Abstract
BACKGROUND AND PURPOSE
Previous studies have reported the occurrence of increased mortality rates among individuals with mild cognitive impairment (MCI), but possible links between MCI subtypes and cause-specific mortality need to be explored. To examine short-term mortality (5-years), long-term mortality (13-years) and cause-specific mortality of individuals over 65 years of age) suffering from mild cognitive impairment (MCI) compared to cognitively unimpaired individuals in the NEDICES (Neurological Disorders in Central Spain) cohort.
METHODS
MCI was classified using standardized psychometric and functional assessment in accordance with diagnostic convention. Cox's proportional hazards models, adjusted by sociodemographic and comorbidity, were used to assess the risk of death at 5 and 13 years of MCI subtypes compared with a reference group of elders without cognitive impairment (N = 2,329). Causes of death were obtained from the National Population Register of Spain.
RESULTS
There were 1,484 deceased individuals at 13 years. MCI subtypes were defined as amnestic-single domain (N = 259), amnestic-multiple domain (N = 197), and non-amnestic (N = 641). After adjusting for covariates, only amnestic-multiple domain MCI showed an increased hazard ratio (HR) for mortality at 5 years versus reference group. However, the HR for mortality at 13-years was increased for all MCI subtypes. The HR by MCI subtype was 1.19 in non-amnestic (95% CI: 1.05 to 1.36), 1.31 in amnestic-single domain (95% CI: 1.10 to 1.56) and 1.67 in amnestic multiple domain (95% CI: 1.38 to 2.02). In terms of cause specific mortality, the chance of death from dementia was statistically higher in all MCI subtypes.
CONCLUSION
Amnestic-multiple domain MCI showed the greatest risk of mortality in comparison with other MCI subtypes at different intervals. Dementia was the only cause-specific mortality that was increased in MCI individuals.
Keywords: Cause-specific mortality, mild cognitive impairment, memory, mortality, population-based study
INTRODUCTION
Mild cognitive impairment (MCI) is a specific intermediate state, commonly used to describe cognitive problems, sometimes considered to be a transition state between normal aging and mild dementia [1]. MCI research has become highly relevant during the last decade, especially as people with MCI have a higher risk of developing dementia than age matched population controls [2]. One of the original definitions of MCI was proposed by Petersen et al [3], and this was subsequently refined [4,5]. All definitions require objective impairment on neuropsychological tasks as a core criterion, but there is no consensus on how the presence of cognitive impairment should be operationalized or the degree of cognitive impairment that is sufficient [6]. The heterogeneity in case definition has led to divergent results in terms of prevalence and outcomes, such as progression to dementia and risk of death [2,7,8].
It is well-known that people with dementia have a less favorable survival rate than normal elders [9]. However, longitudinal studies which have compared the natural history of MCI with similar persons without cognitive impairment are less common [8]. Furthermore, rates of mortality might change due to methodological discrepancies between studies, such as variations in MCI definition, years of follow-up and types of covariates examined [10,11]. In this context, there is no information on long-term (≥10 years) mortality in MCI subtypes. Another important issue is whether there is any link between MCI subtypes and cause-specific mortality. Several medical conditions usually co-occur with MCI and may influence future negative outcomes [12].
Our aim was to determine whether population dwelling individuals with different MCI subtypes (amnestic-single domain, amnestic-multiple domain, non-amnestic) show a higher risk of death at 5-years and 13-years when compared with cognitively unimpaired older people living in the same population. We also tried to address the hypothesis that health-related factors might partly explain any increased mortality rates in MCI individuals. Finally, cause-specific mortality was examined at short and long term intervals in this cohort.
METHODS
Study population
This investigation was part of the Neurologic Disorders in Central Spain (NEDICES), a population-based survey of the prevalence, incidence, and determinants of major age-associated conditions of the elderly (age 65 years and older) [13,14]. Two waves were collected in 1994–1995 (basal cohort; 1st May 1994 was recorded as prevalence day) and 1997–1998, whilst May 1st 2007 was established as follow-up date for registration of deceased individual.
Standard protocol approvals, registrations, and patient consents
Investigators obtained ethics approval from the Human Research Ethics Committee of the University Hospitals “12 de Octubre” (Madrid) and “La Princesa” (Madrid). All enrollees signed written informed consent.
Baseline evaluation
We have reported elsewhere a detailed account of the baseline evaluation [13,14]. Each participant received either a face-to-face evaluation or a questionnaire (mailed to participants who were unavailable for face-to-face interviews). During the face-to-face interview, we collected data on demographics, current medications, medical conditions, and life style questions.
The screening instruments for dementia included a Spanish adaptation of a cognitive test (a 37-item version of the Mini-Mental State Examination [MMSE]) and the Pfeffer FAQ scale for instrumental activities of daily living [15,16]. This screening protocol for dementia was designed and validated in the World Health Organization Aging Study [17–20].
Neurological Examination
Every person had an initial screening for cognitive impairment and when tested positive, underwent a neurological examination at National Health Service clinics or at home. The initial screen was considered positive if: (1) the individual scored < 24 points on the 37-item version of the MMSE and > 5 points on the Pfeffer FAQ scale; or (2) the individual could not provide an answer on the 37-item version of the MMSE or on the Pfeffer FAQ scale (direct screening) or (3) the individual or proxy gave information suspicion of cognitive decline.
For the diagnosis of dementia, we applied the Diagnostic and Statistical Manual of Mental Disorders (DSM)–IV criteria [17,18]. The neurological examination comprised a clinical history concerning cognitive decline, a general neurological examination and a mental status interview.
Follow-up data on mortality was collected up until May 1st 2007. The cause and date of death were obtained from the National Population Register of Spain. In Spain, all deceased individuals receive a death certificate completed by a doctor, at the time of death. The certificate is then sent to the local police authority in the municipality where the person had been living, and the information is collected in the National Register. The cause of death (using the International Classification of Diseases –ICD-, 9th Revision, http://www.cdc.gov/nchs/icd/icd9.htm) was classified into 6 main categories: dementia, cerebrovascular disorders, cardiovascular disorders (pulmonary embolism, congestive heart failure, myocardial infarction, heart or aortic rupture, and asystole), respiratory diseases, cancer, and other causes (infections, trauma, genitourinary or gastrointestinal disorders).
Definition of MCI
MCI was diagnosed based on the general MCI published criteria [4]. Accordingly, the person with MCI showed evidence of cognitive impairment, with preserved or ‘minimally impaired‘ activities of daily living (defined as Pfeffer FAQ scale scores ≤ 5), but did not meet conventional diagnostic criteria for dementia [17,18]. Deficits in a cognitive domain (scores 1.5 SD below the mean for the general population) were required [21]. However in this analysis, subjective memory complaints were not included as criteria for MCI classification [22].
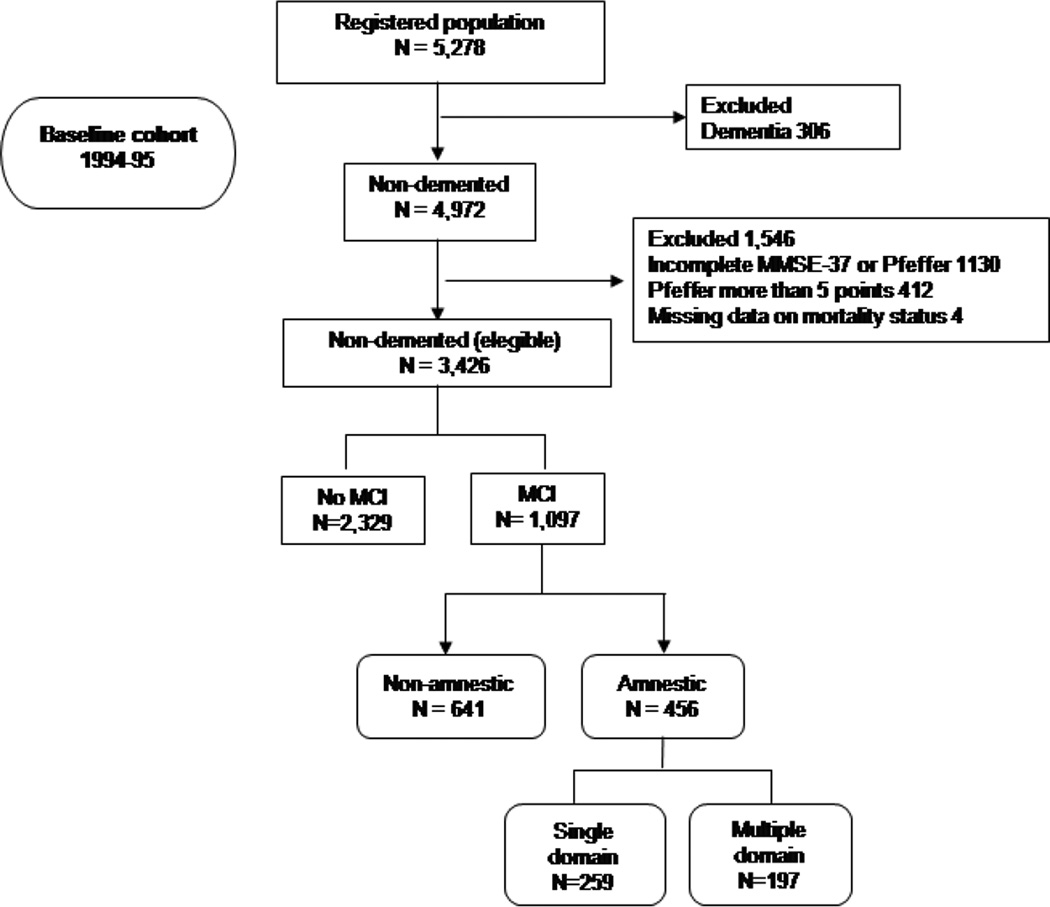
The presence of cognitive impairment was evaluated using different domains of the MMSE-37 test. Composite scores [spatial-temporal orientation, attention-concentration (serial subtraction 7 from 100 and digits backwards), memory (words recall), language (naming, repeating, comprehension and writing), and visuo-constructive abilities (visual reproduction of figures)] were calculated summing items’ performances of the individuals and then standardized in z-scores [15,23]. Cut-off scores were calculated from the baseline cohort without dementia, where cases with missing data or individuals under dementia or suspicion of dementia based were excluded (see figure 1). As literacy has a profound effect on performance of MMSE [24], specific cut-off points by domain were calculated for illiterates subjects. Amnestic vs. non-amnestic single and multiple domain MCI, as proposed by Petersen et al. [25], was used to classify the MCI subtypes according to the presence or absence of memory impairment and the number of cognitive domains affected.
Figure 1.
Flow-chart of the study
Exclusions and final sample for analyses
Figure 1 shows a flow chart of this study. Beginning in January 1994, letters explaining the survey and inviting participation were mailed to 6,395 subjects. Of these, 5,914 subjects were deemed eligible for screening and 5,278 subjects (89.2%) were screened. The remaining 636 subjects refused (292, 45.9%), could not be located due to an address change (292, 45.9%) or had died (52, 8.2%). Of the 5,278 participants screened at the baseline evaluation (1994–1995), we excluded 306 participants who had dementia (n = 306). This left a sample of 4,972 participants without dementia. In addition, we excluded 1,130 participants who (i) had not completed either the 37-item version of the MMSE or the Pfeffer FAQ scale, (ii) 412 who had Pfeffer FAQ scale score more than five points, (iii) 4 who had missing data on mortality status. This left an eligible sample of 3,426 for these analyses (Figure 1).
We compared the final eligible sample of 3,426 participants to 1,852 excluded participants. Those participants who entered in the study were younger (72.8 ± 5.9 [median = 72] vs. 77.0 ± 7.8 [median = 77] years, t-Student test, p < 0.001) and a higher proportion were men (1,529 [44.6%] vs. 709 [38.3%]; chi-square [X2] = 19.6, p < 0.001).
Data Analyses
Statistical analyses were performed in SPSS Version 20.0 (SPSS, Inc., Chicago, IL). All p-values are two-tailed, and were considered p < 0.05 as significant. Baseline characteristics of the subtypes with and without MCI were compared using ANOVA tests for numerical variables and Chi-square tests for categorical variables. We used Cox’s proportional-hazards models to estimate the relative risk of mortality associated with MCI subtypes at 5 years and 13 years follow-up. This generated hazard ratios (HR) with 95% confidence intervals (CI). All participants alive on or after May 1st, 1999 (5-year interval) for the short-term interval, and death or 1 May 2007 (13-year interval) for the long-term were censored in the respective analyses. The time variable was person-years of observation, defined as the interval between screening date at baseline evaluation (1994–95) and death or May 1st, 1999 (5-years interval) and death or May 1st, 2007 (13-years interval). Several potential confounding variables (baseline age, gender, education and comorbidity) were included. The index of comorbidity included the following diseases: hypertension, diabetes mellitus, hyperlipidemia, heart disease, cancer, anemia, chronic obstructive pulmonary disease, psychiatric disorders, osteoarthritis, osteoporosis, hearing loss, cataracts, and peripheral vascular disease. Comorbidities were assessed at the baseline study, at the same time of completion of the questionnaire.
We used the Kaplan-Meier method to calculate mean survival times. Logrank tests were performed in order to evaluate the significance of the difference between survival curves for different MCI subtypes (amnestic-single domain, amnestic-multiple domain and non-amnestic) and cognitively unimpaired subjects. For analysis of death causes, comparison of proportions was performed by chi-square analysis. In order to examine the association between MCI subtypes and the dichotomous outcome of multiple causes of death, logistic regression (odds ratios [OR] and 95% confidence intervals) was used adjusted by sociodemographic characteristics.
RESULTS
Of the 3,426 participants, 2,329 were cognitively unimpaired and 1,097 had MCI according to our definition. Of these 1,097, 259 were categorized as amnestic-single domain, 197 as amnestic-multiple domain, and 641 as non-amnestic MCI. Four hundred twenty-seven (12.5%) of 3,426 participants died over a median follow-up of 5 years (range 0.04–5.0 years), including 261 (11.2%) deaths among 2,329 cognitively unimpaired participants, 41 (15.8%) among 259 participants with amnestic-single domain, 41 (20.8%) deaths among 197 participants with amnestic-multiple domain MCI, and 84 (13.1%) among 641 participants with non-amnestic MCI. Furthermore, 1,484 (43.3%) of 3,426 participants died over a median follow-up of 12.8 years (range 0.05–13.48 years), including 903 (38.8%) among 2,329 cognitively unimpaired participants, 150 (57.9%) among 259 participants with amnestic-single domain, 128 (65.0%) deaths among 197 participants with amnestic-multiple domain MCI, and 303 (47.3%) among 641 participants with non-amnestic MCI.
Baseline demographic characteristics are shown in Table 1. Tukey’s post hoc test (p < 0.012) indicated that the cognitively unimpaired group was significantly younger than MCI groups. Similarly, the non-amnestic MCI subtype showed a higher comorbidity index compared with normal elders, but other subtypes did not significantly differ in this variable. Finally, distributions of education (cognitively unimpaired group was more educated than MCI groups) and gender (percentage of women was higher in non-amnestic and amnestic multiple domain MCI) were also statistically different between groups.
Table 1.
Baseline sociodemographic variables
| Cognitively unimpaired (N = 2,329) |
Non- amnestic MCI (N = 641) |
Amnestic- single domain MCI (N = 259) |
Amnestic- multiple domain MCI (N = 197) |
P value | |
|---|---|---|---|---|---|
| Age (mean and SD in years) | 72.0 (5.5) | 73.8 (6.1) | 74.4 (6.0) | 76.4 (6.4) | <0.001 |
| Gender (% women) | 53.5% | 66.8% | 37.8% | 62.9% | <0.001 |
| Education | <0.001 | ||||
| Little or no formal education (≤ 5 years) | 49.5% | 61.6% | 52.1% | 61.4% | |
| Primary studies (6–11 years) | 32.1% | 30.3% | 42.9% | 31.0% | |
| ≥ Secondary studies (≥12 years) | 18.5% | 8.1% | 5.0% | 7.6% | |
| Mean of diseases (SD) (comorbidity)* | 2.9 (1.7) | 3.2 (1.7) | 2.9 (1.9) | 2.9 (1.7) | <0.001 |
Comorbidity included 13 diseases: hypertension, diabetes mellitus, hyperlipidemia, heart disease, cancer, anemia, chronic obstructive pulmonary disease, psychiatric disorders, osteoarthritis, osteoporosis, hearing loss, cataracts, peripheral vascular disease..
Risk of mortality at 5-years
Cox’s proportional-hazards model at 5-years showed that only the amnestic-multiple domain-MCI had an increased risk of mortality (HR = 1.51; CI 95% = 1.07–2.12; p = .01) after controlling for age, gender and education. When comorbidity was included in the Cox’s regression, the amnestic-multiple domain- MCI group remained significant. Table 2 shows different HRs for death at 5 years adjusted by all covariates.
Table 2.
Hazard ratios (HR) for death with multiple adjustments for all variables shown in Cox proportional Hazards Models at 5 and 13 years.
| Variables | Hazard Ratio (95% CI) at 5 years |
P value | Hazard Ratio (95% CI) at 13 years |
P value |
|---|---|---|---|---|
| MCI subtypes | ||||
| Cognitively unimpaired‡ | 1 | 0.34 | 1 | <.01 |
| Non-amnestic | 1.12 (0.78–1.44) | 0.71 | 1.19 (1.05–1.36) | <.01 |
| Amnestic-single domain | 1.06 (0.76–1.48) | <.01 | 1.31 (1.10–1.56) | <0.001 |
| Amnestic-multiple domain | 1.59 (1.13–2.24) | 1.67 (1.38–2.02) | ||
| Age | 1.07 (1.05–1.09) | <0.001 | 1.09 (1.08–1.10) | <0.001 |
| Gender | ||||
| Women‡ | 1 | <0.001 | 1 | <0.001 |
| Men | 2.70 (2.20–3.32) | 2.20 (1.97–2.45) | ||
| Education | ||||
| ≥ Primary studies‡ | 1 | <.05 | 1 | 0.72 |
| Little or no formal education (≤ 5 years) | 0.82 (0.68–0.99) | 0.98 (0.88–1.08) | ||
| Comorbidity Index * | 1.17 (1.11–1.23) | <0.01 | 1.13 (1.10–1.16) | <0.001 |
A hazard ratio of less than 1.00 represents a decreased likelihood of death, whereas a hazard ratio greater than 1.00 represents an increased likelihood of death.
This group served as the reference category.
Hazard ratio for each disease included (additive effect for each disease).
CI = Interval of Confidence. MCI = Mild Cognitive Impairment.
Risk of mortality at 13-years
In all Cox’s proportional-hazards models at 13-years, every MCI subtype showed an increased risk of mortality after controlling for age, gender and education. The HRs were 1.23 (CI 95% = 1.08–1.40; p < 0.01) for the non-amnestic MCI subtype; 1.32 (CI 95% = 1.11–1.57; p < 0.01) for the amnestic-single domain MCI subtype; and 1.62 (CI 95% = 1.34–1.96; p <0.001) for the amnestic-multiple domain MCI subtype. When comorbidity was entered in the model, all MCI subtypes remained significant (Table 2).
The Kaplan–Meier curves for overall survival at 5 and 13 years of follow up (supplementary figure 2 and supplementary figure 3) indicate the MCI subtypes to be at increased risk of death at 5 (log-rank P = 0.001) and 13 years of follow up (log-rank p < 0.001).
Causes of death at 5 and 13 years
Table 3 and 4 show the causes of death at 5 and 13-years for each MCI subtype and the cognitively unimpaired elders. Chi-square test showed that the distribution of cause specific mortality was similar at 5 years (χ2= 8.96, df =15, p = 0.87), but statistical significant differences emerged between groups at 13-years (χ2= 39.02, df =15, p = 0.001) (Table 3 and 4). In particular, MCI significantly influenced the likelihood of death from dementia compared with other death causes (Wald = 33.46, df = 3, p < 0.001). Amnestic-multiple domain MCI (OR = 6.57; CI 95% = 3.45–12.51, p <.01) > amnestic-single domain MCI (OR = 2.73; CI 95% = 1.29–5.77) > non-amnestic MCI (OR = 2.14; CI 95% = 1.15–3.96). However, the risk of death by other causes was not different between the cognitively unimpaired elders and MCI subtypes (data not shown).
Table 3.
Primary cause of death (The International Classification of Diseases, 9th) by diagnostic groups at 5 years
| Cognitively unimpaired N (%) |
Non-amnestic MCI N (%) |
Amnestic-single domain MCI N (%) |
Amnestic-multiple domain MCI N (%) |
|
|---|---|---|---|---|
| Dementia | 2 (0.8%) | 1 (1.2%) | 0 (0.0%) | 1 (2.4%) |
| Cerebrovascular disorders | 18 (7.0%) | 5 (6.0%) | 3 (7.3%) | 1 (2.4%) |
| Cardiovascular diseases | 65 (25.3%) | 20 (23.8%) | 11 (26.8%) | 10 (24.4%) |
| Respiratory diseases | 32 (12.5%) | 12 (14.3%) | 6 (14.6%) | 10 (24.4%) |
| Cancer | 98 (38.1%) | 31 (36.9%) | 14 (34.1%) | 10 (24.4%) |
| Other causes | 42 (16.3%) | 15 (17.9%) | 7 (17.1%) | 9 (22.0%) |
| Total | 257 (100%)* | 84 (100%) | 41 (100%) | 41 (100%) |
Cause-specific mortality was missed in 4 cognitively unimpaired subjects
Table 4.
Primary cause of death (The International Classification of Diseases, 9th) by diagnostic groups at 13 years
| Cognitively normal N (%) |
Non-amnestic MCI N (%) |
Amnestic-single domain MCI N (%) |
Amnestic-multiple domain MCI N (%) |
|
|---|---|---|---|---|
| Dementia | 29 (3.2%)** | 20 (6.6%)** | 10 (6.7%)* | 18 (14.1%)*** |
| Cerebrovascular disorders | 67 (7.4%) | 22 (7.3%) | 9 (6.0%) | 10 (7.8%) |
| Cardiovascular diseases | 247 (27.4%) | 77 (25.4%) | 44 (29.3%) | 25 (19.5%) |
| Respiratory diseases | 134 (14.8%) | 46 (15.2%) | 19 (12.7%) | 25 (19.5%) |
| Cancer | 265 (29.3%) | 80 (26.4%) | 47 (31.3%) | 27 (21.1%) |
| Other causes | 161 (17.8%) | 58 (19.1%) | 21 (14.0%) | 23 (18.0%) |
| Total | 903 (100%) | 303 (100%) | 150 (100%) | 128 (100%) |
p<.001;
p < .01;
p<.05
DISCUSSION
The current study analysed the mortality and causes of death associated with MCI subtypes from the baseline NEDICES cohort. Essentially, we compared the HRs at 5 and 13 years follow-up for different forms of MCI (amnestic-single domain, amnestic-multiple domain and non-amnestic). The cumulative survival analyses showed that subjects with MCI had a less favorable rate of survival at 5 and 13 years. After adjusting for covariates (age, sex education and health-factors), Cox’s regression indicated that when compared cognitively unimpaired individuals, all patients with MCI subtypes from NEDICES cohort had higher significant risk of mortality in the long-term. However, only the amnestic-multiple domain MCI was significant at 5-years. Consequently, it seems that the effect of MCI on mortality is strengthened over time, when both (short and long) intervals are compared.
These results support the association between MCI and increased risk of death, but the effect was strongest for multiple-domain MCI [26]. Other researchers have found that both the presence of cognitive impairment and the severity are associated with risk of dementia and mortality [11,27]. Hunderfund et al. [28] found an increased rate of mortality for both amnestic MCI subtypes (multiple domain than higher mortality than single domain) at 5.7 years follow-up according to pre-specified criteria of Petersen et al. [3], consistent with our results. Similarly, Villarejo et al. [29] found an association between recall of words (raw scores) and risk of mortality in elderly people without dementia, but the presence of impairment in non-amnestic domains was not excluded. It may be important to note the significant effects of age and gender on mortality [26], whilst the effect of education on mortality has yielded inconsistent results depending on the influence of other covariates such as cognition [30].
Essentially, it appears that there is a parallelism between progression to dementia and mortality outcomes in people with MCI, the risk of non-amnestic being inferior to amnestic single domain, and amnestic multiple domain MCI at the highest risk. Thus, the risk of dementia is generally lower in single domain type of MCI compared with multiple domain forms [28,31]. Furthermore, amnesic types of MCI are linked to increased progression to dementia more than non-amnesic types [32].
Mortality associated with dementia was the only significant cause of death in MCI individuals (vs. cognitively normal elders) at 13 years follow-up. Indeed the increased risk of death in MCI is probably best explained by dementia (and dementia related illness) as cause of death. The likelihood of death from dementia, as a specific cause of death, was evidently higher in amnestic-multiple domain MCI compared with other MCI subtypes. These results are consistent with observations that dementia is a major contributor of death in MCI individuals [33], and that amnestic-multiple domain emerges as the strongest subtype of MCI that predicts onset of dementia [27,31]. Although other causes of death were more common in MCI subgroups, no significant differences emerged in comparison with the reference group. It should be also considered that a short interval of 5-years was not enough to confirm the effect of dementia on mortality in MCI individuals. In this sense, MCI is sometimes a transition state between normal aging and dementia [1].
A number of limitations to the study should be acknowledged. We recognize that assessment using the MMSE-37 is not comparable to a comprehensive neuropsychological assessment, but the criteria to confirm the presence of cognitive impairment in MCI are not clearly defined [6,34,35]. Previous studies have suggested that MMSE domains may be useful and more sensitive than the general scores for MCI detection [21,36]. Although it is always possible that certain MCI individuals could be misclassified, MCI diagnosis was also based the absence of functional impairment by FAQ scale [37], and the exclusion of dementia by a standard two-phase procedure which included the assessment of expert neurologists [18]. In this study, subjective memory complaint was not considered a necessary part of MCI diagnosis due to its value is controversial [25,38], and new criteria from DSM-V propose subjective memory complaints are not required. It should also be noted that dementia is often an under-reported condition of death [39]. Nevertheless, in this study dementia was a unique cause of death in MCI cases, which supports an acceptable validity of the label in this cohort. The exclusion of participants who did not complete screening, as well as the low socioeconomic status and education of this population may limit the generalization of the results. Finally, changes in cognition, function, and comorbidities were not measured over time, but the large sample size and complete data on cause of death over 13 years are strengths of the study.
To conclude, associations between cognition and prognosis of various medical conditions have been previously reported [40,41]. This study supports the notion that MCI measured by simple cognitive tests, is a risk factor of long-term mortality. However, only amnestic-multiple domain MCI showed an increased risk of short-term mortality. Finally, dementia was the only specific cause of death significantly increased in all MCI subtypes who were examined in this cohort. Causes of death in those with MCI require further investigation in the future.
Supplementary Material
Acknowledgments
Additional information about collaborators and detailed funding of the NEDICES Study can be found on the web (http://www.ciberned.es/estudio-nedices).
Funding: The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Drs. Benito-León and Bermejo-Pareja are supported by NIH R01 NS039422 from the National Institutes of Health, Bethesda, MD, USA. Dr. Sánchez-Ferro is supported by a grant “Contrato de Investigación Río Hortega” from Instituto de Salud Carlos III, Spain.
Footnotes
Contributors: Dr. Contador (icontador@usal.es) collaborated in: 1) the conception, organization and execution of the research project; 2) the statistical analysis design, and; 3) the writing of the manuscript first draft and the review and critique of the manuscript. Dr. Bermejo-Pareja (fbp.gijon@yahoo.es) collaborated in: 1) the conception, organization and execution of the research project; and 2) the review and critique of the manuscript. Dr. Mitchell (ajm80@le.ac.uk) collaborated in: 1) the conception, organization and execution of the research project; and 2) the review and critique of the manuscript. Ms Trincado (rtrincado@ono.com) collaborated in: 1) the statistical analysis design; and 2) the review and critique of the manuscript. Dr. Villarejo (avgalende@yahoo.es) collaborated in: 1) the conception, organization and execution of the research project; and 2) the review and critique of the manuscript. Dr. Sánchez-Ferro (alvarosferro@hotmail.com) collaborated in: 1) the conception, organization and execution of the research project; and 2) the review and critique of the manuscript. Dr. Benito-León (jbenitol@meditex.es) collaborated in: 1) the conception, organization and execution of the research project; and 2) the review and critique of the manuscript.
Disclosure of Conflict of Interest: None
REFERENCES
- 1.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 2.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Allegri RF, Glaser FB, Taragano FE, et al. Mild cognitive impairment: believe it or not? Int Rev Psychiatry. 2008;20:357–363. doi: 10.1080/09540260802095099. [DOI] [PubMed] [Google Scholar]
- 6.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- 8.Guehne U, Angermeyer MC, Riedel-Heller S. Is mortality increased in mildly cognitively impaired individuals? A systematic literature review. Dement Geriatr Cogn Disord. 2006;21:403–410. doi: 10.1159/000092846. [DOI] [PubMed] [Google Scholar]
- 9.Villarejo A, Benito-León J, Trincado R, et al. Dementia-associated mortality at thirteen years in the NEDICES Cohort Study. J Alzheimers Dis. 2011;26:543–551. doi: 10.3233/JAD-2011-110443. [DOI] [PubMed] [Google Scholar]
- 10.Lopez OL, Becker JT, Chang Y-F, et al. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology. 2012;79:1599–1606. doi: 10.1212/WNL.0b013e31826e25f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Aggarwal NT, Barnes LL, et al. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66:767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephan BCM, Brayne C, Savva GM, et al. Occurrence of medical comorbidity in mild cognitive impairment: implications for generalisation of MCI research. Age and Ageing. 2011;40:501–507. doi: 10.1093/ageing/afr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales JM, Bermejo FP, Benito-León J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health. 2004;118:426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Vega S, Benito-León J, Bermejo-Pareja F, et al. Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J Clin Epidemiol. 2010;63:215–222. doi: 10.1016/j.jclinepi.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Prieto G, Contador I, Tapias-Merino E, et al. The Mini-Mental-37 test for dementia screening in the Spanish population: an analysis using the Rasch Model. Clin Neuropsychol. 2012;26:1003–1018. doi: 10.1080/13854046.2012.704945. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo-Pareja F, Benito-León J, Vega S, et al. Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci. 2008;264:63–72. doi: 10.1016/j.jns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Bermejo-Pareja F, Benito-León J, Vega S, et al. Consistency of clinical diagnosis of dementia in NEDICES: A population based longitudinal study in Spain. J Geriatr Psychiatry Neurol. 2009;22:246–255. doi: 10.1177/0891988709335794. [DOI] [PubMed] [Google Scholar]
- 19.Amaducci L, Baldereschi M, Amato MP, et al. The World Health Organization cross-national research program on age-associated dementias. Aging (Milano) 1991;3:89–96. doi: 10.1007/BF03323983. [DOI] [PubMed] [Google Scholar]
- 20.Villanueva-Iza C, Bermejo-Pareja F, Berbel-Garcia A, et al. Validation of a clinical protocol for the detection of dementia in the population. Rev Neurol. 2003;36:1121–1126. [PubMed] [Google Scholar]
- 21.Loewenstein DA, Acevedo A, Ownby R, et al. Using different memory cutoffs to assess mild cognitive impairment. Am J Geriatr Psychiatry. 2006;14:911–919. doi: 10.1097/01.JGP.0000229651.62137.e2. [DOI] [PubMed] [Google Scholar]
- 22.Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54:335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor ML, Edwards JD, Wadley VG, et al. Changes in mobility among older adults with psychometrically defined mild cognitive impairment. J Gerontol B Psychol Sci Soc Sci. 2010;65B:306–316. doi: 10.1093/geronb/gbq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardila A, Bertolucci PH, Braga LW, et al. Illiteracy: the neuropsychology of cognition without reading. Arch Clin Neuropsychol. 2010;25:689–712. doi: 10.1093/arclin/acq079. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 26.Guehne U, Luck T, Busse A, et al. Mortality in individuals with mild cognitive impairment. Results ofthe Leipzig Longitudinal Study of the Aged (LEILA75+) Neuroepidemiology. 2007;29:226–234. doi: 10.1159/000112479. [DOI] [PubMed] [Google Scholar]
- 27.Baars MAE, Van Boxtel MPJ, Dijkstra JB, et al. Predictive value of mild cognitive impairment for dementia. The influence of case definition and age. Dement Geriatr Cogn Disord. 2009;27:173–181. doi: 10.1159/000200465. [DOI] [PubMed] [Google Scholar]
- 28.Hunderfund AL, Roberts RO, Slusser TC, et al. Mortality in amnestic mild cognitive impairment: a prospective community study. Neurology. 2006;67:1764–1768. doi: 10.1212/01.wnl.0000244430.39969.5f. [DOI] [PubMed] [Google Scholar]
- 29.Villarejo A, Bermejo-Pareja F, Trincado R, et al. Memory impairment in a simple recall task increases mortality at 10 years in non-demented elderly. Int J Geriatr Psychiatry. 2011;26:182–187. doi: 10.1002/gps.2512. [DOI] [PubMed] [Google Scholar]
- 30.St John PD, Montgomery PR, Kristjansson B, et al. Cognitive scores, even within the normal range, predict death and institutionalization. Age and Ageing. 2002;31:373–378. doi: 10.1093/ageing/31.5.373. [DOI] [PubMed] [Google Scholar]
- 31.Han JW, Kim TH, Lee SB, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement. 2012;8:553–559. doi: 10.1016/j.jalz.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68:761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 34.Kochan NA, Slavin MJ, Brodaty H, et al. Effect of different impairment criteria on prevalence of “objective” mild cognitive impairment in a community sample. Am J Geriatr Psychiatry. 2010;18:711–722. doi: 10.1097/jgp.0b013e3181d6b6a9. [DOI] [PubMed] [Google Scholar]
- 35.Matthews FE, Stephan BCM, McKeith IG, et al. Two-year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56:1424–1433. doi: 10.1111/j.1532-5415.2008.01820.x. [DOI] [PubMed] [Google Scholar]
- 36.Diniz BSO, Yassuda MS, Nunes PV, et al. Mini-mental State Examination performance in mild cognitive impairment subtypes. Int Psychogeriatr. 2007;19:647–656. doi: 10.1017/S104161020700542X. [DOI] [PubMed] [Google Scholar]
- 37.Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Ger Psychiatry. 2008;23:1191–1202. doi: 10.1002/gps.2053. [DOI] [PubMed] [Google Scholar]
- 39.Ganguli M, Rodriguez EG. Reporting of dementia on death certificates: a community study. J Am Geriatr Soc. 1999;47:842–849. doi: 10.1111/j.1532-5415.1999.tb03842.x. [DOI] [PubMed] [Google Scholar]
- 40.Colsher PL, Wallace RB. Epidemiologic considerations in studies of cognitive function in the elderly: methodology and nondementing acquired dysfunction. Epidemiol Rev. 1991;13:1–27. doi: 10.1093/oxfordjournals.epirev.a036065. [DOI] [PubMed] [Google Scholar]
- 41.Shipley BA, Der G, Taylor MD, et al. Cognition and mortality from the major causes of death: the Health and Lifestyle Survey. J Psychosom Res. 2008;65:143–152. doi: 10.1016/j.jpsychores.2008.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.