Abstract
Background
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC) are frequently used to treat appendiceal carcinomatosis. Some patients require multivisceral resection because of the volume of disease. It is unclear whether extent of CRS impacts survival in appendiceal carcinomatosis.
Methods
We analyzed 282 patients undergoing attempted CRS/HIPEC for appendiceal carcinomatosis. Patients were defined as having undergone Extensive CRS (n = 60) if they had >3 organ resections or >2 anastomoses; a subgroup of Extreme CRS patients (n = 10) had ≥5 organ resections and ≥3 anastomoses. Kaplan–Meier survival curves and multivariate Cox-regression models were used to identify prognostic factors affecting outcomes.
Results
Relative to the comparison group, patients undergoing Extensive CRS had a higher median peritoneal carcinomatosis index, operative duration, blood loss, and length of stay. No difference in completeness of cytoreduction, severe morbidity, or 60-day mortality was evident. Subgroup analysis of 10 patients undergoing extreme CRS likewise revealed no increase in severe morbidity or mortality. Median progression-free (PFS) and overall survival (OS) were 23.5 and 74 months in the comparison group; 18.5 (p = 0.086) and 51 (p = 0.85) months in the Extensive CRS group; and 40 months and not reached in the Extreme CRS subgroup. In a multivariable analysis, extent of CRS was not independently associated with PFS or OS.
Conclusions
Extensive CRS is associated with greater OR time, blood loss, and length of stay, but is not associated with higher morbidity, mortality, or inferior oncologic outcomes in patients with appendiceal carcinomatosis.
Cytoreductive surgery combined with hyperthermic intraperitoneal chemoperfusion (CRS–HIPEC) is an established treatment modality for patients with peritoneal carcinomatosis originating from mucinous appendiceal neoplasms. In appropriately selected patients, this aggressive locoregional approach can be undertaken with low mortality and is associated with long-term survival in a population of patients who otherwise experience progressive disease leading to bowel obstruction and ultimately death. Well-characterized risk factors for relapse and death in patients undergoing CRS–HIPEC include extent of peritoneal disease, high-grade tumor histology, inability to achieve complete cytoreduction, and lymph node metastasis.1–9 Because complete cytoreduction is associated with prolonged progression-free and overall survival, an aggressive surgical approach is often advocated in order to maximize the duration of disease-free interval. In many patients, multivisceral resection is necessary in order to attain complete cytoreduction.
Morbidity following CRS–HIPEC has been well characterized. In a previous study from our institution, patients undergoing CRS–HIPEC for a variety of primary tumor sites experienced a severe (grade > 3) morbidity rate of approximately 30 %, with the number of enteric anastomoses and incomplete cytoreduction being independent significant predictors of morbidity.4,10 Likewise, major morbidity rates published from a number of other high-volume centers have ranged from 12 to 55 %.11–20 These studies have consistently identified extent of disease, number of organs resected, number of enteric anastomoses, and duration of surgery as predictors of morbidity following CRS–HIPEC.
Although the relationship between extent of resection and morbidity is well established, the impact of extent of resection on oncologic outcomes is poorly characterized. Critics of the aggressive surgical approach to carcinomatosis of appendiceal origin argue that the high published morbidity rates of CRS–HIPEC outweigh its purported benefits. In order to examine whether multivisceral resections in combination with HIPEC are prohibitively morbid with respect to oncologic outcomes, we sought to characterize the association of extent of resection with morbidity and survival duration in patients undergoing CRS–HIPEC for appendiceal carcinomatosis. We also describe our experience in a set of patients with large-volume carcinomatosis in whom an extremely aggressive surgical approach combined with HIPEC was undertaken.
PATIENTS AND METHODS
Patients presenting to our institution with PC of appendiceal origin were entered into a prospective database; all patients selected for treatment with CRS–HIPEC between May 2001 and July 2010 were included in this study. Approval was obtained from the University of Pittsburgh Institutional Review Board. Patients were selected for CRS–HIPEC following evaluation in a multidisciplinary peritoneal surface malignancy clinic composed of surgical oncologists, medical oncologists, and radiologists.
Patients underwent exploratory laparotomy in a high-volume tertiary referral center with the intent of performing complete cytoreduction and HIPEC. The Dutch simplified peritoneal carcinomatosis index (SPCI) was used to quantify disease burden at the time of laparotomy as previously described.21 Cytoreduction was performed via a previously described method, with the intent being complete cytoreduction.22 Completeness of cytoreduction was graded in the standard manner: CC-0, no visible residual disease; CC-1, residual disease up to 2.5 mm; CC-2, residual disease up to 2.5 cm; CC-3, residual disease >2.5 cm. HIPEC was performed in ~90 % of patients following cytoreductive surgery, according to a previously described institutional protocol.10 HIPEC was omitted in a minority of cases at the discretion of the operating surgeon, typically in cases of gross residual disease, excessive operative duration or blood loss, or hemodynamic instability. We use a closed technique, with a roller-pump heat exchanger (ThermoChem HT-100, ThermaSolutions, Melbourne, FL) to target flow rates of >800 mL/min and a intraperitoneal temperature of 42 °C. A dose of 30 mg mitomycin C is given at the beginning of perfusion, followed by a second dose of 10 mg mitomycin C after 60 min, with a total perfusion duration of 100 min. Postoperatively, patients were admitted to the intensive care unit for at least 24 h, with transfer to a dedicated surgical oncology nursing unit when clinically appropriate. Perioperative complications were graded according to the Dindo–Clavien system, and major morbidity was defined as an event of grade 3 or greater.23
Following discharge, ongoing follow-up care was provided in the multidisciplinary outpatient clinic. At each visit, vital status and disease status were assessed and entered into a prospective database. Overall survival (OS) was defined as the duration from the date of CRS–HIPEC to the date of death. Progression-free survival (PFS) was defined as the duration between the date of CRS–HIPEC and the date of clinically documented tumor recurrence. In cases of incomplete cytoreduction, progression was defined as the date of clinically documented tumor progression relative to baseline postoperative radiologic findings and tumor marker levels. For all outcomes, patients were censored at the time of most recent follow-up.
For purposes of comparison, patients were divided into 2 groups: an Extensive CRS group who underwent >3 organ resections or >2 enteric anastomoses and a comparison group who did not meet these criteria. For descriptive purposes, a subset of the Extensive CRS group, in which ≥5 organ resections and ≥3 enteric anastomoses were performed, was defined as the Extreme CRS group. These groups formed the basis of comparison for baseline patient characteristics (age, gender, ASA, BMI, preoperative serum albumin level, presence of symptoms, prior surgery, or chemotherapy), tumor characteristics (initial presentation vs. recurrent, histologic grade, presence of signet cells, lymph node involvement, SPCI), completeness of cytoreduction, perioperative morbidity (estimated blood loss, complication rates, length of stay, length of ICU stay, reoperation rate, readmission rate), and measures of survival (OS and PFS).
Statistical analysis was performed using SPSS. Statistical significance was defined as p < 0.05. Categorical variables were compared among groups using χ2 test or Fisher exact test as appropriate. Continuous variables were compared among groups using t test or the Mann–Whitney U test as appropriate. Kaplan–Meier curves were constructed to estimate median and actuarial survival times. Survival times among subgroups were compared using the log-rank test. Multivariate analysis, to identify predictors of survival, was performed by constructing stepwise Cox proportional hazard models incorporating variables selected on the basis of results of univariate analysis.
RESULTS
Preoperative baseline patient characteristics are presented in Table 1. Relative to the comparison group, the Extensive CRS group exhibited no significant differences in patient age, gender, body mass index, American Society of Anesthesiology (ASA) score, or serum albumin concentration. Approximately half of the patients in each group were treated during their initial presentation with PC, while half were treated for recurrent disease after previous treatment. Most patients had not undergone prior systemic or peritoneal chemotherapy. Patients in the Extensive CRS group were more likely to be symptomatic at presentation (77 vs. 58 %, p = 0.01) and were more likely to have CT-evident disease (75 vs. 62 %, p = 0.07) or a dominant tumor mass on CT (57 vs. 27 %, p = 0.0001).
TABLE 1.
Clinicopathologic features of patients undergoing extensive cytoreductive surgery
| Comparison group (n = 222) |
Extensive CRS (n = 60) |
p value | |
|---|---|---|---|
| Age years, mean ± SD | 54.7 ± 11.8 | 55.5 ± 10.3 | 0.6 |
| Gender | 0.9 | ||
| Male | 110 (49.5 %) | 29 (48.3 %) | |
| Female | 112 (50.5 %) | 31 (51.7 %) | |
| BMI, mean ± SD | 27.1 ± 5.1 | 28.0 ± 7.5 | 0.3 |
| ASA | 0.2 | ||
| 1 (n = 2) | 1 (0.7 %) | 1 (2.1 %) | |
| 2 (n = 35) | 30 (21.3 %) | 5 (10.4 %) | |
| 3 (n = 134) | 88 (62.4 %) | 36 (75.0 %) | |
| 4 (n = 28) | 22 (15.6 %) | 6 (12.5 %) | |
| Preoperative serum albumin (g/dL) |
3.7 ± 0.8 | 3.5 ± 0.8 | 0.2 |
| Primary vs recurrence | 0.9 | ||
| Primary (n = 134) | 104 (49.1 %) | 30 (50.8 %) | |
| Recurrence (n = 137) | 108 (50.9 %) | 29 (49.2 %) | |
| Symptomatic | 129 (58.1 %) | 46 (76.7 %) | 0.01 |
| Prior chemotherapy | 89 (40.1 %) | 20 (33.3 %) | 0.4 |
| Prior HIPEC | 17 (7.8 %) | 4 (6.8 %) | 1.0 |
| CT-evident disease | 136 (61.5 %) | 45 (75.0 %) | 0.07 |
| CT-evident mass | 59 (26.8 %) | 34 (56.7 %) | 0.0001 |
| Duration, minutes, median (IQR) |
460 (390–750) | 555 (460–660) | 0.0004 |
| Estimated blood loss, mL, median (IQR) |
750 (400–2,500) | 1,225 (750– 2,050) |
0.0006 |
| Units RBCs transfused | 0.007 | ||
| 0 (n = 73) | 64 (37.0 %) | 9 (17.3 %) | |
| 1–2 (n = 56) | 42 (24.3 %) | 14 (26.9 %) | |
| >3 (n = 96) | 67 (38.7 %) | 29 (55.8 %) | |
| SPCI, median (IQR) | 13 (8–16) | 16 (14–18) | <0.0001 |
| Complete cytoreduction (CC-0/1) |
185 (84.5 %) | 45 (75.0 %) | 0.1 |
| HIPEC performed | 198 (93.4 %) | 53 (88.3 %) | 0.8 |
| Histologic grade | 1.0 | ||
| Low grade (n = 180) | 141 (64.1 %) | 39 (65.0 %) | |
| High grade (n = 100) | 79 (35.9 %) | 21 (35.0 %) | |
| Signet ring cells | 0.8 | ||
| Not present (n = 245) | 192 (86.5 %) | 53 (88.3 %) | |
| Present (n = 37) | 30 (13.5 %) | 7 (11.7 %) | |
| Positive lymph nodes | 0.4 | ||
| Not identified (n = 154) |
121 (78.1 %) | 33 (71.7 %) | |
| Identified (n = 47) | 34 (21.9 %) | 13 (28.3 %) |
SD standard deviation, IQR interquartile range
Patients undergoing Extensive CRS experienced longer median operative duration (554.5 vs. 460 min, p = 0.0004), greater median estimated blood loss (1,225 vs. 750, p = 0.0006), and a higher probability of red blood cell transfusion (83 vs. 63 %, p = 0.007). Disease burden, quantified according to the simplified peritoneal carcinomatosis index (SPCI), was greater in the Extensive CRS group (median 16 vs. 13, p < 0.0001), while complete cytoreduction (CCR score 0–1) was achieved in 75 % of patients in the Extensive CRS group and 84.5 % of the comparison (p = 0.1). HIPEC was performed in the vast majority (~90 %) of patients in each group.
Excluding peritonectomy and omentectomy, the most common organ resection procedures in the Extensive CRS group were colectomy (92 %), splenectomy (82 %), cholecystectomy (65 %), and small bowel resection (57 %). Less frequently performed were hysterectomy and salpingooophorectomy (28 %), distal pancreatectomy (27 %), gastrectomy (23 %), and partial hepatectomy (15 %). By virtue of the study design, all procedures were more commonly performed in the Extensive CRS group relative to the comparison group.
No significant difference between the Extensive CRS and comparison groups was noted in the distribution of histologic grade, with a strong majority of patients in both groups having low-grade disease (p = 1.0). Likewise, no significant differences between groups were noted with respect to presence of signet ring cells (p = 0.8), presence of lymph node metastases (p = 0.4), or presence of K-ras mutation (p = 0.4).
Morbidity results are presented in Table 2. Postoperative complications occurred in 70 % of patients undergoing Extensive CRS, with 32 % experiencing major morbidity (grade 3 or 4). These rates were not significantly different than the overall (59 %, p = 0.1) and grade 3–4 (23 %, p = 0.2) morbidity rates in the comparison group. One patient (1.7 %) in the Extensive CRS group and two patients (0.9 %) in the comparison group died within 60 days of surgery (p = 0.1). The most frequent major complications were delayed gastric emptying, surgical site infection, pulmonary insufficiency, and prolonged ileus (>21 days). Anastomotic leaks occurred in two patients (3.3 %) in the Extensive CRS group and four patients (1.8 %) in the comparison group (p = 0.6). Cardiac complications and hemorrhagic complications were significantly more common in the Extensive CRS group (5 vs. 0.5 %, p = 0.03 and 5 vs. 0 %, p = 0.009, respectively). Median ICU length of stay was longer in the Extensive CRS group (3 vs. 2 days, p = 0.006), as was median time to tolerance of regular diet (9.5 vs. 7 days, p = 0.0006) and median hospital length of stay (14.5 vs. 11.5 days, p = 0.0008). No significant difference in ICU readmission rate (3.3 vs. 4.0 %, p = 1.0), reoperation rate (8.3 vs. 3.2 %, p = 0.1), rate of percutaneous drainage for deep surgical site infections (10 vs. 8.6 %), or hospital readmission rate (16.7 vs. 13.1 %, p = 0.5) was observed between the groups.
TABLE 2.
Morbidity in patients undergoing extensive cytoreductive surgery
| Comparison group (n = 222) |
Extensive CRS (n = 60) |
p value |
|
|---|---|---|---|
| Intraoperative complications | 3 (1.4 %) | 1 (1.7 %) | 1.0 |
| Overall morbidity | 130 (58.6 %) | 42 (70 %) | 0.1 |
| Grade 3–4 morbidity | 51 (23.0 %) | 19 (31.7 %) | 0.2 |
| 60-day mortality | 2 (0.9 %) | 1 (1.7 %) | 0.1 |
| Specific complications, grade ≥3 | |||
| Delayed gastric emptying | 35 (15.8 %) | 4 (6.7 %) | 0.09 |
| Surgical site infection, superficial |
30 (13.5 %) | 9 (15.0 %) | 0.8 |
| Surgical site infection, deep | 19 (8.6 %) | 6 (10 %) | 0.8 |
| Pulmonary insufficiency | 15 (6.8 %) | 6 (10.0 %) | 0.4 |
| Ileus >21 days | 11 (5.0 %) | 5 (8.3 %) | 0.3 |
| Sepsis | 5 (2.2 %) | 2 (3.3 %) | 0.6 |
| Anastomotic leak | 4 (1.8 %) | 2 (3.3 %) | 0.6 |
| Thromboembolic event | 4 (1.8 %) | 0 (0 %) | 0.6 |
| Renal insufficiency | 2 (0.9 %) | 1 (1.7 %) | 0.1 |
| Enterocutaneous fistula | 1 (0.5 %) | 0 (0 %) | 1.0 |
| Pancreatic leak | 1 (0.5 %) | 0 (0 %) | 1.0 |
| Biliary leak | 1 (0.5 %) | 0 (0 %) | 1.0 |
| Cardiac event | 1 (0.5 %) | 3 (5.0 %) | 0.03 |
| Hemorrhage | 0 (0 %) | 3 (5.0 %) | 0.009 |
| ICU length of stay, days, median (IQR) |
2 (1–4) | 3 (2–4) | 0.006 |
| Time to regular diet, days, median (IQR) |
7 (6–10) | 9.5 (8–12) | 0.0006 |
| Reoperation | 7 (3.2 %) | 5 (8.3 %) | 0.1 |
| Hospital length of stay, median (IQR) |
11.5 (9–15) | 14.5 (12–18) | 0.0008 |
| Hospital readmission | 29 (13.1 %) | 10 (16.7 %) | 0.5 |
IQR interquartile range
On univariate analysis, severe morbidity was associated with symptomatic disease (p = 0.006), number of enteric anastomoses (p = 0.006), and high-grade disease (p = 0.005). ASA score (p = 0.08), blood transfusion (p = 0.06), SPCI (p = 0.08), operative duration (p = 0.07), and incomplete cytoreduction (0.06) approached but did not meet statistical significance as univariate predictors of severe morbidity. On multivariable analysis, none of these factors carried an independent association with severe morbidity.
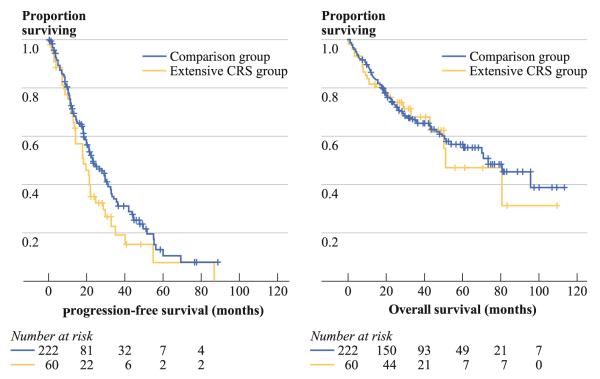
Kaplan–Meier curves for progression-free survival (152 events) and overall survival (105 events) are presented in Fig. 1. Median progression-free survival was 18.5 months in the Extensive CRS group versus 23.5 months in the comparison group, a trend that did not meet statistical significance (p = 0.09). Median overall survival was not significantly different between the Extensive CRS (51 months) and comparison (74 months) groups (p = 0.8). Results of multivariate analysis for progression-free and overall survival are presented in Table 3. Independent predictors of decreased progression-free survival were cytoreduction for recurrent disease, higher disease volume (SPCI), incomplete cytoreduction, high-grade histology, and lymph node metastasis. Independent predictors of decreased overall survival were high-grade histology and higher SPCI. Neither Extensive CRS nor severe morbidity was independently associated with progression-free or overall survival.
FIG. 1.
Kaplan–Meier curves for progression-free and overall survival in patients undergoing extensive cytoreductive surgery. Median progression-free survival was 18.5 months in the Extensive CRS group versus 23.5 months in the comparison group (p = 0.09). Median overall survival was 51 months in the Extensive CRS and 74 months in the comparison group (p = 0.8)
TABLE 3.
Univariate and multivariate analysis of progression-free and overall survival
| Factor | Progression-free survival |
Overall survival |
||
|---|---|---|---|---|
|
p value, univariate |
HR (95 % CI) and p value, multivariate |
p value, univariate |
HR (95 % CI) and p value, multivariate |
|
| Age (per year) | 0.3 | 1.02 (1.0–1.04) p = 0.06 |
0.04 | 1.02 (1.0–1.04) p = 0.1 |
| Female gender | 0.6 | 0.97 (0.7–1.5) p = 0.9 |
0.02 | 0.9 (0.5–1.4) p = 0.6 |
| Recurrent disease | 0.002 | 1.6 (1.1–2.5) p = 0.02 |
0.3 | 1.2 (0.7–2.0) p = 0.5 |
| Symptomatic disease |
0.1 | 1.2 (0.8–1.9) p = 0.4 |
0.2 | 0.9 (0.5–1.6) p = 0.7 |
| High histologic grade |
<0.0001 | 2.7 (1.7–4.3) p < 0.0001 |
<0.0001 | 7.1 (3.9–13.0) p < 0.0001 |
| Preoperative SPCI (per point) |
0.0002 | 1.08 (1.03–1.13) p = 0.0007 |
0.002 | 1.1 (1.04–1.2) p = 0.0009 |
| Incomplete cytoreduction |
<0.0001 | 1.9 (1.2–3.0) p = 0.005 |
<0.0001 | 1.3 (0.8–2.4) p = 0.3 |
| Lymph node metastasis |
<0.0001 | 1.9 (1.2–3.2) p = 0.01 |
<0.0001 | 1.0 (0.5–1.8) p = 1.0 |
| Extensive CRS | 0.09 | 0.9 (0.6–1.4) p = 0.5 |
0.8 | 0.9 (0.5–1.6) p = 0.7 |
| Blood transfusion | 0.09 | 1.5 (0.9–2.4) p = 0.1 |
0.03 | 1.4 (0.8–2.6) p = 0.3 |
| Severe morbidity | 0.5 | 0.9 (0.6–1.4) p = 0.8 |
0.09 | 0.9 (0.5–1.6) p = 0.7 |
HR hazard ratio, SPCI simplified peritoneal carcinomatosis index
A subgroup of 10 patients undergoing Extreme CRS was identified in whom ≥5 organ resections and ≥3 anastomoses were performed prior to HIPEC. With respect to the comparison group, patients undergoing Extreme CRS had higher median SPCI (16, p = 0.01), longer surgical duration (645 min, p = 0.0001), greater median estimated blood loss (1,300 ml, p = 0.008), and longer hospital length of stay (17 days, p = 0.02). Complete cytoreduction was achieved in 9 of 10 patients. Major morbidity occurred in five patients (p = 0.07), with no 60-day mortality. Median progression-free survival was 40 months, while median overall survival had not been reached at the time of analysis in the Extreme CRS subgroup.
DISCUSSION
The rarity of appendiceal carcinomatosis and the variety of histologic entities comprising it have challenged efforts to develop uniform treatment recommendations for this progressive disease. Despite the publication of numerous retrospective series over the past several decades, the optimal treatment of patients with PMP remains the subject of controversy. In the absence of randomized trials comparing proposed treatment regimens, consensus has nevertheless emerged among some experts favoring combined aggressive cytoreductive surgery and intraperitoneal chemotherapy over systemic chemotherapy and/or palliative CRS procedures alone.24
Although CRS–HIPEC has shown promising results in non-randomized trials compared with palliative surgery and/or chemotherapy, critics of this approach have questioned its oncologic benefit and cautioned that the associated morbidity may warrant a less aggressive approach in which complete cytoreduction is de-emphasized in favor of other endpoints, including preservation of function and palliation of symptoms.25 On the other hand, proponents of CRS–HIPEC argue that it is a potentially curative approach, citing 10-year overall survival rates exceeding 50 %, compared with 20–30 % in series advocating a palliative approach.3 Multiple series from various institutions have confirmed the dominant impact of histologic grade and completeness of cytoreduction on survival among patients selected for aggressive locoregional treatment for PMP.1–9
Given the importance of complete cytoreduction in optimizing outcome, a central question is whether an approach of cytoreduction-at-all-costs is warranted in appendiceal carcinomatosis. In this study, we examined the question of whether extent of surgery influences morbidity and oncologic outcome in appendiceal carcinomatosis in an institution in which an aggressive cytoreductive approach is pursued. We did not find a statistically significant increase in the rate of overall morbidity or severe morbidity on the basis of extent of cytoreductive surgery. Nevertheless, patients undergoing Extensive CRS did experience higher complication rates in the cardiac and hemorrhagic subgroups, longer ICU and hospital stays, and longer time to tolerance of regular diet. A trend toward decreased progression-free survival among patients undergoing Extensive CRS on univariate analysis was not confirmed on multivariate analysis, whereas no difference in overall survival was noted on the basis of extent of cytoreductive surgery. Similarly, a subset of 10 patients undergoing extreme cytoreduction with numerous organ resections and anastomoses experienced acceptable morbidity and no perioperative mortality. In short, we found no evidence that Extensive CRS or its associated morbidity negatively impact oncologic outcomes in this cohort of patients.
The decision to treat an individual appendiceal carcinomatosis patient with cytoreductive surgery and HIPEC is complex and involves an assessment of the grade of disease, the likelihood of a complete cytoreductive surgery, the presence of symptoms, and the patient’s medical suitability for aggressive surgery. Judgment is required to balance the potential benefit of a complete cytoreduction with the potential for morbidity and altered quality of life inherent in multivisceral resections. Clearly, not all organ resections are equivalent, and certain procedures (e.g., total gastrectomy, cystectomy, proctectomy) would be anticipated to have a larger impact on quality of life than others. Therefore, a great deal of subjectivity remains at the time of cytoreductive surgery in determining how aggressive to be in an individual patient. However, on the basis of our findings we suggest that the number of organ resections and anastomoses required to obtain an optimal cytoreduction do not independently influence oncologic outcome in appendiceal carcinomatosis and therefore should not be used in isolation to withhold treatment from a patient who is otherwise fit for an aggressive approach.
A number of previous studies have examined the relationship between extent of cytoreduction and morbidity in pseudomyxoma peritonei and other forms of carcinomatosis that have been treated with cytoreductive surgery and HIPEC. Multiple large series, from our institution and others, have identified a relationship between morbidity and number of organ resections and anastomoses.11,14,16–18,26 Other related variables, including duration of surgery and extent of disease, have also been repeatedly identified as predictors of morbidity, as has incomplete cytoreduction.11,14,16–18 We have previously investigated the impact of extent of cytoreduction on morbidity and survival in patients being treated for carcinomatosis from colorectal cancer. In this group of patients, estimated blood loss and number of bowel anastomoses, but not multivisceral resection, were independently associated with morbidity on multivariable analysis. No association between extent of resection and overall survival was seen.26
Quality of life following CRS–HIPEC is a central element in the discussion of whether patients with carcinomatosis are well served by an aggressive locoregional treatment approach.27,28 Studies designed to measure elements of quality of life prior to and following CRS– HIPEC have documented postoperative declines in physical well-being and physical functioning, as well as significant depressive symptoms that persist for at least a year in up to a third of patients.17,29–31 While emotional well-being was reported to improve after CRS–HIPEC, and physical well-being and function were found to return to baseline between 6 and 12 months after surgery, these studies were limited by reporting bias, since patients with poor quality of life may have selectively dropped out during the follow-up period. Clearly, this is an area that deserves intense study given the fact that the vast majority of patients with appendiceal carcinomatosis will not be cured by CRS–HIPEC, emphasizing the importance of maintaining quality of life in this patient population. The effect of extent of cytoreduction on quality of life remains to be studied.
This study is limited by its retrospective nature and by the fact the results were obtained in carefully selected patients treated by a multidisciplinary team in a high-volume tertiary cancer center and may not apply in other treatment settings. Nevertheless, we conclude that aggressive cytoreductive surgery entailing multivisceral resection can be performed with acceptable morbidity and mortality, and there does not appear to be a relationship between the number of resected organs and measures of oncologic outcome. Unfortunately, in spite of aggressive cytoreduction, most patients with appendiceal carcinomatosis will experience subsequent progression, emphasizing the need for innovation in regional and systemic therapies for this disease.
REFERENCES
- 1.Youssef H, Newman C, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum. 2011;54:293–9. doi: 10.1007/DCR.0b013e318202f026. [DOI] [PubMed] [Google Scholar]
- 2.Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol. 2007;14:2289–99. doi: 10.1245/s10434-007-9462-0. [DOI] [PubMed] [Google Scholar]
- 3.González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;9:304–11. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 4.Austin F, Mavanur A, Sathaiah M, Steel J, Lenzner D, Ramalingam L, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19:1386–93. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart JH, 4th, Shen P, Russell GB, Bradley RF, Hundley JC, Loggie BL, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 6.Chua TC, Yan TD, Smigielski ME, Zhu KJ, Ng KM, Zhao J, et al. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann Surg Oncol. 2009;16:1903–11. doi: 10.1245/s10434-009-0341-8. [DOI] [PubMed] [Google Scholar]
- 7.Elias D, Honoré C, Ciuchendéa R, Billard V, Raynard B, Lo Dico R, et al. Peritoneal pseudomyxoma: results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2008;95:1164–71. doi: 10.1002/bjs.6235. [DOI] [PubMed] [Google Scholar]
- 8.Baratti D, Kusamura S, Nonaka D, Langer M, Andreola S, Favaro M, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:526–34. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 9.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FAN. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–9. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15:754–63. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 11.Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106:1144–53. doi: 10.1002/cncr.21708. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13:635–44. doi: 10.1245/ASO.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–6. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–9. doi: 10.1245/aso.2003.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–86. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FAN. Toxicity of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2004;85:61–7. doi: 10.1002/jso.20013. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt U, Dahlke MH, Klempnauer J, Schlitt HJ, Piso P. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:53–8. doi: 10.1016/j.ejso.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–6. doi: 10.1007/s10434-999-0790-0. [DOI] [PubMed] [Google Scholar]
- 19.Younan R, Kusamura S, Baratti D, Oliva GD, Costanzo P, Favaro M, et al. Bowel complications in 203 cases of peritoneal surface malignancies treated with peritonectomy and closed-technique intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2005;12:910–8. doi: 10.1245/ASO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Saxena A, Yan TD, Chua TC, Morris DL. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2010;17:1291–301. doi: 10.1245/s10434-009-0875-9. [DOI] [PubMed] [Google Scholar]
- 21.Portilla AG, Shigeki K, Dario B, Marcello D. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol. 2008;98:228–31. doi: 10.1002/jso.21068. [DOI] [PubMed] [Google Scholar]
- 22.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15:204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei) J Surg Oncol. 2008;98:277–82. doi: 10.1002/jso.21054. [DOI] [PubMed] [Google Scholar]
- 25.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300–8. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, Zeh HJ, 3rd, et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15:3065–72. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 27.McQuellon R, Gavazzi C, Piso P, Swain D, Levine E. Quality of life and nutritional assessment in peritoneal surface malignancy (PSM): recommendations for care. J Surg Oncol. 2008;98:300–5. doi: 10.1002/jso.21050. [DOI] [PubMed] [Google Scholar]
- 28.Piso P, Glockzin G, von Breitenbuch P, Popp FC, Dahlke MH, Schlitt HJ, et al. Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies. J Surg Oncol. 2009;100:317–20. doi: 10.1002/jso.21327. [DOI] [PubMed] [Google Scholar]
- 29.McQuellon RP, Danhauer SC, Russell GB, Shen P, Fenstermaker J, Stewart JH, et al. Monitoring health outcomes following cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:1105–13. doi: 10.1245/s10434-006-9304-5. [DOI] [PubMed] [Google Scholar]
- 30.McQuellon RP, Russell GB, Shen P, Stewart JH, 4th, Saunders W, Levine EA. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol. 2008;15:125–33. doi: 10.1245/s10434-007-9678-z. [DOI] [PubMed] [Google Scholar]
- 31.Hill AR, McQuellon RP, Russell GB, Shen P, Stewart JH, 4th, Levine EA. Survival and quality of life following cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colonic origin. Ann Surg Oncol. 2011;18:3673–9. doi: 10.1245/s10434-011-1793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]