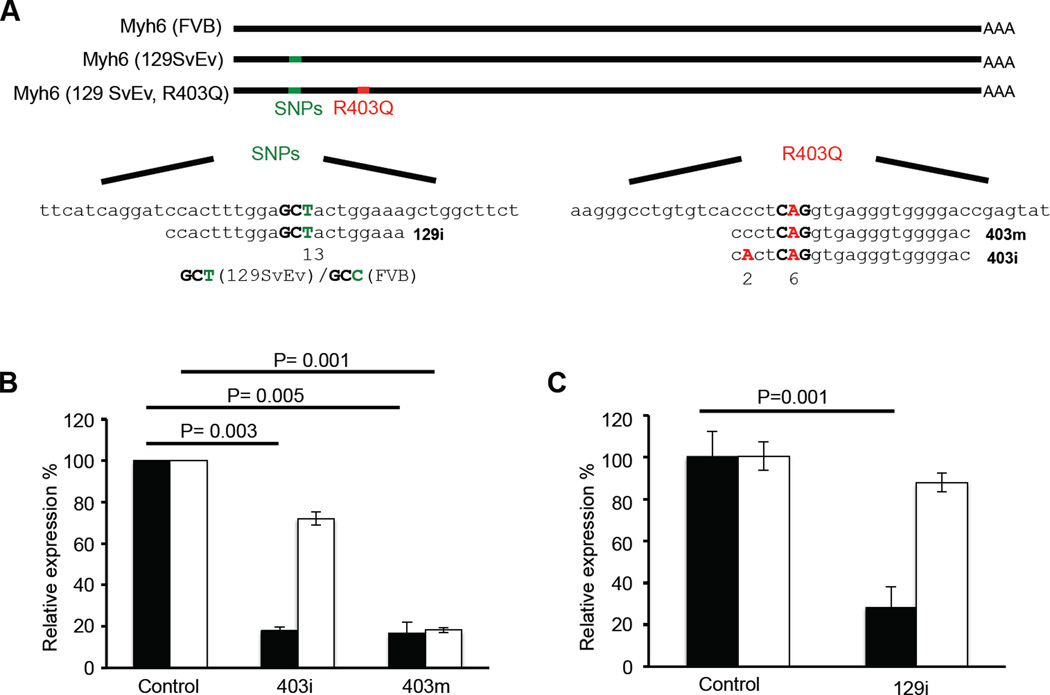
We produced 17 unique RNAi constructs, co-transfected each with a plasmid carrying the Myh6 R403Q mutant gene into 293T human embryonic kidney cells (fig.S1a). One RNAi construct, designated 403m, significantly reduced Myh6 R403Q expression (Fig. 1A, B). To assess its specificity, we transfected wild-type or mutant Myh6 into 293T cells with 403m constructs. Because there was significant silencing (~80%) of both wild-type and mutant Myh6 expression, we introduced an additional mismatch into the 403m RNAi construct (designated 403i; Fig. 1A). 403i had modest reduction (~20%) of wild-type Myh6 expression, but retained approximately 80% reduction in the expression of Myh6 R403Q transcript in 293T cells (Fig. 1B).
Fig. 1. Selective silencing of Myh6 R403Q expression by AAV-9-mediated RNAi.
(A) Schematic representation of FVB, 129SvEv, 129SvEv mutant (R403Q) transcript and RNAi sequences. (B) Quantitative real-time PCR analysis of wild-type Myh6 (white bar) and mutant Myh6 R403Q (black bar) expression after transduction of the 403m and 403i constructs (n=4). Levels of the transcripts were normalized to control LacZ RNAi. (C) Quantitative real-time PCR analysis of FVB Myh6 (white bar) and 129SvEv Myh6 R403Q (black bar) expression after transduction of the 129i construct (n=4). Levels of the transcripts were normalized to control LacZ RNAi. Data are presented as the mean ± s.d.
To ascertain the cardiac selectivity of AAV-9-cTnT vector, we used enhanced green fluorescent protein (EGFP) (fig.S1b). Virus was injected (5×1013 vector genomes (vg)/kg) into the thoracic cavity of one-day old mice (Supplementary Methods) and after 3 weeks, all organs were dissected and EGFP expression was assessed by fluorescence microscopy. EGFP expression occurred exclusively in the heart and was absent in other organs including the brain, lung and spleen (fig. S2). EGFP expression was present within 48 hours after virus transduction and remained robust for 12 months (fig. S2, 3).
We next engineered 403i shRNA or control shRNA (denoted 403i RNAi and control RNAi, respectively) into the AAV-9-cTnT-EGFP-RNAi vector so that all cells expressing EGFP would also express shRNAs. To assess the efficacy of 403i shRNA in vivo, we injected variable amounts of 403i RNAi-encoding viruses (5×109, 5×1011 and 5×1013 vg/kg) into the thoracic cavity of one-day old mice. Two weeks after viral transduction, total RNA extracted from each left ventricle (LV) was individually analyzed by RNA-seq(19). Sequencing reads that corresponded to Myh6 R403Q or wild-type Myh6 were counted and visualized using Integrative Genomics Viewer (IGV, Broad Institute, MA). The expression of Myh6 was comparable in LV tissues after transduction with control RNAi (12,118 reads per million transcripts) and 403i RNAi (11,675 reads per million transcripts), indicating that the wild-type allele was not silenced in vivo. In contrast, the ratio of Myh6 R403Q to Myh6 (wild-type) reads varied between 403i RNAi titers. Only the highest titer (5×1013 vg/kg) resulted in a significant reduction (28.5 %) in the relative expression of Myh6 R403Q compared to wild-type Myh6 transcripts (P = 2.5E-5) (fig.S1c).
To assess the impact of silencing Myh6 R403Q on HCM development, we injected virus encoding the 403i RNAi cassette (n= 8) or control RNAi cassette (n=7) into the thoracic cavity of one-day old male MHC403/+ mice. At 5 to 6 weeks of age, all mice were given cyclosporine A (CsA) for 3 weeks to accelerate the emergence of HCM histopathology, as described(13). Mice were serially evaluated by echocardiography and at sacrifice, the hearts were analyzed by histopathology. After CsA treatment, control RNAi-transduced mice had LV hypertrophy and severe HCM histopathogy (Fig. 2A), similar to non-transduced, CsA-treated MHC403/+ mice (13).
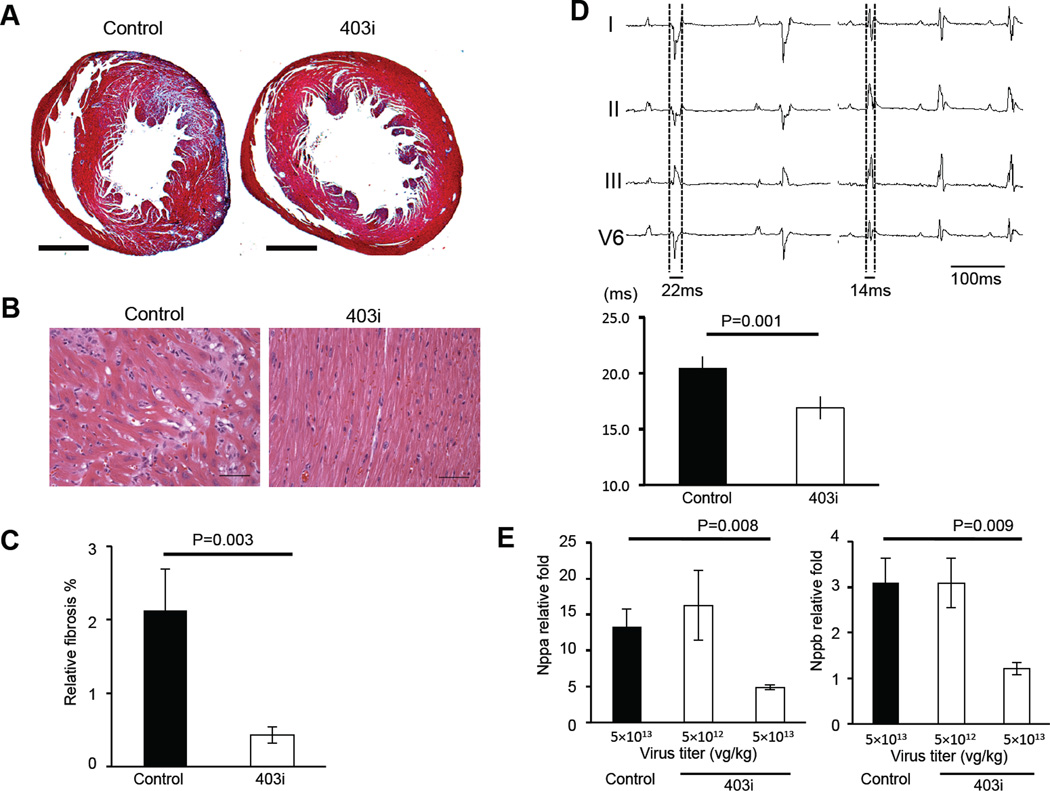
Fig. 2. In vivo effect of Myh6 R403Q silencing.
(A) Cardiac histopathology from MHC403/+ mice transduced with control RNAi (left) and 403i RNAi (right). Masson trichrome staining reveals marked fibrosis (blue) in MHC403/+ mice transduced with control RNAi. Bar =1 mm. (B) Hematoxylin and eosin staining shows myocyte disarray in MHC403/+ mice transduced with control RNAi (left) and normal myocyte architecture in mice transduced with 403i RNAi (right). Bar = 100 µm. (C) Quantification of myocardial fibrosis in MHC403/+ mice transduced with control RNAi (black bar, n=4) and 403i RNAi (white bar, n=4). (D) An electrocardiogram of MHC403/+ mice transduced with control RNAi (left top panel) and 403i RNAi (right top panel). Mice transduced with control RNAi have prolonged QRS (ventricular conduction) interval and high voltage P waves consistent with LV hypertrophy and atrial enlargement. The bottom panel presents QRS intervals from mice transduce with control RNAi (black bar, n=5) and 403i RNAi (white bar, n=6). (E) Quantitative real-time PCR analysis of Nppa (left panel) and Nppb (right panel) expression after transduction of control RNAi (black bar) and two different doses of 403i constructs (white bar) (n=5). Levels of the transcripts were normalized to transcript levels from age matched wild-type hearts. Data are presented as the mean ± s.d.
In contrast, CsA-treated MHC403/+ mice transduced with 403i RNAi did not develop HCM (Table 1, upper panel). The left ventricular wall thickness (LVWT) of 403i RNAi-transduced mice (0.84 ± 0.10 mm) was significantly less than that of mice transduced with control RNAi (1.52 ± 0.25 mm, P = 1.9E-5) and comparable to the LVWT of wild-type mice (0.74 ± 0.05mm, NS) (12). Myocardial disarray (Fig. 2B) was absent and fibrosis (Fig. 2C) was significantly reduced in 403i RNAi-transduced mice (0.43 ± 0.11%) compared to control RNAi-transduced hypertrophic MHC403/+ mice (2.12 ± 0.57%, P = 0.003). QRS interval prolongation, an electrocardiographic feature of LV hypertrophy, was present in mice transduced with control RNAi (20.5 ± 1.2 ms) but not in mice transduced with 403i RNAi (16.9 ± 1.4 ms, P = 0.001) (Fig. 2D). Additionally, the expression of prototypic LV hypertrophy markers Nppa and Nppb were 2.5 fold higher in mice transduced with control RNAi compared to 403i RNAi (Fig. 2E).
Table 1.
RNAi effects on cardiac morphology and function in HCM mice. To accelerate hypertrophic remodeling in MHC403/+ mice CsA was administered for the number of weeks indicated either after (Post) RNAi transduction on day 1 or for 3 weeks prior (Pre) to RNAi transduction on day 21. No CsA denotes MHC403/+ mice not treated with CsA. Age, denotes age at time of cardiac evaluation; (#) number of mice studies. LVDD, LV diastolic dimensions; LVWT, LV wall thickness; FS, percent fractional shortening. Cardiac dimensions and function with associated P values, calculated by T-test, reflect comparisons to MHC403/+ transduced with control RNAi. Values for wildtype 129SvEv mice, not treated with CsA, are shown for comparison. Data are presented as the mean ± s.d
| CsA | RNAi | Age (#) | LVDD | P value | LVWT | P value | FS (%) | P value |
|---|---|---|---|---|---|---|---|---|
| 3 weeks, Post | Control | 8 weeks (n=7) | 2.53 ± 0.41 | 1.52 ± 0.25 | 32.68 ± 6.68 | |||
| 403i | 8 weeks (n=8) | 3.04 ± 0.21 | 0.01 | 0.84 ±0.10 | 1.90E-05 | 40.37 ± 5.39 | 0.04 | |
| 3 weeks, Pre | None | 6 weeks (n=3) | 2.92 ± 0.28 | 1.40 ± 0.11 | 28.38 ± 5.16 | |||
| 403i | 14 weeks (n=3) | 3.50 ± 0.16 | NS | 1.42 ± 0.06 | NS | 30.61 ± 4.59 | NS | |
| No CsA | Control | 6 months (n=6) | 3.54 ± 0.27 | 0.93 ± 0.11 | 33.30 ± 4.02 | |||
| 403i | 6 months (n=5) | 3.75 ± 0.21 | NS | 0.68 ±0.09 | 0.005 | 33.42 ± 7.61 | NS | |
| Control | 11 months (n=6) | 3.66± 0.25 | 0.98 ± 0.16 | 37.8 ± 11.11 | ||||
| 403i | 11 months (n=5) | 3.93 ± 0.48 | NS | 0.87 ± 0.11 | NS | 36.11± 8.86 | NS | |
| 2 weeks, Post | Control | 6 weeks (n=5) | 2.62± 0.20 | 1.37 ± 0.07 | 33.75 ± 4.51 | |||
| 129i | 6 weeks (n=4) | 3.85 ± 0.22 | 0.001 | 0.73 ± 0.03 | 1.60E-06 | 28.21 ± 9.34 | NS | |
| WT, no CsA | None | 6 months (n=4) | 3.75 ± 0.28 | 0.74 ± 0.05 | 30.01 ± 3.73 | |||
To assess if the early age at transduction and/or viral titer influenced HCM development, we injected high (5×1013 vg/kg) and low (5×1012 vg/kg) titer viruses of 403i RNAi in 3-week old MHC403/+ mice (n=5). At 4 weeks, mice were treated with CsA for 3 additional weeks followed by echocardiography to assess LVWT and diastolic (relaxation) performance (left atrial diameter normalized to the aortic root diameter(20)) which becomes abnormal early in HCM (21) Mice transduced with high viral titers of control RNAi or low viral titers of 403i RNAi had both LV hypertrophy and diastolic dysfunction (Table 2). In contrast, mice transduced with high titer 403i RNAi virus had neither hypertrophy (LVWT= 0.72 ± 0.05 mm, P = 1.9E-6 compared to control RNAi) nor diastolic dysfunction (1.17 ± 0.09, P = 0.009 compared to control RNAi).
Table 2.
Viral dosage need for RNAi effects on cardiac morphology and function in HCM mice. MHC403/+ mice (n= number) were transduced with RNAi at vector genomes per kg (titer) per kg on day 1 and treated with CsA for 3 weeks to accelerate hypertrophic remodeling. LVDD (LV diastolic dimension; LVWT, LV wall thickness; FS%, percent fractional shortening and left atria (LA) dimension normalized to the aortic root (Ao) are provide. Cardiac dimensions and function with associated P values, calculated by T-test, reflect comparisons to MHC403/+ transduced with control RNAi. Data are presented as the mean ± s.d
| RNAi | titer | LVDD (mm) |
P value | LVWT (mm) |
P value | FS (%) | P value | LA/Ao root | P value |
|---|---|---|---|---|---|---|---|---|---|
| Control (n=5) | 5×1013 | 3.18 ± 0.24 | 1.34 ± 0.09 | 31.18 ± 7.27 | 1.46 ± 0.12 | ||||
| 403i (n=5) | 5×1013 | 3.33 ± 0.15 | NS | 0.72 ± 0.05 | 1.9E-6 | 38.09 ± 4.64 | NS | 1.17 ± 0.09 | 0.009 |
| 403i (n=5) | 5×1012 | 3.06 ± 0.34 | NS | 1.26 ± 0.26 | 30.33 ± 3.64 | 1.39 ± 0.12 |
Using the high titer virus we next asked whether 403i RNAi-transduction could alter established HCM, by pretreating MHC403/+ mice with CsA for 3 weeks to induce hypertrophy (LVWT, 1.40 ± 0.11 mm) prior to viral transduction. Echocardiography assessments at two months after 403i RNAi (n=3) transduction showed no change in LVWT (Table 1, middle panel), suggesting the treatment was ineffective in reversing established disease.
To determine whether 403i RNAi-transduction affected the pathologic LV remodeling that slowly emerges in MHC403/+ mice with age in the absence of CsA, we monitored LV hypertrophy in mice transduced with a single high titer dose of 403i RNAi (n=5) or control RNAi (n=6) on day one of life. At 6 months, mice transduced with control RNAi had LV hypertrophy (LVWT, 0.93 ± 0.11 mm). There was no LV hypertrophy in mice transduced with 403i RNAi (LVWT= 0.68 ± 0.09 mm, P = 0.004) and LVWT was indistinguishable from wild-type mice (LVWT, 0.74 ± 0.05 mm, P = NS). Importantly, the protective effect of the 403i RNAi-vector dissipated over time. LV hypertrophy emerged in 403i RNAi-transduced mice by 11 months of age (LVWT, 0.87 ± 0.11 mm) and was comparable to that observed in control RNAi-transduced mice. The inadequacy to fully suppress hypertrophy in MHC403/+ mice life-long is presumably due to diminished AAV-mediated transgene expression that occurs 7 months after transduction (22) and sub-therapeutic 403i RNAi levels.
Finally, we considered whether a single RNAi might silence different, patient-specific mutations in the same gene, by targeting a nearby single nucleotide polymorphism (SNP) that distinguished the mutant from wild-type alleles. To test this model, we produced male F1 offspring from 129SvEv MHC403/+ and wild-type FVB crosses. We constructed an RNAi that targeted a 129SvEv SNP on the Mhy6 allele (designated 129i, Fig. 1A) and transfected this with Mhy6 R403Q (129SvEv) or wild-type Myh6 (FVB) plasmids into 293T cells. The 129i RNAi decreased Mhy6 R403Q levels by 75% and reduced wild-type Myh6 (FVB) by only 15% (Fig. 1C). We produced AAV-9-cTnT-EGFP-129i virus and transduced (5×1013 vg/kg) one-day old male F1 MHC403/+ mice with 129i RNAi (n= 4) or control RNAi (n=5). At 4 weeks of age, mice were treated with CsA for 2 weeks and studied by echocardiography. Control RNAi-transduced mice developed LV hypertrophy (LVWT=1.37 ± 0.03 mm) but not MHC403/+ mice transduced with 129i RNAi (LVWT = 0.73 ± 0.07 mm; P = 1.6E-6, Table 1). We conclude from these studies that one RNAi construct which targeted a SNP that demarcates mutant and wild-type alleles could be used to silence distinct HCM mutations in a gene or to augment mutation-specific RNAi.
AAV-9 mediated RNAi preferentially suppresses the expression of the Myh6 R403Q allele in a mouse model of HCM, by directly targeting the mutation or a nearby SNP. It is noteworthy that in a mouse model characterized by accelerated onset and severity of HCM, reduction in the expression levels of the mutant allele by only 28.5% (fig. 1Sc) was sufficient to abrogate hypertrophy and histopathologic remodeling for several months. Indeed, the LVWT in 403i-treated MHC403/+ mice, with or without CsA, was comparable to that in wild-type mice. Based on these findings and evidence for unequal expression of mutated and wildtype MYH7 mRNAs in human HCM hearts (23), we suggest that variable penetrance and severity of HCM may reflect, at least in part, the proportion of wild-type and mutant transcripts and proteins.
The capacity for RNAi to attenuate expression of Myh6 R403Q transcripts and abrogate HCM in mice raises the possibility that mutation silencing might benefit human cardiomyopathy patients. While our study provides proof-of-concept and does not address the many important potential problems associated with viral-mediated gene therapy (including the potential for immune response, and long-term off-target effects), some adeno-associated virus protocols are sufficiently safe for human clinical trials (24). Moreover, the potential to develop RNAi molecules that target allelic-specific, common SNPs rather than each patient’s specific mutation, overcomes the daunting challenge of producing thousands of RNAi that would be required to silence each unique HCM or DCM mutation. There are also new opportunities to deliver gene silencing therapies to the heart. For example, the septal perforating artery can be selectively cannulated to target interventions to the interventricular septum (25), which in the majority of patients is the most profoundly hypertrophied segment in HCM heart and can cause obstruct ventricular outflow of blood, and increase the risk for sudden death. Adaptation of this approach could facilitate prophylactic, localized silencing of HCM mutations in this region where its benefit could be measurable and clinically meaningful. When continued advances in viral (and other) delivery systems (26, 27), allele-selective silencing holds considerable potential to retard the onset and progression of HCM and other genetic cardiomyopathies.
Supplementary Material
Acknowledgements
We thank Joshua Gorham, David Conner, and Steve DePalma for technical and bioinformatic assistance and Constance Cepko for assistance with viral protocols. This work was supported by grants from the NIH (U01HL098166; R01HL084553 to JGS and CES) and the Howard Hughes Medical Institute (CES).
References
- 1.Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med. 1997 Mar 13;336:775. doi: 10.1056/NEJM199703133361107. [DOI] [PubMed] [Google Scholar]
- 2.Ho CY. Hypertrophic cardiomyopathy in 2012. Circulation. 2012 Mar 20;125:1432. doi: 10.1161/CIRCULATIONAHA.110.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013 Jan 19;381:242. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009 Mar 3;119:1085. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 5.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001 Feb 23;104:557. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 6.Teekakirikul P, Padera RF, Seidman JG, Seidman CE. Hypertrophic cardiomyopathy: translating cellular cross talk into therapeutics. J Cell Biol. 2012 Oct 29;199:417. doi: 10.1083/jcb.201207033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopes LR, Rahman MS, Elliott PM. A systematic review and meta-analysis of genotype-phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart. 2013 May 14; doi: 10.1136/heartjnl-2013-303939. [DOI] [PubMed] [Google Scholar]
- 8.Geisterfer-Lowrance AA, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62:999. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 9.Geisterfer-Lowrance AA, et al. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996 May 3;272:731. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 10.Tyska MJ, et al. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res. 2000 Apr 14;86:737. doi: 10.1161/01.res.86.7.737. [DOI] [PubMed] [Google Scholar]
- 11.Fatkin D, et al. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin Invest. 2000 Dec;106:1351. doi: 10.1172/JCI11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JB, et al. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science. 2007 Jun 8;316:1481. doi: 10.1126/science.1137325. [DOI] [PubMed] [Google Scholar]
- 13.Teekakirikul P, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010 Oct;120:3520. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semsarian C, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002 Apr;109:1013. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones WK, et al. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest. 1996 Oct 15;98:1906. doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007 Jun;6:443. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005 Jun;5:285. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 18.Prasad KM, Xu Y, Yang Z, Acton ST, French BA. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2011 Jan;18:43. doi: 10.1038/gt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christodoulou DC, Gorham JM, Herman DS, Seidman JG. Construction of normalized RNA-seq libraries for next-generation sequencing using the crab duplex-specific nuclease. Curr Protoc Mol Biol. 2011 Apr;4(4):12. doi: 10.1002/0471142727.mb0412s94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang RM, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18:1440. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Ho CY, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002 Jun 25;105:2992. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- 22.Bish LT, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008 Dec;19:1359. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathi S, et al. Unequal allelic expression of wild-type and mutated beta-myosin in familial hypertrophic cardiomyopathy. Basic Res Cardiol. 2011 Nov;106:1041. doi: 10.1007/s00395-011-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011 May;12:341. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 25.Elmariah S, Fifer MA. Medical, surgical and interventional management of hypertrophic cardiomyopathy with obstruction. Curr Treat Options Cardiovasc Med. 2012 Dec;14:665. doi: 10.1007/s11936-012-0206-5. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Lebron E, Paulson HL. Allele-specific RNA interference for neurological disease. Gene Ther. 2006 Mar;13:576. doi: 10.1038/sj.gt.3302702. [DOI] [PubMed] [Google Scholar]
- 27.Yu D, et al. Single-stranded RNAs use RNAi to potently and allele-selectively inhibit mutant huntingtin expression. Cell. 2012 Aug 31;150:895. doi: 10.1016/j.cell.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.