Abstract
Purpose of review
This review outlines the concept of cell-based therapy to restore tissue function, and addresses four key points to consider in cell transplantation: source, surveillance, safety, and site. While each point is essential, additional attention should be given to transplantation sites if cell therapy is going to be successful in the clinic. Various ectopic locations are discussed, and the strengths and weaknesses of each are compared as suitable candidates for cell therapy.
Recent findings
Studies in rodents often demonstrate cell transplantation and engraftment in ectopic sites, with little evidence to suggest why it may also work in humans. For example, transplantation to the subcapsular space of the kidney is often performed in rodents, but has not been a good predictor of clinical success. Recent work shows that the lymph node may be a good site for transplantation of multiple tissue types, and several reasons are highlighted as to why it should be considered for future studies.
Summary
The use of cell-based therapy in the clinic has been hampered by the lack of appropriate sites for transplantation. The lymph node is a promising alternative for cell transplantation, and offers hope for clinical application.
Keywords: Ectopic organogenesis, cell therapy, cell transplantation, stem cells, regenerative medicine
Introduction
Transplantation of hematopoietic stem cells (HSCs) has been the “gold standard” for successful cell therapy in patients. On the other hand, transplantation of other cell types, such as hepatocytes and pancreatic islets, has been remarkably difficult thus far. A major reason why HSC transplantation has been superior to alternative cell therapies is the ability to adequately address what we term the four S's: source, surveillance, safety, and site. This review briefly discusses each of these components, with the main focus on the site of transplantation as a major factor to achieve clinical success. Transplantation of HSC, hepatocyte, pancreatic islet, and mesenchymal stem cells (MSCs) are primarily discussed because they have been transplanted extensively in both the laboratory and the clinic.
Defining the four S's of cell-based therapy – source, surveillance, safety, and site
There are several important factors that must be considered before cell therapy can move from a laboratory achievement to clinical reality (Figure 1).
Figure 1.
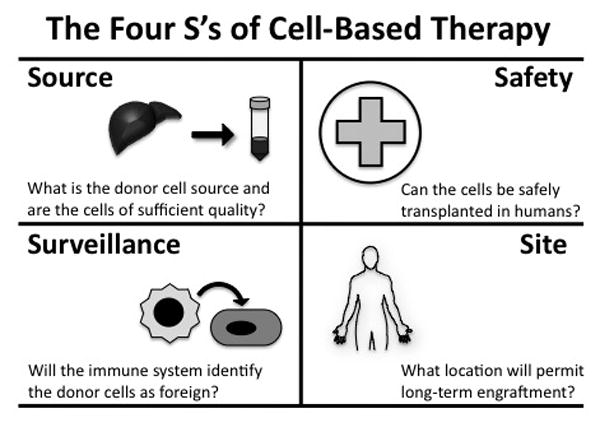
The four S's of cell-based therapy. Cell source, surveillance, safety, and site must all be considered for cell-therapy to be successful in the clinic.
Source – for any cell therapy, donor material must be obtained of sufficient quality, quantity, and function to permit transplantation into a recipient. There are many possibilities to consider as a donor cell source, including living donor tissue, cadaveric tissue, embryonic stem (ES) cells, and induced pluripotent stem (iPS) cells. For HSC and MSC transplantation, living donors provide an ample cell supply. However, there are major shortages of donor tissue available for hepatocyte[1*] and islet cell transplantations[2**], so there has been a lot of attention recently on pluripotent stem cell approaches to overcome this issue[3*].
Surveillance – the next major hurdle for successful cell therapy is the ability to overcome rejection induced by immune surveillance. Anti-rejection protocols have helped minimize immune rejection of donor cells, but the potential always exists that the recipient may one day mount an immune response and reject the donor cells. A major reason iPS cells have garnered so much attention is due to their potential to avoid rejection since an autologous transplantation can be performed[4**,5**,6].
Safety – if cell transplantation is going to become commonplace in the clinic, then patient safety has to be maximized with minimal risk. The risk currently associated with ES or iPS transplantation is one of the biggest hurdles facing their clinical use because they have the potential to form multiple cell types and may become tumorigenic[7*].
Site – a person may have an unlimited supply of donor cells that can be safely transplanted with little chance of rejection, but without an accommodating site for transplantation, cell therapy will not be successful. It is important to consider the potential benefits and limitations of various transplantation sites, and studies in rodents suggest that organogenesis in ectopic locations could be harnessed for cell-based therapy in patients.
Orthotopic versus ectopic tissue
It is reasonable to think that transplanting cells orthotopically (in their native home) would provide the best environment for cell survival and function. However, cell therapy is an approach to restore loss of tissue function or damaged tissue, so the native environment may not be hospitable or readily accessible. For example, fibrotic and cirrhotic livers accompanied with portal hypertension have an altered architecture due to scarring. Placing healthy hepatocytes in this diseased setting may hinder engraftment, not support it. So where should healthy cells be transplanted in a diseased recipient? One possibility can be inferred from nature's approach to overcome an uninhabitable environment by developing ectopic organs.
The majority of HSCs reside in the bone marrow, which is the site of hematopoiesis. In the case of myelofibrosis, however, the bone marrow environment is damaged to the extent that HSCs find a new home and hematopoiesis occurs in an alternative ectopic location. When tissues are found outside their normal location, it is collectively referred to as ectopic tissue. Ectopic tissue can be generated during development or in response to an injury. For example, there have been clinical reports of ectopic liver[8,9], kidney[10], and pancreatic tissue[11,12] observed in patients. Importantly, ectopic tissue has been found to function similar to tissue located in the native site[13*]. Together, these observations demonstrate that functional tissue can form in an alternative location under certain conditions. Therefore, in the context of cell therapy, ectopic organogenesis may be a useful approach to restore or replace normal tissue in a diseased patient (Figure 2). Ectopic transplantation involves the seeding of healthy cells or tissues to a favorable ectopic location. The cells then communicate with the surrounding environment in need of function to promote long-term survival in the ectopic niche. As a result, ectopic organs provide compensatory function for the damaged or diseased native organ.
Figure 2.
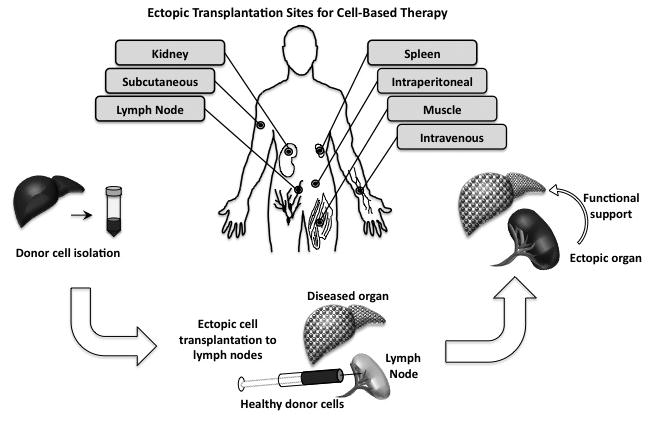
Ectopic transplantation sites. Several ectopic sites for cell therapy are shown. The generation of an ectopic organ involves transplanting healthy cells into an ectopic site such as the lymph node to provide support to a diseased or damaged organ.
Common ectopic cell transplantation sites – analyzing the pros and cons
One of the most common sites of ectopic transplantation in rodents is under the kidney capsule. This site is routinely used to transplant pancreatic islets to show engraftment and function[14]. Pros: The subcapsular space of the kidney is advantageous because it provides a relatively large “pocket” to contain transplanted cells that can easily be examined at later times to verify engraftment. Moreover, the kidney provides access to a blood supply and supports angiogenesis of transplanted tissue (although not as efficiently as other sites), which is necessary to sustain any long-term engraftment[15*,16*]. Cons: While ectopic transplantation under the kidney capsule is suitable in rodents, it is not a feasible approach clinically because the human kidney is anatomically different to the extent that the capsule cannot be easily separated from the parenchyma to safely permit cell transplantation[14]. In addition, it is unclear if extensive organogenesis (e.g. ectopic liver) adjacent to the kidney would disrupt normal kidney function.
The subcutaneous space is another site of transplantation often used in rodents. Pros: Similar to the kidney subcapsular space in mice, there is a large area to accommodate transplantation of high cell numbers. It is also beneficial because it is simple to locate the transplanted cells months after transplantation has occurred. It is easy to examine tissue growth over time in subcutaneous sites, because this location can be observed by eye without the need for special imaging technology. Another advantage of subcutaneous transplantation is the ease of the injection procedure. The subcutaneous space and direct access make it a common choice for transplantation of extracellular matrix enriched devices seeded with cells[17-20]. Cons: A drawback of the subcutaneous space is the general lack of access to the vasculature[21]. Angiogenesis typically does not occur fast enough in this site to sustain cell survival and engraftment of normal tissues. Furthermore, some tissues such as the liver require a significant amount of tissue mass, and it is unlikely that a person would want a sizeable growth protruding from their skin for aesthetic reasons or if it interfered with routine tasks.
The intraperitoneal (IP) space, which includes the peritoneum and mesentery, has been used as a site for cell transplantation of many different tissue types. Pros: The IP space is unique in that it is large enough to support the transplantation of a high number of cells, and it is anatomically similar between rodents and humans. It was shown that hepatocytes engraft in the lymphatic system after IP transplantation, and that angiogenesis occurs during organogenesis of the ectopic tissue[22]. A recent report also showed that human iPS-derived liver buds grew well-vascularized hepatic tissue on the mesentery of immunodeficient mice[23**]. Hepatocytes may be capable of migrating to favorable locations in the IP space, while other cell types such as pancreatic islets may not, since islets do not engraft well after IP transplantation[14]. Cons: There are a number of concerns about cell transplantation to the IP space that limit its potential in a clinical setting. There is little control over where the transplanted cells end up, which makes it difficult to track engraftment over time. An encapsulated device helps overcome this issue, but this often results in poor vascularization[2**]. There is also a concern regarding the potential impact on other organ function, since it is difficult to control the location of engraftment. Finally, for islet transplantation, many more islets need to be transplanted to achieve a functional benefit compared to other sites, which makes the problem of donor material even more significant[14].
One of the more common sites of hepatocyte transplantation is the spleen. Pros: The spleen provides the necessary space for the transplantation of many cell types. The spleen is anatomically similar in rodents and humans, and it can be accessed without a major incision. In mice, the spleen acts as a conduit to the liver, so splenic transplantation of hepatocytes is essentially transplantation of cells to the liver. This transplantation site also works well in rodents because the transplanted hepatocytes can replace the diseased hepatocytes in the liver without the need for angiogenesis. On the other hand, when large cell clusters (e.g. pancreatic islets) are transplanted, the majority remains in the spleen[24]. Cons: It is not clear if sufficient vasculature is available in the spleen to permit long-term engraftment and it is difficult to know how cell engraftment in the spleen might impact normal splenic function[25]. Furthermore, the clinical benefit of using the spleen as a site for hepatocyte transplantation has not been encouraging so far, with little evidence of long-term engraftment in humans[26,27].
Intravenous transplantation is common for HSCs and pancreatic islets. Pros: For HSC transplantation, intravenous access is a relatively simple procedure in both rodents and humans and long-term engraftment has been demonstrated[28*]. Due to the nature of the transplant, the cells are in close proximity to the vasculature. Cons: For hepatocytes and islets, a more specific venous route is used to direct the cells to a particular organ. For example, islets are transplanted to the portal vein, which is a difficult procedure to perform[14]. The number of cells also has to be carefully controlled because delivery of too many cells may create a blockage and lead to major safety issues[2**]. In general, intravenous transplantation provides little control over where the transplanted cells engraft, so it is difficult to determine their location after transplantation. While HSC transplantation has been successful, other cell and tissue types transplanted intravenously have not shown long-term clinical benefit.
The muscle is another site that has been targeted for MSCs[29], hepatocytes[30], and islet cells[31*] to generate ectopic bone, liver, and pancreas, respectively. Pros: intramuscular injection is a mostly noninvasive procedure that has been used successfully in rodents. Engrafted cells remain localized to the injection site, and there appears to be sufficient vasculature available for islet and hepatocyte transplant[2**,30]. Cons: in general, transplantation to the muscle results in poor long-term engraftment. Available space may be an issue, especially if a large tissue mass needs to be generated. Because muscle function is necessary for normal physical activity, muscle movement may have a negative affect on the ectopic tissue.
A new site for ectopic organogenesis – the lymph node
The lymph node provides a home to circulating T cells and B cells, and can quickly expand to mount an immune response[32,33]. In addition, the lymph node is a common metastatic site for many types of cancer[34]. Cancer metastasis could be considered an abnormal form of ectopic organogenesis, and it is worth considering why the lymph node is a preferred location for cancer cells. So what exactly makes the lymph node accommodating to both immune cells and tumor cells, and could this knowledge be applied to cell transplantation?
Lymph nodes are well vascularized, which allows the transport and rapid expansion of immune cells[35]. Direct access to a blood supply also permits tumor related angiogenesis. Reticular fibroblasts form a supporting niche (reticular network) in the lymph node, and contribute to T cell entry and egress[36]. This network may also support engraftment of both normal and cancer cells in the lymph node. In terms of a cell therapy, there are many lymph nodes in both mice and humans (>500), so there are a number of options to choose as a location for cell transplantation. Normal lymph node function is not compromised after cell transplantation, and multiple tissue types (e.g. hepatocytes, islets, thymus) have been shown to engraft in mouse lymph nodes long-term[37*]. Large animal studies have also shown promise for hepatocyte transplantation in the lymph node (Komori J, Lagasse E, unpublished data). Furthermore, lymph nodes are enclosed, so cell engraftment is isolated and can be observed over time. While the lymph node is not a common location for transplantation, it is not shocking to consider it as an alternative site for cell transplantation[38*].
There are, however, a few limitations to consider with lymph node transplantation. Lymph nodes are small, so there is a limit to the number of cells that can be transplanted to each one. However, more than one lymph node can be targeted at a time to overcome this limitation. Lymph node transplantation is not an easy procedure in rodents, and could be technically difficult in humans as well. Finally, there have not been any studies in humans, so future studies will determine if lymph node transplantation will translate well in the clinic.
Conclusion
The recent discovery that mature cells can be easily reprogrammed to a pluripotent state has reignited the stem cell field and renewed important discussions about the immense potential of stem cells to treat a number of diseases using cell-based therapeutic approaches. However, a dialogue that includes the practical methods and not just the potential outcomes for cell therapy is often lacking. A tremendous amount of effort is put into the techniques to generate stem cells and various functional differentiated cell types. However, once these methods have been shown to be efficient, safe, and reproducible, additional major hurdles stand in the way of a successful therapy that could restore tissue function.
For cell-based therapy to be effective, a hospitable site for transplantation must be determined, even if issues with cell source, safety, and immune surveillance have all been resolved. The transplantation site needs to be relatively accessible, provide sufficient space, offer access to vasculature, and support long-term engraftment. We suggest that the lymph node meets these criteria better than other commonly used transplantation sites. We envision the lymph node acting as an in vivo bioreactor to support ectopic tissue function. Eventually, studies in humans will show just how effective transplantation into the lymph nodes could be, but initial studies in rodents have been promising for hepatocyte and islet transplantation[37*]. Our hope is that future studies will place more emphasis on the site of transplantation, because this is an essential issue to address before any cell therapy can effectively move from the laboratory to the clinic.
Key points.
Successful cell-based therapy requires the consideration of the four S's: source, surveillance, safety, and site.
An accommodating site for cell transplantation should be relatively accessible, be rich in vasculature, have sufficient space, and support cell function.
The lymph node is a new candidate target for cell therapy to restore, maintain, or improve tissue and organ functions.
Acknowledgments
The authors acknowledge financial support from the NIH and the Commonwealth of Pennsylvania.
Abbreviations
- HSC
Hematopoietic stem cell
- MSC
mesenchymal stem cell
- ES
embryonic stem cell
- iPS
induced pluripotent stem cell
- IP
intraperitoneal
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References and recommended reading
- 1*.Palakkan AA, Hay DC, Anil Kumar PR, et al. Liver tissue engineering and cell sources: issues and challenges. Liver Int. 2012;33(5):666–676. doi: 10.1111/liv.12134. This review highlights the urgent need for alternative cell sources for hepatocyte transplantation. The advantages and disadvantages are discussed for many sources. [DOI] [PubMed] [Google Scholar]
- 2**.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2(7):a007823. doi: 10.1101/cshperspect.a007823. This review gives a current perspective on clinical islet transplantation as a cure for type 1 diabetes. A brief history of islet transplantation is given and the many advances since the first transplants are discussed. Ectopic transplant sites are also mentioned, with benefits and drawbacks outlined for each site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14(6):357–368. doi: 10.1038/nrm3584. This review provides a comprehensive overview of ES and iPS properties and offers insight for their potential in regenerative medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2012;494(7435):100–104. doi: 10.1038/nature11807. This article shows that iPS cells have limited immunogenicity, which is important if they are to be used as a means to avoid immune rejection for cell therapy. If iPS cells are immunogenic as some labs have suggested, then it greatly limits their potential for clinical use, but this article shows that it is not a significant concern. [DOI] [PubMed] [Google Scholar]
- 5**.Guha P, Morgan JW, Mostoslavsky G, et al. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–412. doi: 10.1016/j.stem.2013.01.006. This article provides additional support that iPS cells are not immunogenic. Both differentiated and undifferentiated cells were examined for their immunogenicity after transplantation. These data support the idea that iPS cells could be used for cell therapy to overcome the issue of immune rejection. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013;12(4):385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 7*.Cherry AB, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med. 2013;64:277–290. doi: 10.1146/annurev-med-050311-163324. This review gives an overview of ES and iPS cells as a potential cell source in regenerative medicine. Rationale and relative merits are given for their potential use in the clinic, with a focus on HSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez CA, de Resende HC, Jr, Rodrigues MR, et al. Gallbladder-associated ectopic liver: A rare finding during a laparoscopic cholecystectomy. Int J Surg Case Rep. 2013;4(3):312–315. doi: 10.1016/j.ijscr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannon K, Hraemic J, Mittal V. Ectopic liver tissue sequestered in the lung. Am Surg. 2013;79(3):E104–105. [PubMed] [Google Scholar]
- 10.Xu X, Weidner N. Papillary renal carcinoma arising in an ectopic native kidney and status after renal transplant: a report of a unique case and review of the literature. Case Rep Pathol. 2012;831403 doi: 10.1155/2012/831403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukino N, Oida T, Mimatsu K, et al. Diffuse Peritonitis due to Perforated Gastric Ectopic Pancreas. Case Rep Gastroenterol. 2012;6(3):689–694. doi: 10.1159/000345382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YT, Lin H, Guo JC, et al. Laparoscopy-assisted billroth I gastrectomy for ectopic pancreas in the prepyloric region. Case Rep Gastroenterol. 2012;6(3):712–719. doi: 10.1159/000345388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.He B, Mitchell A. Laparoscopic donor nephrectomy for ectopic kidney. Transplant Proc. 2012;44(10):3051–3054. doi: 10.1016/j.transproceed.2012.05.078. This case report shows that ectopic organs are fully functional and can be used as donor organs. This suggests that function from an ectopic organ is suitable to sustain necessary organ function overall. [DOI] [PubMed] [Google Scholar]
- 14.Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95(12):1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 15*.Bartholomeus K, Jacobs-Tulleneers-Thevissen D, Shouyue S, et al. Omentum Is Better Site Than Kidney Capsule for Growth, Differentiation, and Vascularization of Immature Porcine beta-Cell Implants in Immunodeficient Rats. Transplantation. 2013;00:00–00. doi: 10.1097/TP.0b013e3182a6ee41. This article shows that islet transplants under the rodent kidney capsule become revascularized, although to a less extent than omental transplants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Wang X, Cui J, Zhang BQ, et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J Biomed Mater Res A. 2013;00:00–00. doi: 10.1002/jbm.a.34764. This article shows that biomaterial scaffolds seeded with cells become vascularized after rodent kidney subcapsular transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyckmans J, Roberts SJ, Bolander J, et al. Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials. 2013;34(19):4612–4621. doi: 10.1016/j.biomaterials.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner-Ecker M, Voltz P, Egermann M, Richter W. The collagen component of biological bone graft substitutes promotes ectopic bone formation by human mesenchymal stem cells. Acta Biomater. 2013;9(7):7298–7307. doi: 10.1016/j.actbio.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Wittig C, Laschke MW, Scheuer C, Menger MD. Incorporation of bone marrow cells in pancreatic pseudoislets improves posttransplant vascularization and endocrine function. PLoS One. 2013;8(7):e69975. doi: 10.1371/journal.pone.0069975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Teoh SH, Chong MS, et al. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng Part A. 2013;19(7-8):893–904. doi: 10.1089/ten.TEA.2012.0187. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama T, Ohashi K, Kuge H, et al. In vivo engineering of metabolically active hepatic tissues in a neovascularized subcutaneous cavity. Am J Transplant. 2006;6(1):50–59. doi: 10.1111/j.1600-6143.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoppo T, Komori J, Manohar R, et al. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2010;140(2):656–666. e652. doi: 10.1053/j.gastro.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. doi: 10.1038/nature12271. This article shows that hepatocytes can be derived from iPS cells, and subsequently turned into functional mini organs for transplantation. After IP transplantation, the mini organs become vascularized within days and generate normal liver function from the ectopic site. [DOI] [PubMed] [Google Scholar]
- 24.Wohlrab F, Wilke B, Schmidt S, Cossel L. Syngeneic transplantation of rat pancreatic islets into the spleen. Light and electron microscopical findings. Int J Pancreatol. 1988;3(1):59–72. doi: 10.1007/BF02788224. [DOI] [PubMed] [Google Scholar]
- 25.Nagata H, Ito M, Shirota C, et al. Route of hepatocyte delivery affects hepatocyte engraftment in the spleen. Transplantation. 2003;76(4):732–734. doi: 10.1097/01.TP.0000081560.16039.67. [DOI] [PubMed] [Google Scholar]
- 26.Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338(20):1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. J Hepatobiliary Pancreat Surg. 2009;16(2):97–100. doi: 10.1007/s00534-008-0023-0. [DOI] [PubMed] [Google Scholar]
- 28*.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi: 10.1182/blood-2013-02-453175. This review discusses the successful clinical approach of HSC transplantation, focusing on umbilical cord blood as a source for donor HSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Zhi W, Lu X, et al. Comparative studies on ectopic bone formation in porous hydroxyapatite scaffolds with complementary pore structures. Acta Biomater. 2013;9(9):8413–8421. doi: 10.1016/j.actbio.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Alvarez N, Soto-Gutierrez A, Chen Y, et al. Intramuscular transplantation of engineered hepatic tissue constructs corrects acute and chronic liver failure in mice. J Hepatol. 2009;52(2):211–219. doi: 10.1016/j.jhep.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 31*.Capito C, Simon MT, Aiello V, et al. Mouse muscle as an ectopic permissive site for human pancreatic development. Diabetes. 2013;62(10):3479–3487. doi: 10.2337/db13-0554. This article shows that islets engraft after transplantation to the thigh muscle, and the localized engraftment can be examined in detail over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286(5447):2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 33.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 34.Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer. 2009;125(12):2747–2756. doi: 10.1002/ijc.24702. [DOI] [PubMed] [Google Scholar]
- 35.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 36.Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 37*.Komori J, Boone L, DeWard A, et al. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol. 2012;30(10):976–983. doi: 10.1038/nbt.2379. This article identifies the lymph node as a new site for ectopic cell transplantation. Liver, pancreas, and thymus were transplanted into lymph nodes and each tissue type was shown to engraft and restore organ function from the ecopic site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Weir GC. Cellular transplantation into lymph nodes may not be such a crazy idea. Cell Stem Cell. 2012;11(5):587–588. doi: 10.1016/j.stem.2012.10.004. This brief review highlights some of the underappreciated advantages of using the lymph node as a site for cell therapy, and how it could translate well into the clinic. [DOI] [PubMed] [Google Scholar]


