Summary
The outer membrane (OM) of Gram-negative bacteria is replete with a host of β-barrel outer membrane proteins (OMPs). Despite serving a variety of essential functions, including immune response evasion, the exact mechanism of OMP folding and membrane insertion remains largely unclear. The β-barrel assembly machinery (BAM) complex is required for OMP biogenesis. Crystal structures and molecular dynamics (MD) simulations of the central and essential component, BamA, suggest a mechanism involving lateral opening of its barrel domain. MD simulations reported here reveal an additional feature of BamA: a substrate exit pore positioned above the lateral opening site. Disulfide crosslinks that prevent lateral opening and exit pore formation result in a loss of BamA function, which can be fully rescued by the reductant TCEP. These data provide strong evidence that lateral opening and exit pore formation are required for BamA function.
Introduction
The targeting and assembly of membrane proteins is a complex process that requires multiple folding factors, including chaperones and integral membrane components. The biogenesis of α-helical inner membrane proteins is well characterized (Dalbey et al., 2011; du Plessis et al., 2011; Osborne et al., 2005; White and von Heijne, 2008). In the final stages of processing, α-helices are inserted laterally into the membrane by the Sec translocon in a step-wise manner. In contrast, much less is understood about the biogenesis of β-barrel outer membrane proteins found in bacterial, mitochondrial, and chloroplast outer membranes (Chacinska et al., 2009; Tommassen, 2010; Walther et al., 2009; Webb et al., 2012). β-barrel outer membrane proteins (OMPs) contain short amphipathic β-strands that assemble into a β-barrel through hydrogen bonding between the first and last β-strands. A folded β-barrel has a hydrophilic pore and a hydrophobic exterior, allowing it to reside in a lipid bilayer. Further complicating membrane insertion is the large structural diversity of OMPs, which range from 8–24 strands and can contain multiple functional domains at the N- or C-termini or within the loops connecting β-strands (Fairman et al., 2012).
OMP processing prior to membrane insertion is well characterized. In Gram-negative bacteria, OMPs are synthesized in the cytoplasm and transported across the inner membrane by the Sec translocon. Once in the periplasm, molecular chaperones such as Skp and SurA escort nascent OMPs to the OM inner surface (Pugsley, 1993; Sklar et al., 2007). There they are recognized by the beta-barrel assembly machinery (BAM) complex, consisting of BamA (an OMP itself) and four lipoproteins called BamB-E (Hagan et al., 2011; Kim et al., 2012; Knowles et al., 2009; Ricci and Silhavy, 2012). BamA has a periplasmic extension which consists of five polypeptide translocation associated (POTRA) domains. Current understanding suggests that the four lipoproteins assemble onto the POTRA scaffold to create a BAM complex containing one copy of each protein. Despite significant functional studies (Hagan et al., 2010; Leonard-Rivera and Misra, 2012; Malinverni et al., 2006; Rigel et al., 2013) and crystal structures for BamB, BamC, BamD, BamE (Albrecht and Zeth, 2011; Dong et al., 2012; Heuck et al., 2011; Kim et al., 2011a, 2012; Kim et al., 2011b; Kim et al., 2011c; Kim and Paetzel, 2011; Knowles et al., 2011; Noinaj et al., 2011; Sandoval et al., 2011; Warner et al., 2011), and the periplasmic POTRA domain of BamA (Gatzeva-Topalova et al., 2008; Gatzeva-Topalova et al., 2010; Kim et al., 2007; Knowles et al., 2008; Zhang et al., 2011), the mechanism for how the BAM complex collectively coordinates recognition, folding, and insertion of nascent OMPs is still not well understood. The structure of the membrane domain of BamA was thought to be the missing piece of this mechanistic puzzle.
Accordingly, we recently reported the crystal structures of BamA from H. ducreyi (HdBamA) and N. gonorrheae (NgBamA) (Noinaj et al., 2013). Unlike FhaC, a transporter within the two-partner secretion (TPS) pathway that secretes polypeptide substrates across the outer membrane through its barrel pore, the extracellular loops of BamA form a capping dome over the barrel domain occluding surface access and preventing free diffusion across the outer membrane. Additionally, loop 6 of BamA and the well conserved VRGF/Y motif were located in the upper region of the barrel domain, unlike FhaC where loop 6 and the VRGF/Y motif extend toward the periplasm (Clantin et al., 2007). Further analysis of the BamA structure suggested that the barrel domain alone could significantly destabilize the outer membrane, potentially priming it for insertion of new OMPs. This was recently verified by electron microscopy observations of BamA-induced membrane thinning in liposomes (Sinnige et al., 2014). Possibly most interesting, our structures revealed that barrel domain strands β1 and β16 are only weakly associated, and molecular dynamics (MD) simulations predicted lateral opening at this interface. The shifted conformation of loop 6 (and VRGF/Y motif) and the possible lateral opening of the barrel domain were further supported by the recent structure determination of TamA, a close homolog of BamA (Gruss et al., 2013). Here we report evidence that not only is lateral opening required for BamA function, but also the formation of a newly discovered substrate exit pore positioned above the lateral opening site. We propose a model whereby nascent OMPs are threaded through the barrel domain of BamA with membrane bound β-strands inserted directly into the membrane through the lateral opening, while extracellular bound domains and loops make their way to the surface via the substrate exit pore.
Results and Discussion
Lateral opening in BamA is required for function
Following the creation of an E. coli BamA (EcBamA) homology model based on HdBamA and NgBamA crystal structures, MD simulations of its conformational stability were performed. After truncating the POTRA domains, the EcBamA model was placed in a DMPE bilayer, which approximates the hydrophobic thickness of the outer membrane. A lateral opening was observed between stand β1 and β16 over the course of a 500-ns simulation (Figure 1A, 1B, and 1C), as was previously seen for HdBamA and NgBamA (Noinaj et al., 2013). This opening reached a maximum of ~9 Å after 120 ns and is caused by changes in the number of backbone hydrogen bonds between strands β1 and β16, which is initially two and fluctuates between zero and four (average of 1.24). Although complete closure was not observed for EcBamA, the lateral opening did decrease over time, and by the end of the simulation the C-terminal β-strand is bent toward the barrel interior. This non-canonical barrel structure, intermediate between fully open and fully closed, leaves the two sides of the proposed gate detached but preserves its ability to prevent an influx of lipids from the membrane.
Figure 1. Lateral opening is essential for BamA function.
A. Model of the ‘closed’ state of EcBamA based on the HdBamA crystal structure. B. Model of the ‘open’ state of EcBamA based on MD simulations. Lateral opening is indicated by the red dashed circle. C. MD simulation plots showing separation between strands β1 and β16 for EcBamA (black), NgBamA (red), and FhaC (grey). D. Disulfide crosslinks were engineered along EcBamA strands β1 and β16 to prevent lateral opening. Strands β1 and β16 of the β-barrel (pink, side chains removed for clarity) are shown with the positions of targeted residues. Dashed lines and colored circles (yellow: disulfides which would point into the lumen of the barrel domain, green: disulfides which would point into the membrane) indicate the engineered disulfide. E. Crosslink mutants were prepared using the C690S/C700S cysteine-less construct, which functions as wild type BamA. Using JCM-166 cells, colony growth assays tested the viability of the disulfide crosslink mutants. In the absence of arabinose, only the C690S/C700S mutant was fully functional. The presence of TCEP rescued mutant EcBamA function.
To determine if flexibility within the C-terminal strand is important for the observed lateral opening, we mutated a highly conserved Gly in strand β16 (E. coli G807) to alanine, valine, and phenylalanine and analyzed these mutants in plate growth assays. Whether mutating G807 fully prevents the conformational dynamics of the C-terminal strand remains to be determined. Even so, we observed no change in phenotype, indicating that strand flexibility alone does not play a significant role in BamA function (Table 1). To probe the possible role of lateral opening, we next engineered paired cysteine mutants between strands β1 and β16 to prevent barrel opening via disulfide crosslinking (Figure 1D). All crosslink mutants were made using the C690S/C700S no cysteine construct (C2S positive control) which has previously been shown to function like wild type BamA (Noinaj et al., 2013; Rigel et al., 2013). Assaying for colony growth on rich media LB agar, only the I806C/Y432C mutant supported any growth, albeit very minimal. However, the addition of reductant (TCEP) rescued functionality for all disulfide mutants, indicating that preventing lateral opening of the barrel domain of BamA renders it non-functional (Figure 1E and Table 1, and Figure S1 and Figure S2).
Table 1.
Mutations targeting the lateral gate and exit pore of BamA. The functional importance of terminal β-strand (strand 16) mobility was tested by paired mutations in strand β1 and β16 (see also Figure 1). Mutations were designed to immobilize strand β16 by cysteine crosslinking. Additional point mutations in strand β16 were also designed to decrease its predicted flexibility. The ability of mutants to support JCM-166 cell growth was assessed by colony formation on LB-agar. For Colony Growth, (+) indicates colony formation equivalent to wild type BamA transformants in the absence of arabinose and (−) indicates no colony formation.
| Mutant Construct | Structural Group | Colony Growth |
|---|---|---|
| G807A | Strand 16 flexibility | + |
| G807V | " | + |
| G807F | " | + |
| C690S/C700S | Loop 6 cysteines | + |
| N805C | Lateral opening (strand β1)1 | + |
| I806C | " | + |
| G807C | " | + |
| K808C | " | + |
| T809C | " | + |
| W810C | " | - |
| G429C | Lateral opening (strand β16)1 | + |
| I430C | " | + |
| G431C | " | + |
| Y432C | " | + |
| G433C | " | + |
| N805C/G433C | Lateral opening (crosslinks)1 | − / + 2 |
| I806C/Y432C | " | − / + 2 |
| G807C/G431C | " | − / + 2 |
| K808C/I430C | " | − / + 2 |
| T809C/G429C | " | − / + 2 |
| E435C | Exit Pore1 | + |
| S665C | " | + |
| E435C/S665C | " | − / + 2 |
| T659C | Loop 6 (crosslink)1 | + |
| V713C | " | + |
| T659C/V713C | " | + |
Mutations made in the C690S/C700S cysteine-less EcBamA mutant plasmid.
Colony growth only observed in the presence of reductant (for I806C/Y432C, minimal growth was observed in absence of arabinose and reductant). A dominant negative phenotype was also observed in the presence of arabinose where cells grew slower and often more sparse on the plates.
To verify that the crosslink mutants were properly folded into the OM, we grew transformed JCM-166 cells under conditions to express both wild type and mutant BamA constructs. Heat modifiability assays determined whether the crosslink mutants were folded properly and proteinase K treatment of whole cells assessed their presentation at the surface of the cells. For heat modifiability, folded samples run faster on semi-native gels than unfolded samples (boiled). All disulfide mutants were heat modifiable and susceptible to extracellular proteinase K treatment, demonstrating proper folding and presentation at the cell surface (Figure 2A and 2B). Furthermore, SDS-PAGE/Western blot analysis indicated clear disulfide formation for all mutants, which could be enhanced by treatment with the oxidizing agents copper sulfate (CuP) and copper-o-phenanthroline (CuP) (Figure 2C and 2D). BamA disulfide mutant phenotypes were further characterized with end point growth assays in shaking cultures. These results agreed well with the colony growth plate assays, except for the G431C/G807C mutant which could only be minimally rescued in 5 mM TCEP (Figure 2E). Disulfides oriented into the membrane (Y432C/I806C and I430C/K808C) formed less efficiently as observed by Western blot and were only minimally enhanced with CuS treatment, suggesting the membrane environment may be limiting disulfide formation. This may explain the residual growth of the Y432C/I806C and I430C/K808C mutants in plate and culture assays. CuP was most effective at enhancing crosslinks indicating that it may permeate the membrane better than CuS, however it was found to be toxic in growth assays.
Figure 2. BamA crosslink mutants are folded and incorporated into the outer membrane.
Crosslink mutants were prepared as described in Figure 1. Proteinase K (PK) digestion (panel A) and heat modifiability (panel B) assays were performed on cells grown in LB + 0.01% arabinose. All crosslink mutants were susceptible to PK and showed heat modifiability, indicating presentation on the surface and proper folding. C. To analyze disulfide crosslink formation under native conditions, cell lysates were resolved by SDS-PAGE −/+ DTT. All of the engineered disulfide mutants formed disulfide crosslinks within the cell. Additionally, treatment with 100 µM copper sulfate (CuS) or 100 µM copper o-phenanthroline (CuP) enhanced disulfide formation (panel D). Notably, those crosslinks pointing into the lumen of the β-barrel were formed more efficiently than those pointing into the membrane. E. End-point growth assays agreed well with results observed in plate assays (Fig. 1E), with the exception of the G431C/G807C mutant. F. All mutants formed crosslinks in the DsbA- strain, indicating that crosslinks formed in the membrane. However, DsbA enhanced disulfide formation for some mutants. G. Treatment of the DsbA- cells with CuS or CuP enhanced crosslinking.
DsbA is a periplasmic protein that assists in disulfide formation. To determine whether DsbA is required for disulfide formation in the EcBamA crosslink mutants, experiments were repeated in a DsbA- strain and its parent strain (DsbA+). DsbA was not required for disulfide formation, however its presence enhanced crosslinking efficiency for some of the mutants (Figure 2F). Similar to results in the JCM-166 cells, CuS and CuP treatment enhanced disulfide formation for all mutants (Figure 2G). Taken together, our structural and functional analysis of the BamA crosslink mutants leads us to conclude that lateral opening of the BamA barrel domain is indeed required for function.
Formation of a putative substrate exit pore in BamA is required for function
MD simulations on the EcBamA homology model revealed an unexpected additional structural feature, a putative substrate exit pore located at the junction between loops 1–3 and loop 6, just above the lateral opening site (Figure 3A and Movie S1). The opening and closing of the exit pore is mediated primarily by an inward/outward flipping motion of loop 1. Plots monitoring the exit pore show that it remained open for much of the EcBamA and NgBamA MD simulations, both of which started with a destabilized C-terminal strand (Figure 3B) (Noinaj et al., 2013). The exit pore in HdBamA (initiated with an ordered C-terminal strand as found in the crystal structure) adopted an open state in only brief instances, suggesting a possible link between lateral opening of the β-barrel and exit pore formation. Still, we clearly observe independent states for each (e.g. lateral gate open and exit pore closed and vice versa) and therefore treat them as separate states experimentally.
Figure 3. Substrate exit pore formation in BamA is necessary for function.
A. Surface view of a putative substrate exit pore in EcBamA (from MD simulations). B. MD simulation plots monitoring the opening/closing of the exit pore for EcBamA (black), NgBamA (red), and HdBamA (green). C. Cartoon representation of EcBamA (green) showing the exit pore in a closed state (left, zoomed view shown on the right). Yellow spheres indicate the locations of residues E435 and S665. D. Using JCM-166 cells, colony growth assays tested the viability of the exit pore disulfide crosslink mutant (E435C/S665C). In the presence of arabinose, all mutants grew similar to the no-cysteine control (C690S/C700S). In the absence of arabinose the mutants E435C, S665C, and C690S/C700S all showed colony growth, but no colonies were observed for the double crosslink mutant E435C/S665C. Function of the E435C/S665C EcBamA was rescued by TCEP, indicating that opening of the exit pore was necessary for functionality. E. Heat modifiability for the E435C/S665C mutant (grown in the presence of 0.01% arabinose) and native disulfide formation (panel F) were both observed.
To investigate the functional role of the exit pore in BamA, we engineered a disulfide crosslink between residues E435 and S665 (Figure 3C). No growth was observed for the E435C/S665C crosslink mutant, despite normal growth for each of the single point mutants (Figure 3D and Table 1). However, normal growth for the E435C/S665C crosslink mutant was recovered in the presence of TCEP. Heat modifiability verified proper folding of the crosslink mutant (Figure 3E), and Western blot analysis clearly showed the oxidized form running slightly faster than the reduced form, yet slower than the oxidized form of the G431C/G807C crosslink mutant (Figure 3F). These results indicate that formation of the exit pore is required for function in BamA.
BamA function does not require a conformational change of loop 6
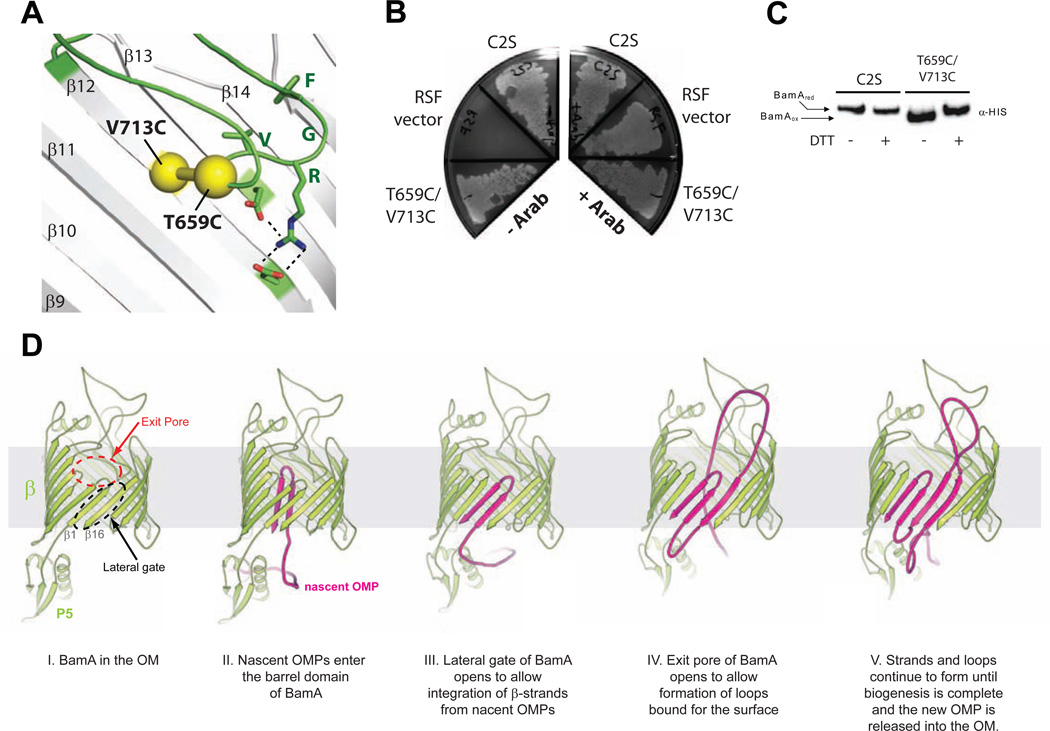
Previous work suggested that loop 6 of BamA may undergo a large conformational change, consistent with a structural comparison to FhaC (Noinaj et al., 2013). Therefore, it is possible that the E435C/S665C mutation could prevent a conformational change in loop 6 that is required for BamA function, which would also explain our observed results. We therefore engineered a disulfide crosslink between T659 (the residue immediately before the conserved VRGF motif) and V713 (inner wall along strand 12), which were ideally positioned to anchor loop 6 in a single conformation (Figure 3A). Normal JCM-166 colony growth was observed for the T659C/V713C crosslink mutant in the absence of arabinose, indicating that the loop 6 crosslink did not significantly affect BamA function (Figure 3B). To verify crosslink formation, samples were run with and without DTT on a 5% SDS-PAGE/MOPS gel followed by Western blot analysis, showing clear 100% disulfide crosslink formation as evident by an increase in mobility compared to the C2S and reduced (+DTT) samples (Figure 3C). Together, these results support the conclusion that loop 6 remains in a mostly fixed conformation and a large conformational change of loop 6 is not required for BamA function. This is further supported by the lack of any conformational changes in our MD simulations and the fact that loop 6 has been observed in the same conformation in all known BamA (and TamA) crystal structures (Gruss et al., 2013; Ni et al., 2014; Noinaj et al., 2013).The conformational differences in loop 6 between FhaC and BamA may be reflective of the differing functions between the two proteins. Notably, recent studies show loop 6 of FhaC remains in a mostly fixed conformation and may not sample large conformational changes as had been proposed (Guerin et al., 2014).
Hybrid model for the role of BamA in OMP biogenesis
Kim et al. recently summarized four models for the role of the BAM complex in OMP assembly: (i) ‘the oligomeric BamA-assisted model’ where oligomers of BamA orchestrate insertion of OMPs into the OM; (ii) ‘the BamA-assisted model’ where BamA primes the OM for ‘spontaneous’ insertion of the OMPs; (iii) ‘the export model’ where OMPs are exported through the barrel domain of BamA to the surface, then inserted directly into the membrane; and (iv) ‘the budding model’ where OMPs use BamA as a template/scaffold to fold, eventually budding from BamA directly into the membrane (Kim et al., 2012). While early studies reported that BamA may function as an oligomer, recent in vitro studies clearly show that BamA (and the BAM complex) is likely monomeric (Hagan et al., 2010). This is supported by the monomeric crystal structures of BamA (Noinaj et al., 2013), suggesting that the oligomeric BamA-assisted model is likely incorrect, especially considering the vast steric clashes that would result from the interaction of multiple fully assembled BAM complexes. We favor the BamA-assisted model largely due to our observation that the barrel domain of BamA can significantly destabilize the local membrane, which may prime the membrane (reduce the kinetic energy required) for direct insertion (Burgess et al., 2008; Gessmann et al., 2014; Moon et al., 2013; Sinnige et al., 2014). Here, BamA may serve as an OM targeting factor for OMPs which can spontaneously fold into membranes efficiently. Rather than favoring two separate export and budding models, we present evidence here that supports a hybrid model. We hypothesize that the β-barrel lateral opening is for direct insertion of the membrane segments into the membrane, loosely similar to what is observed for the Sec translocon and α-helical transmembrane domains (Gumbart et al., 2013). This lateral opening would lead to exposed unpaired β-strands in BamA (β1 and β16) which could be used for nucleating OMP folding/insertion via β-strand templating. We hypothesize that the first step may be the C-terminal strand of the new OMP binding to strand 1 of BamA, essentially replacing the disordered C-terminal strand (strand 16) of BamA. In addition, our MD simulations and mutagenesis reveal the formation of a substrate exit pore which may be required to allow exit of extracellular loops of nascent OMPs during folding by BamA. We thus propose a model whereby nascent OMPs are threaded through the barrel domain of BamA with membrane bound β-strands inserted directly into the membrane through the lateral opening, while extracellular domains and loops make their way to the surface through the substrate exit pore (Figure 4D and Movie S2). A transient BamA:OMP intermediate would form to allow ‘budding’ of the new OMP alongside the barrel domain of BamA. Folding may be terminated once the first strand of the new OMP enters into the barrel domain of BamA, which may have higher affinity for its last strand than does the first strand of BamA, thereby triggering strand exchange and closing of the barrel domain of the new OMP, releasing the new OMP to diffuse into the membrane.
Figure 4. BamA function does not require a large conformational change in loop 6 along the conserved VRGF motif.
A. Zoomed view inside the BamA (gray) showing the engineered disulfide crosslink (T659C/V713C) (yellow) to anchor loop 6 to the inner wall of the barrel domain. Loop 6 is shown in green, residues of the conserved VRGF motif are shown as sticks. B. Plate assays showed normal growth for the loop 6 disulfide crosslink mutant (T659C/V713C) in the presence and absence of arabinose, indicating that the crosslink, if formed, was not significantly affecting BamA function. C. To verify loop 6 crosslink formation, SDS-PAGE/Western blot analysis directly visualized increased electrophoretic mobility of the crosslink mutant compared to the C2S control, which could be reduced by DTT treatment. The loop 6 crosslink forms completely, yet does not affect BamA function. D. Updated model for the role of BamA in OMP biogenesis: I - The β-barrel domain (β) and POTRA 5 domain (P5) of BamA (green) are shown in the outer membrane (OM, gray). For clarity, POTRAs 1–4 are omitted. II - Upon delivery to the BAM complex, nascent OMPs (magenta) interact with the POTRA domains and enter the barrel domain of BamA (open conformation). III - The lateral gate of BamA is utilized for the insertion of membrane-bound strands directly into the OM, a process that may involve strand templating and β-augmentation. IV - Extracellular-bound loops utilize the substrate exit pore of BamA for presentation on the cell surface. V - Strands and extracellular loops of the nascent OMPs continue to form via the lateral gate and exit pore, respectively, until biogenesis is complete and the new OMP is released into the OM.
Experimental Procedures
Cloning and Mutagenesis
The experiments here are based upon our previously reported homology model of the BamA barrel domain from E. coli (EcBamA) (Noinaj et al., 2013). For mutations of EcBamA, the bamA coding sequence containing an N-terminal pelB signal sequence and 10X-His tag was placed into the pRSF-1b vector (EMD Millipore). Mutants were prepared using standard site-directed mutagenesis protocols (primer sequences available upon request). For disulfide crosslinking assays in JCM-166 cells, mutants were prepared in a cysteine-less EcBamA construct (C690S/C700S), which functions like wild type (Rigel et al., 2013). For the DsbA- cells (JW3832-2, Keio Collection) and the DsbA+ parent strain (BW25113, Keio Collection), mutants were prepared using the pET20b vector (EMD Millipore), also in the (C690S/C700S) EcBamA construct.
Plate assays with JCM-166 cells
JCM-166 cells, whose endogenous BamA is under the control of an arabinose promoter (Wu et al., 2005), were transformed via electroporation with pRSF-1b vector coding for a mutant BamA under kanamycin selection, and were then plated on LB+agar plates with 50 µg/mL kanamycin (kan) in the presence or absence of 0.1% L-(+)-arabinose and grown for 12–15 hrs at 37°C. Where noted, plates were saturated with reductants (DTT, βME, or TCEP) prior to plating cells.
JCM-166 cell end-point growth assays
Disulfide mutant colonies from plates grown in the presence of 0.1% arabinose were used for 3 mL starter cultures (0.01% arabinose+kan) and grown to OD600=1 – 2. Then 1 mL of cells at OD600=1.0 were pelleted, washed 3 times in 1X PBS + 10% glycerol, and resuspended in 1 mL 1X PBS + 10% glycerol. Three µL of each sample was then added to 3 mL of LB+kan media supplemented with or without arabinose (0.01%), with TCEP (5 mM), with copper sulfate (50 µM), or with copper o-phenanthroline (50 µM). Culture growths (performed in triplicate) were incubated at 37°C, shaking at 220 RPM, for ~10 hours. OD measurements at 595 nm were performed using a BioRad iMark Microplate Reader. Data was analyzed and prepared using Microsoft Excel.
Heat modifiability assays
To determine if the EcBamA mutants were folded properly, heat modifiability assays were performed using whole cell lysates. One mL of cells at OD600 =1.0 were centrifuged, resuspended in 1X PBS, 1% DDM, 10 mM EDTA, 10 µg/mL lysozyme, supplemented with AEBSF and DNaseI, and rocked at room temperature for 15 min before centrifugation for 10 min at 15,000 rpm using a microcentrifuge at 4°C. SDS-loading buffer was then added and either boiled or left at ~23°C for 5–10 min. Samples were then separated using NativePage 4–12% gels (Invitrogen) with 1X MES SDS-PAGE running buffer by running the gels for 60 min at 150 volts (constant) at 4°C. After transfer to PVDF membrane via the iBlot system (Invitrogen) anti-HIS-HRP (Sigma) antibodies were used for Western blot analysis. Antibody staining was visualized with the ECL Prime kit (GE Healthcare), and imaged with an ImageQuant LAS 4000 imaging system (GE Healthcare).
Proteinase K digestion assays
To assess the surface exposure of EcBamA constructs, proteinase K digestion assays were performed on whole cells as previously reported (Rigel et al., 2013). Briefly, colonies were grown in LB + 0.01% arabinose liquid culture overnight at 37°C. 1.0 mL of cells at OD600 =1.0 were resuspended in 20 mM Tris-HCl 7.4, 100 mM NaCl. Then, 0.5 mg/mL proteinase K was added and incubated at 37°C for 30 minutes. Phenylmethanesulfonylfluoride (PMSF) was added (10 mM), cells were centrifuged again, and supernatant removed. PMSF was added again and the cells were resuspended in 1X LDS loading buffer plus DTT, boiled and separated using NuPAGE analysis. Transfer to PVDF membrane was performed using the iBlot system and anti-HIS-HRP and anti-MBP (NEB) antibodies were used for Western blot analysis, visualized as noted above.
Disulfide crosslinking assays
To observe the oxidized form of the disulfide crosslink mutants, cultures were allowed to grow to an OD600=1.5 before harvesting with and without 50 mM DTT in 1X LDS sample loading buffer and run on a 5% SDS-PAGE gel in 1X MOPS buffer at constant 200V for 60–70 minutes. Western blot analysis was performed as above. To induce oxidation with copper crosslinkers, cells were pelleted, washed, and resuspended in 1 mL of reaction buffer (20 mM Tris-HCl, pH 7.5 and 100 mM NaCl), supplemented with 100 µM of either copper sulfate or copper ophenanthroline, and incubated at 37°C for 15–30 minutes. Cells were pelleted, washed, and then resuspended in 1X LDS sample loading buffer without DTT and analyzed using 5% SDS-PAGE/1X MOPS/Western blot analysis as noted above.
Molecular dynamics simulations
MD simulations have previously been described (Noinaj et al., 2013). For simulations of EcBamA, a homology model was used with the POTRA domains truncated at residue 416 to reduce the system size. While this manuscript was under review, coordinates for two EcBamA crystal structures were released into the Protein Data Bank (PDB codes 4N75 (Ni et al., 2014) and 4C4V), which have an RMSD of 0.6 Å to one another along the barrel domain. A comparison of our model to these coordinates yields RMSDs of 0.9/1.2 Å, respectively, and the backbone/rotamer conformations of the regions/residues highlighted in this study are almost indistinguishable from the crystal structures, verifying that our model of EcBamA is accurate. After equilibration in a DMPE model bilayer using NAMD (Phillips et al., 2005), a 500-ns simulation at 340 K was run using the Anton supercomputer (Shaw et al., 2008). The high temperature was again used to increase the probability of lateral opening, although opening was also observed at 310 K for NgBamA (Noinaj et al., 2013). Strand separation at the putative gate was tracked by projecting the distance between residues 806 on strand β16 and 432 on strand β1 onto the vector normal to strand β16. Exit pore opening was quantified by measuring the distance between residues E435 and G656 for EcBamA, G432 and G655 for HdBamA, and D435 and G655 for NgBamA.
Supplementary Material
Highlights.
The membrane domain of BamA primes the local membrane for OMP insertion
Lateral opening and exit pore formation are required for BamA function
Lateral opening in BamA may mediate strand formation of new OMPs
Substrate exit pore formation in BamA may assist in loop formation of new OMPs
Acknowledgements
We thank H. Bernstein and R. Ieva for providing JCM-166 cells. NN, AK, and SKB are supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. CB is funded by a GAANN fellowship in the Molecular Biophysics Training Program at Georgia Tech. JCG is supported by NIH grants K22-AI100927 and R01-GM67887. Anton computer time provided by the National Resource for Biomedical Supercomputing and the Pittsburgh Supercomputing Center through NIH Grant RC2GM093307, using a machine donated by DE Shaw Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht R, Zeth K. Structural basis of outer membrane protein biogenesis in bacteria. J Biol Chem. 2011;286:27792–27803. doi: 10.1074/jbc.M111.238931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess NK, Dao TP, Stanley AM, Fleming KG. Beta-barrel proteins that reside in the Escherichia coli outer membrane in vivo demonstrate varied folding behavior in vitro. J Biol Chem. 2008;283:26748–26758. doi: 10.1074/jbc.M802754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clantin B, Delattre AS, Rucktooa P, Saint N, Meli AC, Locht C, Jacob-Dubuisson F, Villeret V. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily. Science. 2007;317:957–961. doi: 10.1126/science.1143860. [DOI] [PubMed] [Google Scholar]
- Dalbey RE, Wang P, Kuhn A. Assembly of bacterial inner membrane proteins. Annu Rev Biochem. 2011;80:161–187. doi: 10.1146/annurev-biochem-060409-092524. [DOI] [PubMed] [Google Scholar]
- Dong C, Hou HF, Yang X, Shen YQ, Dong YH. Structure of Escherichia coli BamD and its functional implications in outer membrane protein assembly. Acta Crystallogr D Biol Crystallogr. 2012;68:95–101. doi: 10.1107/S0907444911051031. [DOI] [PubMed] [Google Scholar]
- du Plessis DJ, Nouwen N, Driessen AJ. The Sec translocase. Biochim Biophys Acta. 2011;1808:851–865. doi: 10.1016/j.bbamem.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Fairman JW, Dautin N, Wojtowicz D, Liu W, Noinaj N, Barnard TJ, Udho E, Przytycka TM, Cherezov V, Buchanan SK. Crystal structures of the outer membrane domain of intimin and invasin from enterohemorrhagic E. coli and enteropathogenic Y. pseudotuberculosis. Structure. 2012;20:1233–1243. doi: 10.1016/j.str.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzeva-Topalova PZ, Walton TA, Sousa MC. Crystal structure of YaeT: conformational flexibility and substrate recognition. Structure. 2008;16:1873–1881. doi: 10.1016/j.str.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzeva-Topalova PZ, Warner LR, Pardi A, Sousa MC. Structure and flexibility of the complete periplasmic domain of BamA: the protein insertion machine of the outer membrane. Structure. 2010;18:1492–1501. doi: 10.1016/j.str.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessmann D, Chung YH, Danoff EJ, Plummer AM, Sandlin CW, Zaccai NR, Fleming KG. Outer membrane beta-barrel protein folding is physically controlled by periplasmic lipid head groups and BamA. Proc Natl Acad Sci U S A. 2014;111:5878–5883. doi: 10.1073/pnas.1322473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss F, Zahringer F, Jakob RP, Burmann BM, Hiller S, Maier T. The structural basis of autotransporter translocation by TamA. Nat Struct Mol Biol. 2013;20:1318–1320. doi: 10.1038/nsmb.2689. [DOI] [PubMed] [Google Scholar]
- Guerin J, Baud C, Touati N, Saint N, Willery E, Locht C, Vezin H, Jacob-Dubuisson F. Conformational dynamics of protein transporter FhaC: large-scale motions of plug helix. Mol Microbiol. 2014 doi: 10.1111/mmi.12585. [DOI] [PubMed] [Google Scholar]
- Gumbart JC, Teo I, Roux B, Schulten K. Reconciling the roles of kinetic and thermodynamic factors in membrane-protein insertion. Journal of the American Chemical Society. 2013;135:2291–2297. doi: 10.1021/ja310777k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CL, Kim S, Kahne D. Reconstitution of outer membrane protein assembly from purified components. Science. 2010;328:890–892. doi: 10.1126/science.1188919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CL, Silhavy TJ, Kahne D. beta-Barrel membrane protein assembly by the Bam complex. Annu Rev Biochem. 2011;80:189–210. doi: 10.1146/annurev-biochem-061408-144611. [DOI] [PubMed] [Google Scholar]
- Heuck A, Schleiffer A, Clausen T. Augmenting beta-Augmentation: Structural Basis of How BamB Binds BamA and May Support Folding of Outer Membrane Proteins. J Mol Biol. 2011;406:659–666. doi: 10.1016/j.jmb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Kim KH, Aulakh S, Paetzel M. Crystal structure of beta-barrel assembly machinery BamCD protein complex. J Biol Chem. 2011a;286:39116–39121. doi: 10.1074/jbc.M111.298166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Aulakh S, Paetzel M. The bacterial outer membrane beta-barrel assembly machinery. Protein Sci. 2012;21:751–768. doi: 10.1002/pro.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Aulakh S, Tan W, Paetzel M. Crystallographic analysis of the C-terminal domain of the Escherichia coli lipoprotein BamC. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011b;67:1350–1358. doi: 10.1107/S174430911103363X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kang HS, Okon M, Escobar-Cabrera E, McIntosh LP, Paetzel M. Structural characterization of Escherichia coli BamE, a lipoprotein component of the beta-barrel assembly machinery complex. Biochemistry. 2011c;50:1081–1090. doi: 10.1021/bi101659u. [DOI] [PubMed] [Google Scholar]
- Kim KH, Paetzel M. Crystal Structure of Escherichia coli BamB, a Lipoprotein Component of the beta-Barrel Assembly Machinery Complex. J Mol Biol. 2011;406:667–678. doi: 10.1016/j.jmb.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC, Kahne D. Structure and function of an essential component of the outer membrane protein assembly machine. Science. 2007;317:961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- Knowles TJ, Browning DF, Jeeves M, Maderbocus R, Rajesh S, Sridhar P, Manoli E, Emery D, Sommer U, Spencer A, et al. Structure and function of BamE within the outer membrane and the beta-barrel assembly machine. EMBO Rep. 2011;12:123–128. doi: 10.1038/embor.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles TJ, Jeeves M, Bobat S, Dancea F, McClelland D, Palmer T, Overduin M, Henderson IR. Fold and function of polypeptide transport-associated domains responsible for delivering unfolded proteins to membranes. Mol Microbiol. 2008;68:1216–1227. doi: 10.1111/j.1365-2958.2008.06225.x. [DOI] [PubMed] [Google Scholar]
- Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009;7:206–214. doi: 10.1038/nrmicro2069. [DOI] [PubMed] [Google Scholar]
- Leonard-Rivera M, Misra R. Conserved residues of the putative L6 loop of Escherichia coli BamA play a critical role in the assembly of beta-barrel outer membrane proteins, including that of BamA itself. J Bacteriol. 2012;194:4662–4668. doi: 10.1128/JB.00825-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinverni JC, Werner J, Kim S, Sklar JG, Kahne D, Misra R, Silhavy TJ. YfiO stabilizes the YaeT complex and is essential for outer membrane protein assembly in Escherichia coli. Mol Microbiol. 2006;61:151–164. doi: 10.1111/j.1365-2958.2006.05211.x. [DOI] [PubMed] [Google Scholar]
- Moon CP, Zaccai NR, Fleming PJ, Gessmann D, Fleming KG. Membrane protein thermodynamic stability may serve as the energy sink for sorting in the periplasm. Proc Natl Acad Sci U S A. 2013;110:4285–4290. doi: 10.1073/pnas.1212527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D, Wang Y, Yang X, Zhou H, Hou X, Cao B, Lu Z, Zhao X, Yang K, Huang Y. Structural and functional analysis of the beta-barrel domain of BamA from Escherichia coli. Faseb J. 2014 doi: 10.1096/fj.13-248450. [DOI] [PubMed] [Google Scholar]
- Noinaj N, Fairman JW, Buchanan SK. The Crystal Structure of BamB Suggests Interactions with BamA and Its Role within the BAM Complex. J Mol Biol. 2011;407:248–260. doi: 10.1016/j.jmb.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, Lithgow T, Buchanan SK. Structural insight into the biogenesis of beta-barrel membrane proteins. Nature. 2013;501:385–390. doi: 10.1038/nature12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci DP, Silhavy TJ. The Bam machine: A molecular cooper. Biochim Biophys Acta. 2012;1818:1067–1084. doi: 10.1016/j.bbamem.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigel NW, Ricci DP, Silhavy TJ. Conformation-specific labeling of BamA and suppressor analysis suggest a cyclic mechanism for beta-barrel assembly in Escherichia coli. Proc Natl Acad Sci U S A. 2013;110:5151–5156. doi: 10.1073/pnas.1302662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval CM, Baker SL, Jansen K, Metzner SI, Sousa MC. Crystal structure of BamD: an essential component of the beta-Barrel assembly machinery of gram-negative bacteria. J Mol Biol. 2011;409:348–357. doi: 10.1016/j.jmb.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DE, Deneroff MM, Dror RO, Kuskin JS, Larson RH, Salmon JK, Young C, Batson B, Bowers KJ, Chao JC, et al. Anton, a special-purpose machine for molecular dynamics simulation. Commun ACM. 2008;51:91–97. [Google Scholar]
- Sinnige T, Weingarth M, Renault M, Baker L, Tommassen J, Baldus M. Solid-State NMR Studies of Full-Length BamA in Lipid Bilayers Suggest Limited Overall POTRA Mobility. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes & development. 2007;21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J. Assembly of outer-membrane proteins in bacteria and mitochondria. Microbiology. 2010;156:2587–2596. doi: 10.1099/mic.0.042689-0. [DOI] [PubMed] [Google Scholar]
- Walther DM, Rapaport D, Tommassen J. Biogenesis of beta-barrel membrane proteins in bacteria and eukaryotes: evolutionary conservation and divergence. Cell Mol Life Sci. 2009;66:2789–2804. doi: 10.1007/s00018-009-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LR, Varga K, Lange OF, Baker SL, Baker D, Sousa MC, Pardi A. Structure of the BamC two-domain protein obtained by Rosetta with a limited NMR data set. J Mol Biol. 2011;411:83–95. doi: 10.1016/j.jmb.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CT, Heinz E, Lithgow T. Evolution of the beta-barrel assembly machinery. Trends Microbiol. 2012;20:612–620. doi: 10.1016/j.tim.2012.08.006. [DOI] [PubMed] [Google Scholar]
- White SH, von Heijne G. How translocons select transmembrane helices. Annu Rev Biophys. 2008;37:23–42. doi: 10.1146/annurev.biophys.37.032807.125904. [DOI] [PubMed] [Google Scholar]
- Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gao ZQ, Hou HF, Xu JH, Li LF, Su XD, Dong YH. High-resolution structure of a new crystal form of BamA POTRA4-5 from Escherichia coli. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:734–738. doi: 10.1107/S1744309111014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.