Abstract
Currently approved adjuvants induce protective antibody responses but are more limited for generating cellular immunity. Here we assessed the effect of combining two adjuvants with distinct mechanisms of action on their ability to prime T cells; the TLR3 ligand, polyinosinic:polycytidylic acid (Poly I:C), and immunostimulatory complexes (ISCOMs). Each adjuvant was administered alone or together with HIV Gag protein (Gag) and the magnitude, quality and phenotype of Gag-specific T cell responses were assessed. For CD8 T cells, all adjuvants induced a comparable response magnitude, but combining Poly I:C with ISCOMs induced a high frequency of CD127+, IL-2 producing cells with decreased expression of Tbet compared to either adjuvant alone. For CD4 T cells, combining Poly I:C and ISCOMs increased the frequency of multifunctional cells, producing IFNγ, IL-2 and TNF, and the total magnitude of the response compared to either adjuvant alone. CD8 or CD4 T cell responses induced by both adjuvants mediated protection against Gag-expressing Listeria monocytogenes or vaccinia viral infections. Poly I:C and ISCOMs can alter antigen uptake and/or processing and we therefore used fluorescently labeled HIV Gag and DQ-OVA to assess these mechanisms respectively in multiple DC subsets. Poly I:C promoted uptake and retention of antigen, while ISCOMs enhanced antigen degradation. Combining Poly I:C and ISCOMs caused substantial death of DCs but persistence of degraded antigen. These data illustrate how combining adjuvants, such as Poly I:C and ISCOMs that modulate antigen processing and have potent innate activity, can enhance the magnitude, quality and phenotype of T cell immunity.
Introduction
Preventive vaccination against HIV, malaria and tuberculosis will require induction of potent antibody responses, T cell responses or both for optimal protection (1–4). Since humoral and cellular immune responses can wane following vaccination, continued boosting may be required to maintain responses above a threshold necessary to mediate protection. Protein-based vaccines given with adjuvants are one approach that can be used in combination with other vaccine platforms for priming and/or boosting adaptive immunity. Currently approved clinical adjuvant formulations include alum and oil/water emulsions, which elicit protective humoral immunity but are far less potent for inducing CD4/Th1 or CD8 T cell immunity (reviewed in (5)). Improving cellular immunity with protein-based vaccination will require adjuvants that elicit potent innate cytokines conducive to induction of cellular responses and efficient antigen presentation. Polyinosinic:polycytidylic acid (Poly I:C) and immunostimulatory complexes (ISCOMs) are two adjuvants that show promise in pre-clinical studies and early clinical trials for induction of both antibody and T cell responses (6–9).
Poly I:C is a synthetic double-stranded RNA analog and a ligand for multiple pathogen recognition receptors (PRRs); including toll-like receptor (TLR)3, melanoma differentiation-associated protein 5 (MDA-5), retinoic acid-inducible gene 1 (RIG-I) and dsRNA-dependent protein kinase R (PKR) (10–14). Expression of TLR3 is endosomal and found predominantly in CD8α + dendritic cells (DCs) or langerin+ dermal DCs (dDCs) (15, 16), while MDA-5, RIG-I and PKR localise to the cytosol and are more broadly expressed on antigen presenting cells (APCs) and non-haematopoetic stromal cells (6, 17, 18). Poly I:C stimulates rapid production of IL-6, IL-10, IL-12 p40, MCP-1, TNF, type I IFN and IFNγ, resulting in significant DC and NK cell activation (6, 19). When co-administered with protein antigen, Poly I:C potently primes CD4/Th1 cell and antibody responses (6, 7, 20) and promotes cross-presentation of antigen to CD8 T cells by DCs through TLR3 signaling (21).
ISCOM particles are cage-like structures that assemble from cholesterol, phospholipids and saponins (reviewed in (22)). ISCOMs can enhance antigen delivery to APCs when antigen is incorporated into the particle but ISCOMs do not function solely as delivery vehicles, since certain fractions of saponin possess intrinsic adjuvant activity (23). ISCOMs have been shown to induce caspase-dependent cleavage of IL-1β and robust serum production of IL-5, IL-6, GM-CSF and IL-12 p40 (24, 25). As a result, ISCOMs prime potent long-lived antibody responses with a balanced CD4 Th1/Th2 T cell response (26), and low-level induction of CTLs. ISCOMs lead to cross-presentation most likely as a result of disruption of the integrity of phago-lysosomes after endocytosis, which could permit access of antigen to the cytosol (27, 28). Cross-presentation with ISCOMs in vitro is most efficient with monocyte-derived DCs (28), although ex vivo CD8α+ DCs are responsible for the majority of antigen presentation to CD8 T cells (25).
A combination of Poly I:C and ISCOMs could potentiate the effect of each adjuvant by activating distinct but complimentary innate signaling and antigen processing pathways. Prior studies using combined ligands for distinct TLRs have demonstrated enhanced innate or adaptive immunity in vitro (29) and in vivo (30). Poly I:C has been used in combination with particulate delivery systems, such as liposomes, and combined with other TLR agonists, such as CpG, to enhance innate signaling and priming of T cells (31, 32). ISCOMs have also been used in combination with CpG, which enhanced cross-priming of tumour antigen (33) and induced robust HIV Env-specific humoral immunity (34). However, a combination of Poly I:C with ISCOMs has not been evaluated.
For this study, we hypothesised that combining Poly I:C and ISCOMs would result in more potent T cell immunity than either adjuvant alone. We show that combining the adjuvants increased CD4/Th1 cell responses and enhanced the qualitative and phenotypic profile of CD8 T cell responses by increasing expression of CD127 and IL-2. Combining Poly I:C with ISCOMs also resulted in rapid initial degradation but prolonged retention of antigen, which may represent a mechanism contributing to the observed effects on T cells. Overall, the data presented show how combining adjuvants with distinct effects on innate cytokine production and antigen presentation can enhance T cell immunity with protein-based vaccines.
Materials and Methods
Mice
Balb/c, C57BL/6 or Batf3 −/− (C.129S-Batf3tm1Kmm/J) mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and housed at the Vaccine Research Center Biomedical Research Unit (Bethesda, MD,USA). Mice were 6 to 12 weeks old at the time of vaccination. All experimental animal protocols were approved by the Vaccine Research Center Animal Care and Use Committee.
Formulations and vaccinations
Formulations were prepared using 30 μg of HIV-Gag p41 protein (Gag) (Protein Sciences, Meriden, CT, USA), which was mixed with 12 μg of Abisco100 ISCOMs (Isconova AB, Stockholm, Sweden) and/or 50 μg of Poly I:C (Invivogen, San Diego, CA, USA) immediately prior to vaccination. In Supplemental Figure 3, 50 μg of CpG 1826 (Pfizer, New York, NY, USA) was used. The ISCOM dose was chosen based on manufacturer’s recommendation and the Poly I:C, CpG and protein doses were chosen based on dose titrations to maximise T cell responses.
Following purification of Gag protein, it was treated with Triton-X100 to remove residual endotoxin and was validated at less than 0.1 endotoxin units with an Endpoint Chromogenic LAL Assay (Lonza, Basel, Swizerland), as has been performed for prior studies (35, 36). AbISCO100 is a Matrix formulation that is mixed with antigen in solution and as such the antigen is not incorporated in the structure. All formulations were prepared in PBS and then administered subcutaneously in both hind footpads, given as a 100 μL dose split into 50 μL per footpad. Two doses were administered 3 weeks apart.
To assess antigen uptake and degradation experiments, 30 μg of HIV Gag Alexa Fluor (AF) 488 (conjugated by Molecular Probes/Life Technologies, Grand Island, NY, USA), full-length ovalbumin (OVA)-AF488 (Molecular Probes) or DQ-OVA (Molecular Probes) were used.
To assess boosting of primed CD8 T cell responses, 1 x108 particle units of replication deficient adenovirus rAd5:Gag was given intramuscularly as a 100 μL dose into the left gluteal muscle (37).
Tetramer staining
For assessment of KLRG1 and CD127 expression, splenocytes were harvested and stained as previously described (37) using a PE-labeled H-2Kd tetramer loaded with the immuno-dominant HIV Gag peptide (AMQMLKETI; (38)). For assessment of Tbet expression, splenocytes were stained with LIVE/DEAD® Fixable Violet viability dye (Life Technologies, NY, USA), blocked with anti-FcγRIII antibody (clone 2.4G2; 5 μg/mL; BD Pharmingen, CA, USA), surface stained with PE-labeled tetramer, anti-CD8-APC-Cy7 (clone 53–6.7; Biolegend, CA, USA) and anti-CD62L-PE-Cy7 (clone MEL-14; Abcam, MA, USA), fixed and permeabilised with Fix/Perm and Perm/Wash Buffers (eBiosciences) and intracellularly stained with anti-CD3-PerCP-Cy5.5 5 (clone 145-2C11; BD Pharmingen) and anti-Tbet-AF647 (eBiosciences).
Intracellular cytokine staining
For assessment of antigen-specific cytokine production, splenocytes were harvested and restimulated in vitro as previously described (37) using a peptide pool comprising 15-mers spanning HIV Gag p41 (each at 2 μg/mL) or full-length HIV Gag p41 protein (20 μg/mL). For evaluation of IFNγ, IL-2, TNF and IL-10 production, samples were then stained as previously described (37). Alternatively, for evaluation of IL-17 production, samples were stained as previously described, except anti-IL-2-FITC (clone JES6-5H4; BD Pharmingen) and anti-IL-17A-PE (clone TC11-18H10; BD Pharmingen) antibodies were substituted for anti-IL-10-AF488 and anti-IL-2-PE antibodies during intracellular staining.
DC preparation and staining
Following vaccination, both popliteal lymph nodes were harvested and pooled from 8 (Gag:AF488 staining) or 4 (DQ-OVA staining) individual mice for each formulation. DCs were harvested by enzymatic digestion and enriched for by MACS CD11c positive selection, as previously described (39). Cells were then stained with LIVE/DEAD® Fixable AquaBlue viability dye (Life Technologies) and stained by one of 2 panels: 1. Gag:AF488 staining- Surface staining for B220-Cy7PE (clone RA3-6B2; BD Pharmingen), CD8-APCCy7 (clone 53-6.7; Biolegend), CD11b-AF700 (clone M1/70; BioLegend), Pan-NK-PacificBlue (clone DX5; BioLegend), CD19-PacificBlue (clone 6D5; BioLegend), CD11c-PE (clone HL3; BD Pharmingen), CD103-PerCPCy5.5 (clone 2E7; BioLegend) and DEC205-Biotin (clone NLDC-145; Miltenyi Biotec), followed by streptavidin-TexasRedPE (BD Pharmingen), followed by intracellular staining for CD3-PECy5 (clone 145-2C11; BD Pharmingen), and Langerin-AF647 (clone 929F3.01; Dendritics, Lyon, France); 2. DQ-OVA staining- Surface staining for B220-Cy7PE, CD8-APCCy7, CD11b-AF700, Pan-NK-PacificBlue and CD19-PacificBlue, CD11c-QD605 (clone HL3; conjugated inhouse), followed by intracellular staining for CD3-APC (clone 145-2C11; BD Pharmingen). DC numbers were back calculated to represent cells recovered per lymph node.
Multi-parameter flow cytometry
Samples were resuspended in 0.5% paraformaldehyde before acquisition using a modified LSR II flow cytometer (BD Biosciences). Results were analysed using FlowJo version 9.3, Pestle version 1.6.2 and SPICE version 5.22 software (Mario Roederer, VRC, NIAID, NIH, MD, USA). Background cytokine staining was subtracted, as defined by staining in samples incubated without peptide or protein.
Infections and antibody-mediated depletions
For infectious challenge, attenuated Listeria monocytogenes (ΔactA, ΔintB) or vaccinia virus (thymidine kinase-deficient Western Reserve strain) were used, each expressing Gag from HIV-1 strain HXBX or strain IIIB respectively. Infections were performed as previously described (37) using a 2 × 107 colony forming unit (CFU) dose of Listeria monocytogenes expressing Gag (Listeria:Gag) or a 6.5 × 106 plaque forming unit (PFU) dose of vaccinia virus expressing Gag (rVACV:Gag).
Antibody-mediated depletion of CD8 and/or CD4 T cells, after vaccination and prior to challenge, was performed as previously described (37). Depleting antibodies were kindly provided by Fred Finkelman (University of Cincinnati, Cincinnati, OH, USA).
Statistics
Statistical significance was calculated using a two-tailed Wilcoxon Rank Sum test using SPICE software or a two-tailed Mann-Whitney test using PRISM software.
Results
Vaccination Schedule
To assess the effect of combining Poly I:C and ISCOMs on the generation of T cell responses, we formulated each adjuvant alone or in combination using HIV-Gag protein (Gag) in Balb/c mice (Fig. 1A). ISCOMs were used as ISCOM-Matrix formulations, where there is no defined physical association between the ISCOM and the antigen. Two doses of each formulation were administered subcutaneously in both rear footpads, 3 weeks apart (Fig. 1A). At the peak response (10 days after the second dose), the magnitude, quality and phenotype of Gag-specific CD8 and CD4 T cell responses were assessed using multi-parameter flow cytometry after MHC class I tetramer staining and/or intracellular cytokine staining (Fig. 1A).
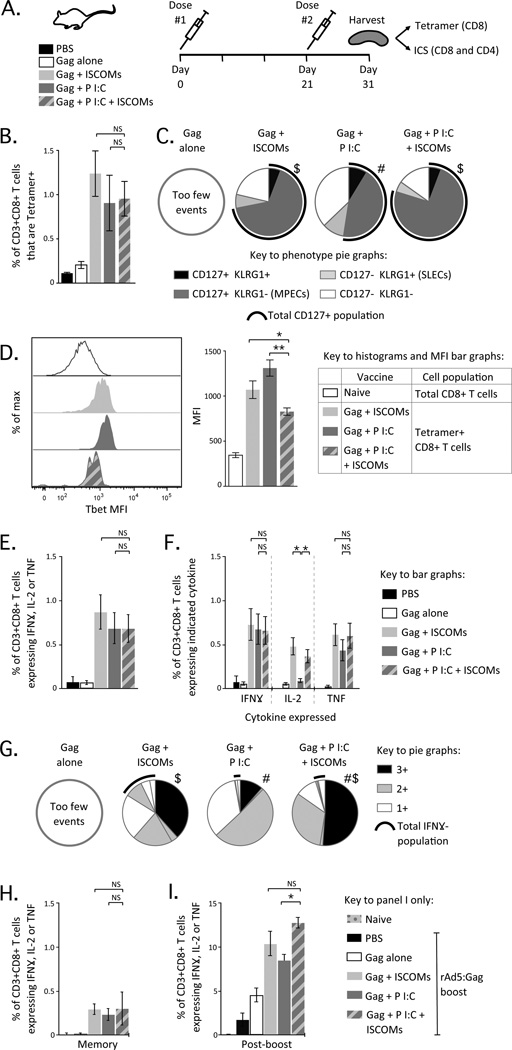
Figure 1.
Characterisation of CD8 T cell responses after vaccination with Poly I:C and ISCOMs. (A) Experimental schema for Figures 1 and 2. Balb/c mice are vaccinated with one of the 5 indicated combinations of Gag (30 μg), Poly I:C (50 μg) and ISCOMs (12 μg) given as two doses, 21 days apart. Splenocytes were harvested 10 days later and used in MHCI tetramer- or ICS-based assays for T cell responses. (B) Frequency of CD3+CD8+ T cells that are Tetramer+. (C) Proportion of CD3+CD8+Tetramer+ T cells that express any combination of CD127+or KLRG1+. The black arc represents the proportion of cells that express CD127. (D) Histograms (left) and average MFI (right) of CD3+CD8+Tetramer+ T cells with each adjuvant as compared to total CD8+ T cells from a naïve animal. (E) Frequency of CD3+CD8+ T cells producing IFNγ, IL-2, or TNF (total cytokine+) by ICS. (F) Frequency of CD3+CD8+ T cells producing IFNγ, IL-2, or TNF individually. (G) Proportion of CD3+CD8+ T cells producing any combination of IFNγ, IL-2, or TNF; where 3+ cells produce IFNγ, IL-2 and TNF (black), 2+ cells produce any two of IFNγ, IL-2 and TNF (grey) and 1+ cells produce IFNγ, IL-2 or TNF alone (white). The black arc represents cells that do not produce IFNγ. (H) Frequency of CD3+CD8+ T cells producing IFNγ, IL-2, or TNF 6 weeks after the second dose. (I) Frequency of CD3+CD8+ T cells producing IFNγ, IL-2, or TNF 2 weeks after boosting with rAd5:Gag. Bars and error bars represent mean ± SEM. Statistical differences for bar graphs are represented as NS= no significant difference, * = p ≤ 0.05 and ** = p ≤ 0.01. Statistical differences for pie graphs are represented as # = p ≤ 0.05 compared to ISCOMs alone, $ = p ≤ 0.05 compared to Poly I:C alone. Each group is representative of at least two independent experiments and 4-8 Balb/c mice per group.
Poly I:C and/or ISCOMs Induce CD8 T Cell Responses of Similar Magnitude but Distinct Phenotype
The frequency of Gag-specific CD8 T cell responses by tetramer staining was similar when Gag was given with Poly I:C alone, ISCOMs alone or Poly I:C with ISCOMs (Fig. 1B). We next assessed the phenotype of tetramer+ CD8 T cells using CD127, the IL-7 receptor α chain, and killer-cell lectin-like receptor subfamily G1 (KLRG1). Differential expression of these markers can delineate populations of short-lived effector cells (SLECs) (40), and memory precursor effector cells (MPECs) (41). In addition, CD127+KLRG1+ CD8 T cells are a population of durable long-term memory cells induced following viral vaccination or prime-boost vaccination (37, 42).
Formulations that contained ISCOMs induced a significantly higher proportion (~70–80%) of CD8 T cells expressing CD127 with or without KLRG1 (Fig. 1C; black arc) compared to Poly I:C alone (~50%).
Expression of the transcription factor Tbet is strongly induced with IL-12 signaling and can alter the differentiation, stability and functional capacity of CD8 T cells (40, 43). Tbet expression was upregulated in Gag-specific CD8 T cells after vaccination with any of the adjuvants relative to the total CD8 T cell population in naïve mice used as a negative control (Fig. 1D). Poly I:C alone induced the highest median fluorescence intensity (MFI) for Tbet expression, followed by ISCOMs alone (Fig. 1D; right panel), but Poly I:C with ISCOMs induced a significantly lower MFI compared to either adjuvant alone (Fig. 1D; right panel). These data show that combining Poly I:C and ISCOMs primes a CD8 T cell population that is distinct in terms of phenotype and transcription factor expression from that induced by either adjuvant alone.
Poly I:C and/or ISCOMs Induce Qualitatively Different CD8 T Cell Responses
Using multi-parameter flow cytometry following in vitro re-stimulation with overlapping Gag peptides, we showed that all adjuvants induced a comparable frequency of Gag-specific CD8 T cells as assessed by total cytokine (IFNγ, IL-2 and TNF) production (Fig. 1E). These data are consistent with the magnitude of Gag-specific CD8 T cell responses observed by tetramer staining (Fig. 1B). Interestingly, formulations that contained ISCOMs significantly increased the frequency of IL-2 producing cells relative to Poly I:C alone (Fig. 1F), highlighting differences in the cytokine profile of Gag-specific CD8 T cells.
To extend these findings, we assessed the quality of Gag-specific CD8 T cell responses using the relative frequency of cells producing any combination of IFNγ, IL-2 and TNF at the single cell level (44, 45). Poly I:C alone induced predominantly IFNγ+ TNF+ or IFNγ+ Gag-specific CD8 T cells (Suppl. Fig. 1A) and a low frequency (Suppl. Fig. 1A) and proportion (Fig. 1G; 3+; black sector) of multifunctional cells, which can produce all three cytokines (IFNγ, IL-2 and TNF). These data are consistent with the low frequency of IL-2 producing CD8 T cells observed in Figure 1F. ISCOMs alone and Poly I:C with ISCOMs induced a high frequency (Suppl. Fig. 1A) and proportion (Fig. 1G; 3+; black sector) of multifunctional cells. However, ISCOMs alone also induced a significantly higher frequency (Suppl. Fig. 1A) and proportion (Fig. 1G; black arc) of CD8 T cells that did not express IFNγ, such as IL-2+ and IL-2+ TNF+ cells, compared to Poly I:C alone and Poly I:C with ISCOMs. Taken together, these data illustrate that for CD8 T cells, Poly I:C promotes production of IFNγ while ISCOMs promote production of IL-2.
CD8 T Cells Primed by Poly I:C and/or ISCOMs are Potently Boosted by rAd5
Given that combining Poly I:C and ISCOMs altered the quality and phenotype of CD8 T cells, we assessed the durability of these cells and their ability to respond to subsequent stimulation in vivo following a boost with a viral vector. CD8 T cell responses were evaluated 6 weeks after priming, and the frequency of Gag-specific CD8 T cells had contracted markedly compared to the peak response, but responses were still comparable across all adjuvants (Fig. 1H). To evaluate the ability of primed CD8 T cells to expand in vivo, mice were boosted with 1 × 108 PU of rAd5:Gag 4 weeks after priming and responses were assessed 2 weeks later. The frequency of Gag-specific CD8 T cells was significantly higher in groups that had been primed with any of the adjuvanted vaccines compared to mice primed with PBS or Gag alone (Fig. 1I). Additionally, the frequency of Gag-specific CD8 T cells in mice primed with Poly I:C with ISCOMs was significantly higher compared to Poly I:C alone and trended higher compared to ISCOMs alone (Fig. 1I). Thus, formulations containing ISCOMs prime CD8 T cell populations that are more responsive to subsequent boosting and this may be further enhanced with inclusion of Poly I:C.
Combining Poly I:C with ISCOMs Increases the Magnitude of Multifunctional CD4 T Cell Responses
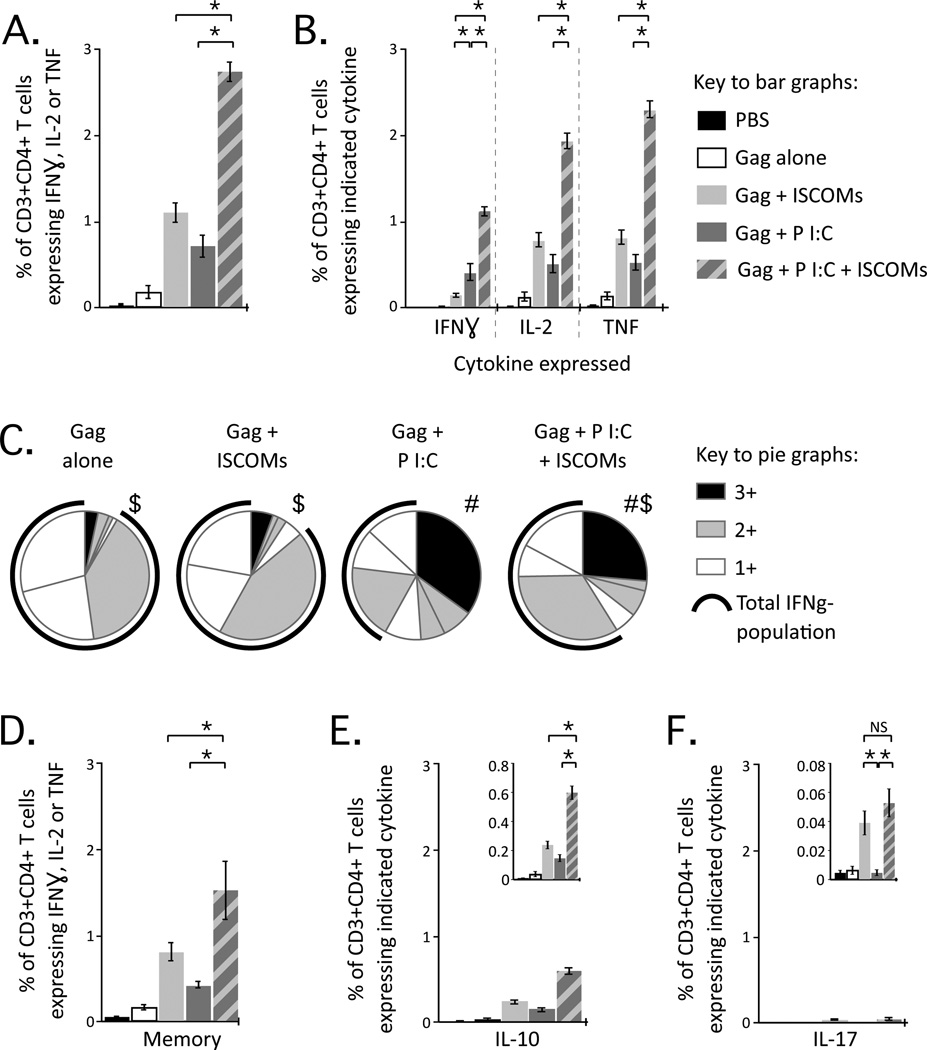
We next assessed the magnitude and qualitative profile of Gag-specific CD4 T cell responses. Poly I:C with ISCOMs primed significantly higher total cytokine responses than either adjuvant alone (Fig. 2A). ISCOMs alone induced a significantly lower frequency of IFNγ producing cells than formulations containing Poly I:C (Fig. 2B). This resulted in marked differences in the quality of CD4 T cell cytokine responses. Poly I:C alone induced a high proportion of multifunctional cells (Fig. 2C; 3+; black sector), while ISCOMs alone primed predominantly IL-2+ TNF+, IL-2+ or TNF+ CD4 T cells (Suppl. Fig. 1B), which are populations that do not produce IFNγ (Fig. 2C; IFNγ-; black arc). Poly I:C with ISCOMs induced a high frequency and proportion of IL-2+ TNF+ and multifunctional CD4 T cells (Sup Fig 1B; Fig. 2C), reflecting the qualitative profiles of both adjuvants. In general, we observed a clear polarisation bias, where Poly I:C promoted a strong Th1 response with a higher proportion of IFNγ producing CD4 T cells compared to ISCOMs. When we assessed the durability of CD4 T cells, responses had contracted modestly (1–2-fold) but comparably across adjuvant formulations by 6 weeks after priming (Fig. 2D).
Figure 2.
Characterisation of CD4 T cell responses after vaccination with Poly I:C and ISCOMs. (A) Frequency of CD3+CD4+ T cells producing IFNγ, IL-2, or TNF by ICS. (B) Frequency of CD3+CD4+ T cells producing IFNγ, IL-2, or TNF individually. (C) Proportion of CD3+CD4+ T cells producing any combination of IFNγ, IL-2, or TNF; 3+, 2+ or 1+ cells and the black arc represents cells that do not produce IFNγ. (D) Frequency of CD3+CD4+ T cells producing IFNγ, IL-2, or TNF 6 weeks after the second dose. Frequency of CD3+CD4+ T cells producing (E) IL-10 or (F) IL-17 at peak. Bars and error bars represent mean ±SEM. Statistical differences for bar graphs are represented as NS= no significant difference and * = p ≤ 0.05. Statistical differences for pie graphs are represented as # = p ≤ 0.05 compared to ISCOMs alone, $ = p ≤ 0.05 compared to Poly I:C alone. Each group is representative of at least two independent experiments and 4-8 Balb/c mice per group.
IFNγ, IL-2 and TNF are critical cytokines for Th1 immunity and protection with a variety of intracellular pathogens. As noted above, Poly I:C potently induced IFNγ production in CD4 T cells, likely due to induction of IL-12 and type I and II IFNs (6). By contrast, CD4 T cell responses induced by ISCOMs were less polarised towards IFNγ production and Th1 immunity. Given the heterogeneity of CD4 T cell responses, we assayed for additional cytokines that may regulate responses or provide alternative effector function.
IL-10 produced by CD4 T cells can regulate and promote resolution of immune responses (reviewed in (46)). IL-10 was detected with all adjuvant formulations (Fig. 2E) and the frequencies of IL-10 producing CD4 T cells were proportionate to the frequencies of total cytokine response for each adjuvant (Fig. 2A). Both vaccines (47) and infections (48) can induce CD4 T cells that simultaneously induce Th1 cytokine and IL-10 production and this may be a mechanism for self-regulation.
ISCOMs induce production of IL-1β (24, 25), which promotes induction of Th17 CD4 T cells in combination with IL-6 (49), and induction of IL-17+ CD4 T cells has been observed after ISCOM vaccination (50). We did not detect IL-17 production after vaccination with Poly I:C alone, but did detect low and comparable frequencies of IL-17+ CD4 T cells after ISCOM vaccination, with or without Poly I:C in several experiments (Fig. 2F). The biologic importance of such responses remains to be determined.
Protection Against Listeria and Vaccinia is Mediated by CD8 and CD4 T Cell Responses Respectively
Given the qualitative and quantitative differences in Gag-specific T cell responses after vaccination, we assessed the ability of these responses to confer protection against bacterial or viral challenge. Mice were vaccinated and then challenged 6 weeks later with recombinant Listeria monocytogenes or vaccinia virus expressing Gag (Listeria:Gag and rVACV:Gag respectively).
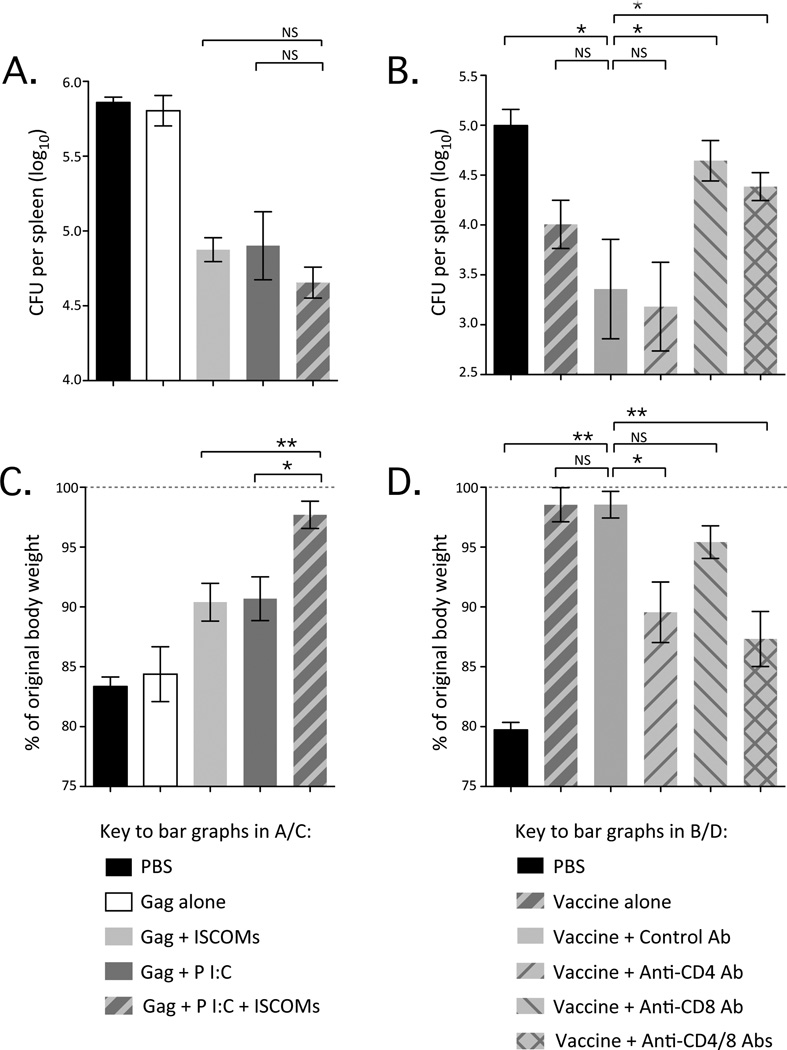
After intravenous challenge with Listeria:Gag, spleens were harvested and bacterial load was evaluated. Mice primed with any of the adjuvants had a significant but comparable reduction in bacterial load compared to PBS control or Gag alone (Fig. 3A). After vaccination with Poly I:C with ISCOMs, depletion of CD8 but not CD4 T cells abrogated protection (Fig. 3B). As all adjuvants induced comparable Gag-specific CD8 T cell responses (Fig. 1B and D), these results are consistent with a prior study showing that the magnitude of CD8 T cell responses correlates with protection in this model (37).
Figure 3.
Protection conferred by vaccination with Poly I:C and ISCOMs against infectious challenge. (A) Bacterial load in the spleen (colony forming units, CFU) after Listeria:Gag challenge of mice vaccinated with formulations containing Gag, ISCOMs and Poly I:C as indicated. (B) Bacterial load in the spleen (CFU) after Listeria:Gag challenge of mice vaccinated with Gag, ISCOMs and Poly I:C (vaccine) and either left untreated or treated with a control antibody (Control Ab), a CD4-depleting antibody (Anti-CD4 Ab), a CD8-depleting Ab (Anti-CD8 Ab) or both of the latter (Anti-CD4/8 Abs). (C) Weight loss as % of original body weight at day 6 after rVACV:Gag challenge in mice vaccinated with formulations containing Gag, ISCOMs and Poly I:C as indicated. (D) Weight loss as % of original body weight at day 6 after rVACV:Gag challenge in mice vaccinated with Gag, ISCOMs and Poly I:C and left untreated or treated the indicated antibodies, as above. Bars and error bars represent geometric mean ±GEM. Statistical differences for bar graphs are represented as NS= no significant difference, * = p ≤ 0.05 and ** = p ≤ 0.01. Each group is representative of at least two independent experiments with 4-6 Balb/c mice per group.
As an alternative viral challenge model, rVACV:Gag was given intranasally and body weight was followed over 6 days as a measure of disease severity. Mice primed with Gag alone or the PBS control exhibited a marked loss of body weight; however, mice primed with either Poly I:C or ISCOMs alone similarly and significantly attenuated loss of body weight (Fig. 3C). Strikingly, mice primed with Poly I:C with ISCOMs maintained their original body weight and were significantly higher than weights observed in mice primed with Poly I:C or ISCOMs alone (Fig. 3C). After vaccination with Poly I:C with ISCOMs, depletion of CD4 T cells caused significantly more weight loss than observed in a control treated animal, although not to the same level as the PBS control, while depletion of CD8 T cells caused a mild but not significant loss of weight (Fig. 3D). These data suggest that protection against rVACV:Gag is predominantly dependent on CD4 T cells, with some contribution by CD8 T cells and possibly antibody responses. This is consistent with the observation that Poly I:C with ISCOMs induced Gag-specific CD4 T cell responses of higher frequency than Poly I:C or ISCOMs alone.
In summary, in these acute infection models, protection correlates directly with magnitude of the relevant T cell response; CD8 for Listeria:Gag and CD4 for rVACV:Gag. It is possible that the qualitative differences in T cell responses induced by different adjuvant formulations would have more impact on protection against more chronic infections.
Poly I:C and ISCOMs Differentially Alter Recruitment of DC Subsets to the draining lymph node
The quantitative and qualitative differences in T cell responses reflect early activation signals received by T cells from APCs and the innate environment at the site of priming. Poly I:C is an strong inducer of type I IFN, which we have previously shown to enhance trafficking and uptake of antigen by DCs after adjuvanting with a TLR7 ligand (39). Moreover, ISCOMs disrupt phago-lysosomes upon endocytosis by APCs and can alter antigen distribution within antigen processing compartments (27, 28). Accordingly, we focused on whether Poly I:C, ISCOMs or the combination of adjuvants altered antigen uptake, processing and presentation by DC subsets that could subsequently influence priming of T cell immunity.
To assess the kinetics of DC subsets in the draining lymph node (dLN) after vaccination and their potential for antigen uptake, we administered each adjuvant formulation with Gag protein fluorescently labeled with AF488 (Gag:AF488). The dLN were harvested, pooled and processed at 24, 48, 72 and 168 hours (1, 2, 3 or 7 days) after vaccination. Samples were stained with a twelve colour multi-parameter flow cytometry panel that enables us to identify 6 DC subsets present in murine skin-draining LNs; the LN resident CD8α+ DCs and plasmacytoid DCs (pDCs), as well as the migratory populations such as monocyte-derived DCs, langerin+ and langerin- dDCs and Langerhans cells (Fig. 4A). The dLN must be pooled to detect rare DC populations, which limits statistical analysis between the groups, but the data presented are representative of three independent experiments with similar results.
Figure 4.
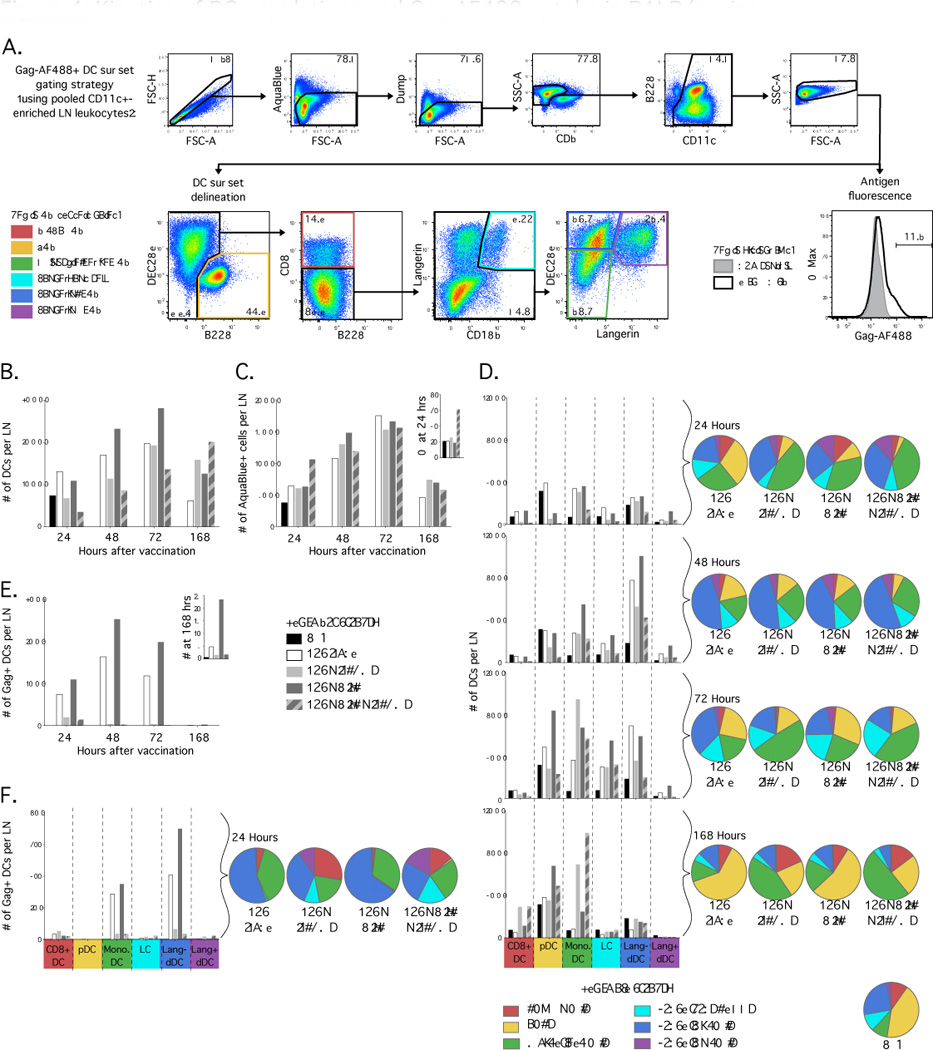
Composition of DC populations and antigen uptake in dLN of Balb/c mice after vaccination with Gag:AF488, Poly I:C and ISCOMs. (A) Flow cytometric plots illustrating gating strategy with CD11c+ enriched samples for identification of DC subsets and evaluation of uptake of AF488 labeled antigen. (B) Number of total live CD11c+ DCs recovered per dLN at indicated times after vaccination. (C) Number of total leukocytes in dLN that stain with AquaBlue, a marker of non-viable cells, and (inset) the proportion of total leukocytes in dLN that are non-viable at 24 hours. (D) Number (bar graphs) and relative proportion (pie graphs) of total live CD11c+ DCs that distribute to each DC subset at indicated times after vaccination. (E) Number of live Gag:AF488+ CD11c+ DCs recovered per dLN and (inset) the number at 168 hours on an expanded y axis. (F) Number and relative proportion of live Gag:AF488+ CD11c+ DCs that distribute to each DC subset at 24 hours after vaccination. Results are representative of three independent experiments, with dLN from 10 mice pooled for each formulation.
After vaccination, there was a gradual increase in the number of total CD11c+ DCs recovered after vaccination with Gag alone, Poly I:C alone and ISCOMs alone compared to the PBS control or naïve (data not shown) mice at 24 hours (Fig. 4B). In contrast, the number of total CD11c+ DCs decreased relative to the PBS control after vaccination with Poly I:C with ISCOMs at 24 hours (Fig. 4B) and there was a higher number (Fig. 4C) and proportion (Fig. 4C inset) of dead leukocytes. This suggests that Poly I:C given with ISCOMs induced death of CD11c+ DCs in the dLN.
Upon assessment of DC subset composition in the dLN, we observed that the number (Fig. 4D; bar graph) and relative frequency (Fig. 4D; pie graph) of pDCs declined markedly by 24 hours after vaccination in all adjuvanted vaccines compared to PBS or Gag alone. This has been observed after vaccination with other adjuvants, such as CpG in a protein subunit vaccine (51), and may represent mobilisation to the blood or death of pDCs. Other DC subsets sequentially increased in number and relative frequency for all formulations; first monocyte-derived DCs at 24 hours, langerin+ and langerin- dDCs at 48 hours then Langerhans cells at 72 hours (Fig. 4D), reflecting sequential migration of skin-derived DC subsets to the dLN after vaccination. Of note, vaccination with ISCOM formulations increase the relative frequency of monocyte-derived DCs at 48 hours and the number and relative frequency of monocyte-derived DCs remain elevated at 168 hours after vaccination (Fig. 4D), which was the last time point assessed. This highlights that ISCOMs induce prolonged recruitment of certain cell subsets, and monocyte-derived DCs in particular, to the dLN.
Adjuvants Modify Uptake of Antigen by DC Subsets Following Vaccination
When uptake of Gag:AF488 was assessed, an increased number of Gag:AF488+ CD11c+ DCs were recovered after vaccination with Poly I:C alone compared to Gag alone at all time points (Fig. 4E), and Gag:AF488 was still detected 168 hours after vaccination (Fig. 4E; inset). This is consistent with our previous study where a TLR7 ligand induced type I IFN production and thereby increased antigen uptake (39). In marked contrast, we recovered low numbers of Gag:AF488+ CD11c+ DCs from mice that received formulations containing ISCOMs at all time points after vaccination (Fig. 4E).
When DC subsets were assessed for antigen uptake at 24 hours after vaccination, Gag:AF488 localised predominantly to langerin- dDCs and monocyte-derived DCs for Gag alone or Poly I:C alone, with very low but detectable uptake by CD8α+ DCs (Fig. 4F). In mice that had received ISCOMs with or without Poly I:C, there was a very low number of total Gag:AF488+ DCs (Fig. 4F; bar graphs) but there were higher proportions of Gag:AF488+ CD8α+ DCs, Langerhans cells and langerin+ dDCs compared to Gag alone or Poly I:C alone (Fig. 4F; pie graphs). At subsequent time points, distribution of detectable antigen remained similar to the 24 hour time point for each formulation (data not shown).
These data were collected using Balb/c mice and fluorescently labeled Gag antigen. Similar results were obtained in C57BL/6 mice with fluorescently labeled ovalbumin (OVA) antigen (Suppl. Fig. 2), demonstrating that changes in dLN DC composition and antigen uptake are not mouse strain- or antigen-specific.
ISCOMs Induce Rapid Processing of Antigen by DCs but Poly I:C Promotes Retention of Peptide
The limited ability to detect Gag:AF488 in CD11c+ DCs after vaccination with ISCOMs could be due to two distinct mechanisms: ISCOMs may limit uptake of antigen by DCs or promote rapid degradation of antigen and loss of the AF488 fluorescent signal. To examine the rate of degradation of antigen in vivo after vaccination, we used DQ-OVA. DQ-OVA has high-density BODIPY fluorophore residues arrayed along the OVA protein, which self-quench when the protein is intact but fluoresce when the protein undergoes degradation (52, 53).
We simplified the analysis to identify three dLN DC populations; CD8α+ DCs, pDCs and migratory DCs, to include the monocyte-derived DCs, Langerhans cells, langerin- and langerin+ dDCs (Fig. 5A). Since Gag:AF488 signal is already low at 24 hours after vaccination with ISCOM formulations (Fig. 4E), dLNs were harvested from Balb/c mice at 10, 24 and 72 hours after vaccination.
Figure 5.
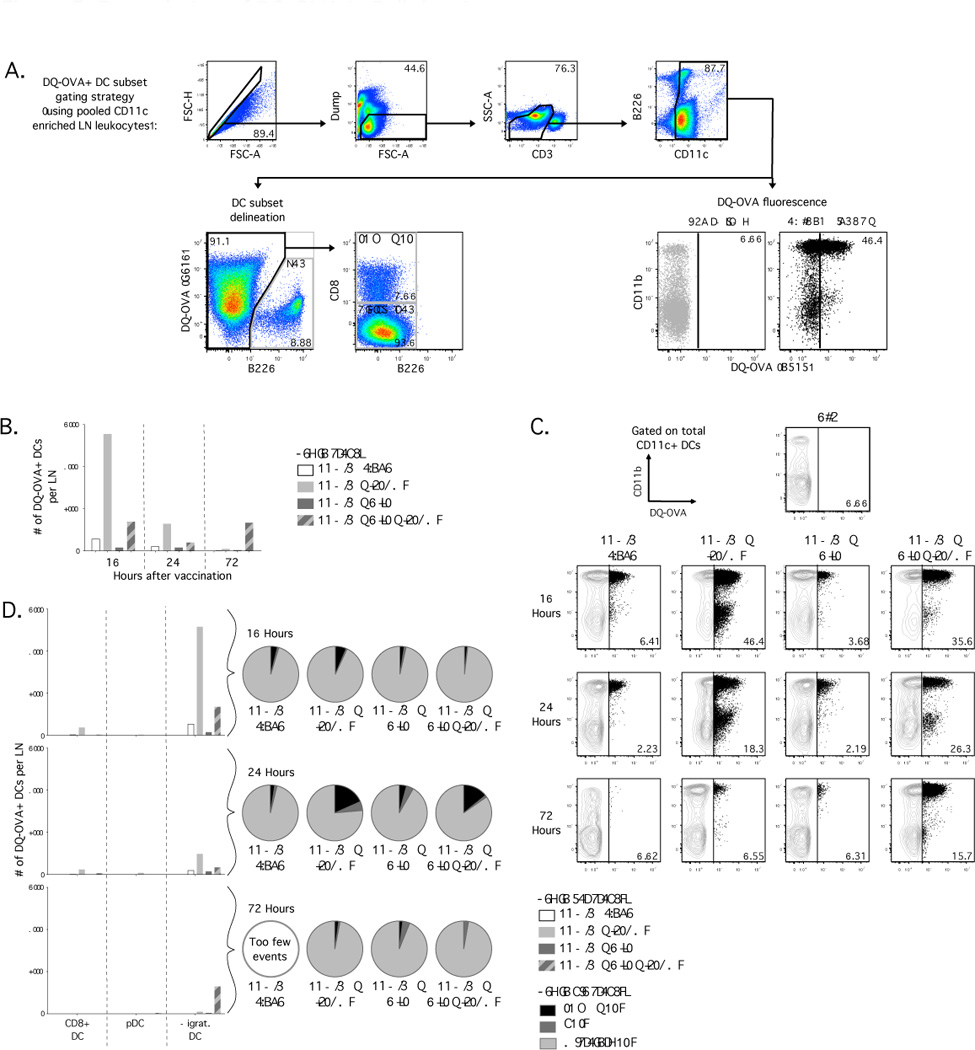
Degradation of antigen by DC subsets in dLN of Balb/c mice after vaccination with DQ-OVA, Poly I:C and ISCOMs. (A) Flow cytometric plots illustrating gating strategy with CD11c+ enriched samples for identification of DC subsets and evaluation of degradation of DQ-OVA, indicated by fluorescence in the B515 channel on an LSRII. (B) Number of live DQ-OVA+ CD11c+ DCs recovered per dLN at indicated times after vaccination. (C) Dot plots with frequency of total live CD11c+ DCs that are DQ-OVA+. (D) Number and relative proportion of live DQOVA+ CD11c+ DCs that distribute to each DC subset at indicated times after vaccination. Results are representative of three independent experiments, with dLN from 4 mice pooled for each formulation.
Mice that received ISCOMs alone had a higher number (Fig. 5B) and frequency (Fig. 5C; top and middle rows) of DQ-OVA+ CD11c+ DCs at 10 and 24 hours after vaccination. Mice that received Poly I:C with ISCOMs had low numbers of DQ-OVA+ DCs compared to ISCOMs alone at 10 and 24 hours after vaccination (Fig. 5B), but both groups had comparable proportions of DCs that contained DQ-OVA (Fig. 5C; top and middle rows). At early time points after vaccination with Poly I:C with ISCOMs, we observed significant cell death in the dLN (Fig. 4C), suggesting that the low number of DQ-OVA+ DCs is likely due to low viability. Interestingly, we consistently observed an elevated number (Fig. 5B) and frequency (Fig. 5C; bottom row) of DQ-OVA+ DCs at 72 hours after vaccination with Poly I:C with ISCOMs compared to Poly I:C alone or ISCOMs alone. Finally, degraded OVA was predominantly detected in migratory DCs, with very low numbers of DQ-OVA+ CD8α+ DCs, but not detected in pDCs (Fig. 5D).
Thus, ISCOMs increase the rate of antigen degradation relative to Poly I:C or protein alone but inclusion of Poly I:C with ISCOMs promotes persistence of degraded antigen.
Poly I:C and ISCOMs Require CD8α+ DCs and/or Langerin+ dDCs for Optimal T Cell Priming
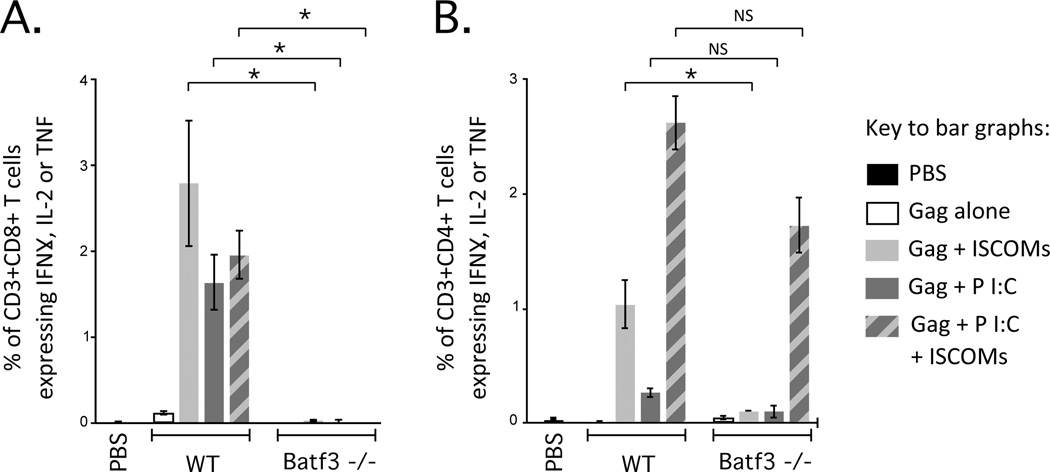
To identify the DC subsets responsible for priming CD8 and CD4 T cell responses in vivo, we used Batf3 deficient (−/−) mice. Batf3 is a transcription factor essential for development of CD8α+ DCs and langerin+ (CD103+) dDCs (54, 55) and therefore Batf3 −/− mice lack these two DC subsets. CD8α+ DCs, langerin+ dDCs and langerin–dDCs have been shown to mediate cross-presentation in vivo (39, 56–58) and, as such, would be likely required to mediate priming of CD8 T cells with a protein-based vaccine. Indeed, Gag-specific CD8 T cell cytokine responses were completely abrogated in Batf3 −/− mice compared to WT mice for all adjuvant formulations (Fig. 6A). For CD4 T cells, Batf3 −/− mice trended towards lower Gag-specific cytokine responses for Poly I:C with or without ISCOMs and were significantly lower for ISCOMs alone compared to WT mice (Fig. 6B). Overall, CD8α+ DCs and langerin+dDCs are essential for CD8 T cell responses with Poly I:C and/or ISCOMs and may play a role for optimising CD4 T cell responses with ISCOMs.
Figure 6.
Role of DC subsets for in vivo priming of T cells after vaccination with Poly I:C and/or ISCOMs. Frequency of (A) CD3+CD8+ T cells or (B) CD3+CD4+ T cells producing IFNγ, IL-2, or TNF by ICS after vaccination of WT Balb/c or Batf3 −/− mice with Gag and each adjuvant formulation. Bars and error bars represent mean ±SEM. Statistical differences for bar graphs are represented as NS= no significant difference and * = p ≤ 0.05. Each group is representative of at least two independent experiments and 4-5 mice per group.
Discussion
Poly I:C and ISCOMs have been used individually in protein-based vaccines to enhance humoral and cellular immunity in pre-clinical and human studies. We focused on Poly I:C as an adjuvant based on recent studies in non-human primates, showing that it elicits potent SIV or HIV Gag T cell responses when given with protein-based or DC targeting vaccines (7, 59). Moreover, Poly I:C formulated with poly-lysine and carboxymethylcellulose to enhance stability has been used as an investigational reagent in humans and shown to have potent innate activity (60). ISCOM-Matrix vaccines and vaccines containing the saponin derivative QS21 have been well tolerated, protective and generated antibody and T cell responses in human clinical trials (8, 9, 61). We also noted a significant increase in Gag-specific serum IgG when Poly I:C and ISCOMs were combined, but we focused in this study on quantitative and qualitative differences in T cell immunity and adjuvant mechanisms that may contribute to these differences, due to the difficulty in inducing potent T cell responses with protein-based vaccines in humans.
Poly I:C potently induces type I IFN and co-administration of Poly I:C with Gag in this study lead to more DCs loaded with detectable antigen (Fig. 4D). These data are consistent with our previous study using a TLR7 ligand adjuvant, which promoted type I IFN-dependent uptake of antigen by DCs (39). In addition to antigen uptake, in vitro production of type I IFN by Poly I:C promotes retention of degraded antigen by DCs (62). Of note, CpG also induces robust type I IFN production and can prolong antigen retention and presentation in vitro (63). Consistent with this in vitro observation, Poly I:C prolonged the presence of degraded antigen in DCs in vivo in this study (Fig. 5B and C) and this correlated with increased CD4 T cell responses after vaccination with the combination of Poly I:C and ISCOMs (Fig 2). Other studies have combined CpG with ISCOMs and observed increased CD4 T cell immunity (33). As CpG is also a potent inducer of type I IFN in vivo, we confirmed that combining CpG with ISCOMs induced quantitative and qualitative changes that were similar to Poly I:C (Sup. Fig. 3). Thus, our results suggest that type I IFN generated by Poly I:C, and possibly CpG, acts on DCs in vivo to prolong retention of degraded antigen and thereby enhance expansion of antigen-specific CD4 T cell responses.
ISCOMs were shown to accelerate antigen degradation in this study, which is likely the result of two possible and not mutually exclusive mechanisms. Antigen co-delivered with ISCOMs can enter the cytosol during disruption, whereupon antigen can be rapidly degraded by tripeptidyl peptidase II (28). Alternatively, disruption of phago-lysosomal integrity could trigger cellular death, during which cellular contents, including antigen, may be degraded, taken up and presented by other APCs. When Poly I:C and ISCOM were combined, we observed a high number and frequency of dead leukocytes soon after vaccination (Fig. 4C). Additionally, we found that Batf3 expression, and presumably CD8α+ DCs and langerin+ dDCs, were required for CD8 T cell responses (Fig. 6A), despite the fact that these DCs were relatively minor populations for antigen uptake and degradation (Fig. 4 and 5). These date imply that a very low number of such DCs are required to directly present antigen or mediate cross-presentation. It was also notable that CD8α+ DCs and langerin+ dDCs were required for optimal CD4 T cell responses after ISCOM vaccination (Fig. 6B). Taken together, we speculate that APCs that initially take up antigen are not optimal for direct presentation to CD4 T cells, possibly due to reduced cell viability. Subsequent uptake of cellular debris with antigen by CD8α+ DCs and langerin+ dDCs could facilitate both cross-presentation and MHC class II presentation.
Aside from DCs and T cells, other innate cells may shape the development of adaptive immunity with Poly I:C and ISCOMs. In particular, elevated numbers of neutrophils have been observed in the dLN after ISCOM vaccination (25), which we also observed in this study (Sup. Fig. 4A). Neutrophils may prime or cross-prime naïve TCR transgenic CD4 and CD8 T cells directly in vivo (64, 65) but neutrophils also undergo cell death in dLNs (66) and may therefore ferry antigen to secondary lymphoid sites. We also observed increased and prolonged recruitment of macrophages and monocytes (Sup. Fig. 4B and C) and sustained recruitment of monocyte-derived DCs with the combination of Poly I:C and ISCOMs. Antigen presentation by these APC subsets has been shown to increase T cell responses and promote qualitative changes such as increased TNF production (67), consistent with our study.
Regardless of the mechanism, the rapid degradation of antigen would provide an immediate bolus of antigen presentation after ISCOM vaccination. High antigen density on APCs can engage T cells with lower affinity T cell receptors (68, 69), but subsequent depletion of antigen can alter CD8 T cell differentiation to increase IL-2 and TNF production and increase CD127 expression, as observed in a L. monocytogenes infection model (70). We speculate that this also occurs after ISCOM vaccination, with rapid presentation of a high density of antigen that is subsequently depleted, leading to CD8 T cell responses with high IL-2 and TNF production and high CD127 expression as observed in this study.
Innate signaling induced by Poly I:C or ISCOMs could directly influence the magnitude of T cell responses. While not directly assessed here, a number of prior studies have clearly shown that Poly I:C is a potent inducer of type I and II IFNs and IL-12 in vivo (6, 11), which leads to polarised Th1 responses and promotes expansion and survival of CD8 T cells (71–73). Alternatively, ISCOMs have been shown to elicit IL-1βproduction (24, 25), which can directly signal to CD4 T cells in vitro to augment proliferation (71). Concurrent treatment of CD4 T cells in vitro with type I IFN and IL-1 α/βhas been shown increase cytokine responses (74); thus cytokines induced by Poly I:C and ISCOMs may work in concert to enhance the magnitude of CD4/Th1 responses and the quality of CD8 T cell responses.
In conclusion, the combination of Poly I:C and ISCOMs resulted in protective T cell immunity against bacterial and viral infections. These data highlight that adjuvants not only have a major role for enhancing T cell responses through innate cytokines but also have profound effects on DC trafficking, antigen uptake and kinetics of antigen presentation. We speculate that differences in antigen degradation with the inclusion of ISCOMs and retention of antigen with the inclusion of Poly I:C may contribute to differences in the magnitude and quality of T cell responses. As a result, the robust T cell responses induced by combining Poly I:C and ISCOMs are likely shaped by the collective effects of antigen presentation and innate signaling. To enhance rational design of subunit vaccines, the net effect of multiple mechanisms altering antigen processing and innate signaling can be harnessed to augment T cell immunity.
Supplementary Material
Acknowledgements
We would like to acknowledge Allison Malloy, Tracy Ruckwardt and Barney Graham (Vaccine Research Center, Bethesda, MD, USA) for helpful discussion. We additionally thank Christine Trumpfheller (Rockerfeller University, New York, NY, USA) for provision of rVACV:Gag and Fred D. Finkelman (University of Cincinnati, Cincinnati, OH, USA) for provision of antibodies used for depletion studies.
This research has been supported in part by a grant from the Foundation for the National Institutes of Health with support from the Collaboration for AIDS Vaccine Discovery (CAVD) award OPP1039775 from the Bill & Melinda Gates Foundation.
Abbreviations
- Poly I:C
polyinosinic:polycytidylic acid
- ISCOM
immunostimulatory complex
- PRR
pathogen recognition receptor
- TLR
toll-like receptor
- MDA-5
melanoma differentiation-associated protein 5
- RIG-I
retinoic acid-inducible gene 1
- PKR
dsRNA-dependent protein kinase R
- DC
dendritic cell
- dDC
dermal dendritic cell
- AF
Alexa fluor
- Gag
HIV Gag protein
- KLRG1
killer-cell lectin-like receptor subfamily G1
- SLECs
short-lived effector cells
- MPECs
memory precursor effector cells
- MFI
median fluorescent intensity
- dLN
draining lymph node
- Gag:AF488
Gag protein labeled with Alexa fluor 488
- pDC
plasmacytoid DC
- OVA
ovalbumin
- −/−
genetically deficient
References
- 1.Kwong PD, Mascola JR, Nabel GJ. The changing face of HIV vaccine research. J. Int. AIDS Soc. 2012;15:17407. doi: 10.7448/IAS.15.2.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein JE, Tewari K, Lyke KEL, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science. 2011;334:475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 4.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau MG, Hendriks J, Weverling GJ, Goudsmit J, Sizemore D, McClain JB, Goetz M, Gearhart J, Mahomed H, Hussey GD, Sadoff JC, Hanekom WA. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am. J. Respir. Crit. Care Med. 2010;181:1407–1417. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn BJ, Kastenmüller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, Esteban M, Park CG, Trumpfheller C, Keler T, Pantaleo G, Steinman RM, Seder R. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc. Natl. Acad. Sci. USA. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drane D, Maraskovsky E, Gibson R, Mitchell S, Barnden M, Moskwa A, Shaw D, Gervase B, Coates S, Houghton M, Basser R. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a phase I study in healthy volunteers. Hum. Vaccin. 2009;5:151–157. doi: 10.4161/hv.5.3.6614. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen GK, Madhun AS, Breakwell L, Hoschler K, Sjursen H, Pathirana RD, Goudsmit J, Cox RJ. T-helper 1 cells elicited by H5N1 vaccination predict seroprotection. J. Infect. Dis. 2012;206:158–166. doi: 10.1093/infdis/jis330. [DOI] [PubMed] [Google Scholar]
- 10.Offermann MK, Zimring J, Mellits KH, Hagan MK, Shaw R, Medford RM, Mathews MB, Goodbourn S, Jagus R. Activation of the double-stranded-RNA-activated protein kinase and induction of vascular cell adhesion molecule-1 by poly (I).poly (C) in endothelial cells. Eur. J. Biochem. 1995;232:28–36. doi: 10.1111/j.1432-1033.1995.tb20777.x. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 13.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh C-S, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 15.Edwards AD, Diebold SS, Slack EMC, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 16.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, Wang Y, Venzon D, Epstein SL, Segal DM. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J. Immunol. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang D-C, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, Fisher PB. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–1800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J. Immunol. 2010;184:2751–2755. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- 19.Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–5132. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, Soundarapandian V, Ahumada A, Keler T, Hoffman SL, Nussenzweig MC, Steinman RM, Seder RA. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and αDEC-CSP in non human primates. Vaccine. 2010;28:7256–7266. doi: 10.1016/j.vaccine.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, DiDonato J, Dziarski R, Akira S, Schoenberger SP, Raz E. A subset of Toll-like receptor ligands induces cross-presentation by bone marrowderived dendritic cells. J. Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 22.Drane D, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev. Vaccines. 2007;6:761–772. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]
- 23.Behboudi S, Morein B, Rönnberg B. Isolation and quantification of Quillaja saponaria Molina saponins and lipids in iscom-matrix and iscoms. Vaccine. 1995;13:1690–1696. doi: 10.1016/0264-410x(95)00107-c. [DOI] [PubMed] [Google Scholar]
- 24.Villacres-Eriksson M, Bergström-Mollaoglu M, Kåberg H, Lövgren K, Morein B. The induction of cell-associated and secreted IL-1 by iscoms, matrix or micelles in murine splenic cells. Clin. Exp. Immunol. 1993;93:120–125. doi: 10.1111/j.1365-2249.1993.tb06507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, Morelli AB, Clausen BE, Dauer M, Eigler A, Anz D, Bourquin C, Maraskovsky E, Endres S, Schnurr M. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J. Immunol. 2011;187:55–63. doi: 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maloy KJ, Donachie AM, Mowat AM. Induction of Th1 and Th2 CD4+ T cell responses by oral or parenteral immunization with ISCOMS. Eur. J. Immunol. 1995;25:2835–2841. doi: 10.1002/eji.1830251019. [DOI] [PubMed] [Google Scholar]
- 27.Maraskovsky E, Schnurr M, Wilson NS, Robson NC, Boyle J, Drane D. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol. Cell. Biol. 2009;87:371–376. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- 28.Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, Cebon J, Maraskovsky E, Endres S. ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II. J. Immunol. 2009;182:1253–1259. doi: 10.4049/jimmunol.182.3.1253. [DOI] [PubMed] [Google Scholar]
- 29.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM, Foged C. Immunity by formulation design: Induction of high CD8(+) Tcell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J. Control. Rel. 2010;150:307–317. doi: 10.1016/j.jconrel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J. Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, Kisser U, Bauer CA, Dauer M, Eigler A, Maraskovsky E, Endres S, Schnurr M. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int. J. Cancer. 2010;128:897–907. doi: 10.1002/ijc.25399. [DOI] [PubMed] [Google Scholar]
- 34.Sundling CE, Forsell MN, O'Dell S, Feng Y, Chakrabarti B, Rao SS, Loré K, Mascola JR, Wyatt RT, Douagi I, Karlsson Hedestam GB. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J. Exp. Med. 2010;207:2003–2017. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn KM, Da Costa A, Yamamoto A, Berry DB, Lindsay RW, Darrah PA, Wang L, Cheng C, Kong W-P, Gall JGD, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gomez CE, Esteban M, Wyatt LS, Moss B, Morgan C, Roederer M, Bailer RT, Nabel GJ, Koup RA, Seder RA. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mata M, Travers PJ, Liu Q, Frankel FR, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 1998;161:2985–2993. [PubMed] [Google Scholar]
- 39.Kastenmüller K, Wille-Reece U, Lindsay RWB, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, Kaplan DH, Kastenmüller W, Germain RN, Seder RA. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Invest. 2011;121:1782–1796. doi: 10.1172/JCI45416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 42.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham Q-M, Zickovich JM, Lefrançois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 44.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 45.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray PJ, Smale ST. Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nat. Immunol. 2012;13:916–924. doi: 10.1038/ni.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darrah PA, Hegde ST, Patel DT, Lindsay RWB, Chen L, Roederer M, Seder RA. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J. Exp. Med. 2010;207:1421–1433. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghoreschi K, Laurence A, Yang X-P, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun H-W, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O'Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 2010;78:2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah JA, Darrah PA, Ambrozak DR, Turon TN, Mendez S, Kirman J, Wu C-Y, Glaichenhaus N, Seder RA. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J. Exp. Med. 2003;198:281–291. doi: 10.1084/jem.20030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santambrogio L, Sato AK, Carven GJ, Belyanskaya SL, Strominger JL, Stern LJ. Extracellular antigen processing and presentation by immature dendritic cells. Proc. Natl. Acad. Sci. USA. 1999;96:15056–15061. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, Vremec D, Robb L, Shortman K, McKenna HJ, Maliszewski CR, Maraskovsky E. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but not CD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 2000;165:49–58. doi: 10.4049/jimmunol.165.1.49. [DOI] [PubMed] [Google Scholar]
- 54.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edelson BT, KC W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung S-SJ, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 57.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 58.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park H, Adamson L, Ha T, Mullen K, Hagen SI, Nogueron A, Sylwester AW, Axthelm MK, Legasse A, Piatak M, Lifson JD, McElrath JM, Picker LJ, Seder RA. Polyinosinic-polycytidylic acid is the most effective TLR adjuvant for SIV Gag protein-induced T cell responses in nonhuman primates. J. Immunol. 2013;190:4103–4115. doi: 10.4049/jimmunol.1202958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet J-P, Haddad EK, Breton G, Trumpfheller C, Pollak S, Shimeliovich I, Duque-Alarcon A, Pan L, Nelkenbaum A, Salazar AM, Schlesinger SJ, Steinman RM, Sékaly RP. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 2011;208:2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.RTS SCTP, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BGNO, Kabwende AL, Adegnika AA, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kaboré B, Sombié O, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Otsyula N, Gondi S, Otieno A, Owira V, Oguk E, Odongo G, Woods JB, Ogutu B, Njuguna P, Chilengi R, Akoo P, Kerubo C, Maingi C, Lang T, Olotu A, Bejon P, Marsh K, Mwambingu G, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Dosoo D, Asante I, Adjei G, Kwara E, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Mahende C, Liheluka E, Malle L, Lemnge M, Theander TG, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Sarfo A, Agyekum A, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Tembo T, Tegha G, Tsidya M, Kilembe J, Chawinga C, Ballou WR, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Olivier A, Vekemans J, Carter T, Kaslow D, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 63.Kuchtey J, Chefalo PJ, Gray RC, Ramachandra L, Harding CV. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J. Immunol. 2005;175:2244–2251. doi: 10.4049/jimmunol.175.4.2244. [DOI] [PubMed] [Google Scholar]
- 64.Culshaw S, Millington OR, Brewer JM, McInnes IB. Murine neutrophils present Class II restricted antigen. Immunol. Lett. 2008;118:49–54. doi: 10.1016/j.imlet.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P, Barnaba V, Jeannin P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 66.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJM, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 67.Abadie V, Bonduelle O, Duffy D, Parizot C, Verrier B, Combadière B. Original encounter with antigen determines antigen-presenting cell imprinting of the quality of the immune response in mice. PLoS ONE. 2009;4:e8159. doi: 10.1371/journal.pone.0008159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 69.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, Von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat. Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J. Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 71.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 72.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 73.Tough DF, Zhang X, Sprent J. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J. Immunol. 2001;166:6007–6011. doi: 10.4049/jimmunol.166.10.6007. [DOI] [PubMed] [Google Scholar]
- 74.Madera RF, Wang JP, Libraty DH. The combination of early and rapid type I IFN, IL-1α, and IL-1β production are essential mediators of RNA-like adjuvant driven CD4+ Th1 responses. PLoS ONE. 2011;6:e29412. doi: 10.1371/journal.pone.0029412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.